Abstract
Objective
To assess the proportion of primary care patients who report a family history (FH) of type 2 diabetes, coronary artery disease, breast cancer, or colorectal cancer (CRC); assess concordance of FH information derived from the electronic medical record (EMR) compared with patient-completed health questionnaires; and assess whether appropriate screening was informed by risk based solely on FH.
Design
Data from the BETTER (Building on Existing Tools to Improve Chronic Disease Prevention and Screening in Primary Care) trial were used. Patients were mailed questionnaires. Baseline FH and screening data were obtained for enrolled patients from the EMR and health questionnaires.
Setting
Ontario and Alberta.
Participants
Randomly selected patients from 8 family practices.
Main outcome measures
Agreement on FH between the EMR and questionnaire was determined; logistic regression was used to assess significant predictors of screening.
Results
In total, 775 of 789 (98%) patients completed the health questionnaire. The mean age of participants was 52.5 years and 72% were female. A minimum of 12% of patients (range 12% to 36%) had a reported FH of 1 of 4 chronic diseases. Among patients with positive FH, the following proportions of patients had that FH recorded in the EMR compared with the questionnaire: diabetes, 24% in the EMR versus 36% on the questionnaire, κ = 0.466; coronary artery disease, 35% in the EMR versus 22% on the questionnaire, κ = 0.225; breast cancer, 21% in the EMR versus 22% on the questionnaire, κ = 0.241; and CRC, 12% in the EMR versus 14% on the questionnaire, κ = 0.510. There was moderate agreement for diabetes and CRC. The presence of FH was a significant predictor of CRC screening (odds ratio 1.9, 95% CI 1.1 to 3.1).
Conclusion
A moderate prevalence of FH was found for 4 conditions for which screening recommendations vary with risk based on FH. Having patients self-complete an FH was thought to be feasible; however, questions about FH accuracy and completeness from both self-report and EMR remain. Work is needed to determine how to facilitate the adoption of FH tools into practice as well as strategies linking familial risk to appropriate screening.
Trial registration number ISRCTN07170460 (ISRCTN Registry).
Résumé
Objectif
Déterminer la proportion de patients d’une clinique de soins primaires qui rapportent des antécédents familiaux (AF) de diabète de type 2, de maladie coronarienne, de cancer du sein ou de cancer colorectal (CCR); déterminer s’il y a concordance entre les informations provenant du dossier médical électronique (DME) et les données du questionnaire auquel a répondu le patient; et vérifier si un dépistage approprié a été effectué lorsque le risque était basé uniquement sur les AF.
Type d’étude
On s’est servi des données du Programme BETTER (Building on Existing Tools to Improve Chronic Disease Prevention and Screening in Primary Care). Les données de base des AF et celles du dépistage ont été obtenues par l’entremise du DME et du questionnaire des patients participants.
Contexte
L’Ontario et l’Alberta.
Participants
Des patients choisis au hasard dans 8 cliniques de santé familiale.
Principaux paramètres à l’étude
On a déterminé s’il y avait concordance entre le DME et le questionnaire à propos des AF; on a utilisé une régression logistique pour déterminer les prédicteurs significatifs du dépistage.
Résultats
Un total de 775 patients sur 789 (98 %) ont répondu au questionnaire. Les participants avaient en moyenne 52,5 ans et 72 % étaient des femmes. Entre 12 % et 36 % des patients avaient mentionné de 1 à 4 maladies chroniques dans leurs AF. Parmi les patients qui avaient des AF positifs, on notait les proportions suivantes pour ceux dont les AF était consignés dans le DME ou dans le questionnaire : diabète, 24 % dans le DME contre 36 % dans le questionnaire, κ = 0.466; maladie coronarienne, 35 % dans le DME contre 22 % dans le questionnaire, κ = 0.225; cancer du sein, 21 % dans le DME contre 22 % dans le questionnaire, κ = 0.241; et CCR, 12 % dans le DME contre 14 % dans le questionnaire, κ = 0.510. Il y avait une concordance modérée pour le diabète et le CCR. La présence des AF était un prédicteur significatif pour un dépistage du CCR (rapport de cotes 1.9, IC à 95 % 1.1 à 3.1).
Conclusion
On a trouvé une prévalence modérée des AF pour 4 conditions pour lesquelles les recommandations de dépistage varient en fonction du risque évalué d’après les AF. On estimait qu’il était possible de demander aux patients de décrire leurs AF; certaines interrogations demeuraient toutefois quant à la précision et à l’exhaustivité des AF provenant de la description du patient et du DME. Il faudra trouver un moyen d’encourager l’adoption de mesures pour que les AF fassent désormais partie de la pratique, de même que des stratégies pour établir un lien entre un risque familial et un dépistage approprié.
Family history (FH) reflects social, behavioural, and environmental factors that influence health and provides insights into the genetic risk of disease.1–3 It is useful in the diagnosis of rare genetic disorders with clear patterns of inheritance or to assess the risk of common chronic diseases. Unfortunately, FH is often incomplete or not documented in primary care records.2,4–8 To address this deficiency, patient-completed FH questionnaires are gaining attention,5,6,9–13 with evidence of reasonable completeness and accuracy compared with a structured interview done by a health care provider.6,10–12,14 There is some evidence that questionnaires substantially improve completeness of FH compared with patient medical records.6,15 Collecting FH will have clinical usefulness in chronic disease prevention and screening if it can be demonstrated that it leads to improved appropriate screening and modification of health behaviour.1–3,16,17
This study was embedded in the BETTER trial (Building on Existing Tools to Improve Chronic Disease Prevention and Screening in Primary Care), a randomized controlled trial to improve the primary prevention of and screening for multiple conditions.18 This study identified high-grade guidelines and clinical risk assessment tools, then created algorithms for risk assessment, screening, and management, incorporating multiple variables that contribute to risk of chronic disease.19 We wanted to look specifically at the FH portion of the risk assessment used in the BETTER trial and it is the focus of this article.
The goal of this FH component of the study was to assess the proportion of patients in primary care who report an FH of type 2 diabetes, coronary artery disease (CAD), breast cancer (BC), and colorectal cancer (CRC); the concordance of FH information derived from the electronic medical record (EMR) compared with that reported on a patient-completed questionnaire; and whether patients at increased risk of disease based solely on FH were more likely to have undergone appropriate screening.
METHODS
This study used baseline data from the BETTER trial. The BETTER trial was a pragmatic 2-way factorial randomized controlled trial conducted in primary care that assessed the effect of both a practice-and a patient-level intervention on primary prevention and screening across a range of chronic diseases, compared with regular care.18 With respect to FH, the included conditions were type 2 diabetes, CAD, BC, and CRC. The intervention sites were 4 family practices in Ontario and 4 in Alberta, with 32 participating family physicians. Eligible patients were aged 40 to 65 and able to provide informed consent. Randomly selected patients in the practices of participating family physicians were mailed a study package including a letter of invitation, a trial-specific health questionnaire with an embedded FH questionnaire, and consent information. Patients were asked to complete the health questionnaire and return it by mail. The FH questionnaire was modified from one used in a previous study20 that was derived from many examples of FH questionnaires in the literature.
Baseline FH and screening data were extracted from patient EMRs using an electronic EMR audit tool and data were extracted manually from the health questionnaires. The EMR audit tool and completion manual were created for this study, and study personnel were trained in the protocol for EMR data retrieval. Risk based solely on FH and screening behaviour for each disease were evaluated by applying algorithms (Table 1).21–25 Multiple factors contribute to the risk of these chronic diseases but this study was designed to focus on FH as an independent risk factor.
Table 1.
Algorithms for assessing disease risk based solely on FH
| DISEASE | LEVEL OF FH RISK: SELF-REPORT | FH RISK ASSESSMENT ALGORITHM: SELF-REPORT | LEVEL OF FH RISK: EMR | FH RISK ASSESSMENT ALGORITHM: EMR | RECOMMENDED SCREENING |
|---|---|---|---|---|---|
| Diabetes21 | Average | Does not fit into elevated category | Average | Does not fit into elevated category | FPG level measured every 3 y starting at age 40 y |
| Elevated | First-degree relative with diabetes | Elevated | First-degree relative with diabetes | FPG level measured every y | |
| CAD22 | Average | Does not fit into elevated category | Average | Does not fit into elevated category | NA |
| Elevated | First-degree relative diagnosed with CAD* at age < 60 y | Elevated | First-degree relative diagnosed with CAD* at age < 60 y | NA | |
| BC23 | Average | Does not fit into elevated category (either moderate- or high-risk category) | Average | No FH of BC or ovarian cancer | Mammogram every 2 y for women aged 50–69 y |
| Elevated: moderate risk | 1 first- or second-degree relative with BC at age 35–49 y OR 2 first- or second-degree relatives on the same side of the family with BC at age < 70 y | Elevated | Any relative with BC or ovarian cancer | Mammogram every y for women aged 40–69 y | |
| Elevated: high risk | 3 relatives on same side of the family with BC OR 2 first- or second-degree relatives on same side of the family with BC at age < 50 y OR 1 first- or second-degree relative with any of BC at age < 35 y or ovarian cancer at any age OR 1 male relative with BC OR first- or second-degree Ashkenazi Jewish relative with BC at age < 50 y | ||||
| CRC24,25 | Average | No FH of CRC | Average | No FH of CRC | FOBT every 2 y or sigmoidoscopy every 5 y or colonoscopy every 10 y starting at age 50 y |
| Low: slightly increased risk | Might have 1 second- or third-degree relative with CRC | ||||
| Elevated: mildly increased risk | 1 first-degree relative with CRC at age > 60 y OR ≥ 2 second-degree relatives from same side of the family with CRC | Elevated | Any relative with CRC | FOBT every 2 y or colonoscopy every 10 y starting at age 40 y | |
| Elevated: moderate to high risk | 1 first-degree relative with CRC at age ≤ 60 y OR ≥2 first-degree relatives with CRC at any age | Colonoscopy every 5 y starting at age 40 y or 10 y earlier than age of youngest family member at time of diagnosis, which ever comes first |
BC—breast cancer, CAD—coronary artery disease, CRC—colorectal cancer, EMR—electronic medical record, FH—family history, FOBT—fecal occult blood testing, FPG—fasting plasma glucose, NA—not applicable.
CAD included angina, myocardial infarction, congestive heart failure, and CAD.
Risk based solely on FH
Family history for each disease was assessed using published Canadian guidelines available at the time of the study.19 From the health questionnaires, we determined if patients had relatives with any of the 4 conditions and collected details regarding number and closeness of relatives and age at diagnosis, which permitted a more detailed assessment of risk based solely on FH. Electronic medical record study data for diabetes and CAD identified first-degree relatives with these conditions; however, for BC and CRC, EMR data only identified if there were any relatives with either cancer. Risk of diabetes and CAD based solely on FH taken from patient health questionnaires was divided into 2 categories: average and elevated.21,22 Patient-reported FH of BC was stratified into average, moderate, and high risk, based on a risk algorithm (Table 1).21–25 Self-reported FH of CRC was stratified into average, low, and elevated risk levels by following an algorithm (Table 1).21–25 The EMR study data only reported whether or not the patient had an FH of BC or CRC recorded in the EMR. For the purpose of the study, patients with any recorded positive FH of BC or CRC in the EMR were categorized as elevated risk and those with a negative FH were categorized as average risk.
In some instances, the health questionnaires were incomplete with respect to age of diagnosis or the side of the family to which a second-degree relative belonged. In these cases it was assumed that the relative was diagnosed at an age sufficiently advanced to bias the patient toward lower familial risk. In the case of unclear lineage of affected relatives, we assumed that all affected relatives were on the same side of the family, biasing the patient toward higher familial risk. In the health questionnaire and EMR, if familial risk for a disease was not recorded, it was assumed that no relevant FH existed.
Appropriate screening based on risk from FH
Appropriate screening based solely on FH-associated risk was assessed according to recommendations from Canadian guidelines.19 Screening was recorded as completed if listed in either the EMR or the health questionnaire to maximize capture. Outcomes included whether the patient had ever undergone screening, whether screening was up-to-date at the time of entry into the study, and for CRC whether the correct screening method was done (eg, colonoscopy for those at elevated FH risk rather than fecal occult blood testing).
Guidelines suggest that meaningful FH of CAD might result in a doubling of the 10-year Framingham CAD risk score, possibly leading to new lipid targets.26,27 Because this requires individual calculation, we were unable to report screening related to FH and CAD.
Analysis
Data were provided by the BETTER trial18 and analyzed using SPSS, version 20. Frequency distributions calculated the percentage of patients in each FH risk category (elevated or average) and each of the screening items (ever had screening, correct type of screening, and screening up-to-date). Fisher exact tests were used to examine differences between elevated-and average-risk patients (based on FH) in appropriate screening variables, and Cohen κ statistics were used to determine concordance of the data sources. We hypothesized a “moderate” level of agreement on FH between data obtained from the EMR and data self-reported on the questionnaire. We fit a series of a priori–specified multivariable logistic regressions to assess predictors of appropriate screening for 3 of the 4 conditions (ie, not CAD), which included the following 15 covariates: age 55 and older, female sex, smoking, alcohol use, exercise, good health, mental health diagnoses, born in Canada, recent immigrant, completed college or university, married, employed, income equal to or greater than $100 000, presence of FH on the health questionnaire, and increased risk based solely on FH. Assuming a response distribution for our dichotomous outcomes in which at least 20% of patients were screened, simulation studies on power for logistic regression models suggested we had sufficient power to detect departures from the null hypothesis, should they exist, for any of the 15 predictors included in our respective logistic regression model fits.28,29
The trial was approved by the Ontario Cancer Research Ethics Board (REB), the University of Alberta REB, and all relevant REBs in both provinces and at each primary care team site.
RESULTS
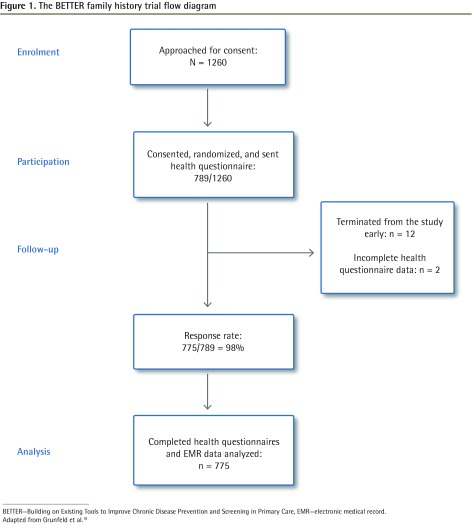
The BETTER trial flow diagram is shown in Figure 1.18 Participant demographic characteristics are shown in Table 2.
Figure 1.
The BETTER family history trial flow diagram
BETTER—Building on Existing Tools to Improve Chronic Disease Prevention and Screening in Primary Care, EMR—electronic medical record.
Adapted from Grunfeld et al.18
Table 2.
BETTER trial participant demographic characteristics: N = 775.
| CHARACTERISTIC | VALUE |
|---|---|
| Mean (SD) age, y | 52.5 (6.8) |
| Age range, y | 40–65 |
| Female sex, n (%) | 555 (72) |
| Married, common law, or living with partner, n (%) | 587 (76) |
| Completed college or university, n (%) | 519 (67) |
| Employed full or part time, n (%) | 585 (75) |
| Total household income ≥ $100 000, n (%) | 378 (49) |
| Born in Canada, n (%) | 611 (79) |
| Recent immigrant (in Canada < 10 y), n (%) | 14 (2) |
| General health excellent, very good, or good, n (%) | 677 (87) |
| Smoker | 82 (11) |
BETTER—Building on Existing Tools to Improve Chronic Disease Prevention and Screening in Primary Care.
Comparison of FH collection methods
Family history data were obtained using 2 different methods: EMR data and self-reports from the health questionnaire. When these methods were compared, 24% of patients were identified as having an FH of diabetes from EMR data, while significantly more self-identified an FH of diabetes (36%, P < .001). Data from the EMR showed 35% of participants had an FH of CAD, while 22% (P < .001) had a self-reported FH of CAD. Slightly more than 20% of women were identified as having an FH of BC by either EMR (21%) or self-report (22%, P = .41). The proportion of patients with an FH of CRC was 12% in the EMR and 14% by self-report (P = .13). The κ interrater agreement scores between EMR FH and self-reported FH are shown in Table 3.30 The κ statistics were fair for CAD and BC, and moderate for diabetes and CRC.30
Table 3.
Concordance between FH from EMR data and self-reported FH
| CONDITION | FH FROM EMR DATA | SELF-REPORTED FH | Κ STATISTIC* (95% CI) | |
|---|---|---|---|---|
|
| ||||
| NO | YES | |||
| Diabetes (n = 775) | No | 459 | 41 | 0.466 (0.40–0.53) |
| Yes | 134 | 141 | ||
| Coronary artery disease (n = 775) | No | 433 | 175 | 0.225 (0.15–0.30) |
| Yes | 73 | 94 | ||
| Breast cancer (women only, n =555) | No | 365 | 69 | 0.241 (0.15–0.34) |
| Yes | 73 | 48 | ||
| Colorectal cancer (n = 775) | No | 571 | 5 | 0.510 (0.44–0.58) |
| Yes | 114 | 85 | ||
EMR—electronic medical record, FH—family history.
Strength of agreement30: < 0 = less than chance; 0.01–0.20 = slight agreement; 0.21–0.40 = fair agreement; 0.41–0.60 = moderate agreement; 0.61–0.80 = substantial agreement; and 0.81–0.99 = almost perfect agreement.
Patients at elevated risk based solely on FH
Because we had more details from the health questionnaire, including age at diagnosis and closeness of affected relatives, we were able to determine which patients were at increased risk of the 4 disorders based solely on FH (Table 1).21–25 For BC, 18% were at high risk, 4% were at moderate risk, and 78% were at average risk. For CRC, 4% were at moderate to high risk, 10% were at mildly elevated risk, and 86% were at low or average risk.
Patients appropriately screened
Table 4 shows patients with completed risk-appropriate screening according to either the health questionnaire or the EMR at baseline. Significantly more patients at average risk based only on FH were up-to-date on diabetes screening than those at elevated FH risk (average 91%, elevated 65%, P < .001). Significantly more women at average risk of BC based solely on FH were up-to-date with screening than those at elevated FH risk (average 90%, elevated 70%, P < .001). For CRC, patients at elevated risk based only on FH were more likely than those at average FH risk to have had screening (elevated 80%, average 62%, P < .001) and to have undergone the correct screening method (elevated 99%, average 84%, P < .001), but were not more likely to be up-to-date on screening.
Table 4.
Risk level based solely on FH and screening from self-report health questionnaire or EMR data: Familial risk level is taken from self-reported data; screening information is taken from either self-reported data or EMR data.
| CONDITION | AVERAGE RISK BASED ON FH, N/N (%) | ELEVATED RISK BASED ON FH, N/N (%) | FISHER EXACT P VALUE |
|---|---|---|---|
| Diabetes (n = 775) | |||
| • Ever had screening | 378/500 (76) | 221/275 (80) | .076 |
| • Up-to-date screening | 342/378 (91) | 144/221 (65) | < .001 |
| Breast cancer (n = 555) | |||
| • Ever had screening | 381/434 (88) | 107/121 (88) | .50 |
| • Up-to-date screening | 341/381 (90) | 75/107 (70) | < .001 |
| CRC (n = 775) | |||
| • Ever had screening* | 416/669 (62) | 85/106 (80) | < .001 |
| • Had correct type of screening† | 349/416 (84) | 84/85 (99) | < .001 |
| • Up-to-date screening | 288/349 (83) | 74/84 (88) | .14 |
CRC—colorectal cancer, EMR—electronic medical record, FH—family history.
For CRC screening, average risk includes average and low risk; elevated risk includes mildly increased risk and moderate to high risk.
Correct CRC screening for moderate to high risk includes colonoscopy only.
Predictors of screening
Table 5 shows the predictors of screening from the logistic regression analysis. Very few covariates predicted screening. Of note, for BC, being born in Canada significantly predicted being screened (odds ratio [OR] = 2.4, 95% CI 1.2 to 4.6, P = .013), while those with an FH of BC were significantly less likely to have up-to-date screening (OR = 0.45, 95% CI 0.29 to 0.71, P < .001). For CRC, being a recent immigrant was significantly less likely to predict having CRC screening (OR = 0.23, 95% CI 0.06 to 0.87, P = .031). A high-risk FH of CRC was a significant predictor of being up-to-date and undergoing the correct CRC screening method (OR = 2.7, 95% CI 1.4 to 5.2, P = .002). The presence of FH was a significant predictor of CRC screening (OR = 1.9, 95% CI 1.1 to 3.1, P = .016).
Table 5.
Predictors of screening from self-reports or electronic medical records: Covariates in the logistic regression model include age ≥ 55 y, female sex, smoking, alcohol use, exercise, good health, mental health diagnoses, born in Canada, recent immigrant, completed college or university, married, employed, income ≥ $100 000, FH reported on health questionnaire, and high risk based on FH.
| PREDICTOR VARIABLE | ODDS RATIO (95% CI) | P VALUE |
|---|---|---|
| Diabetes (n = 775) | ||
| Ever had screening | ||
| • Age ≥ 55 y | 1.6 (1.1–2.3) | .018 |
| • Completed college or university | 0.6 (0.37–0.85) | .006 |
| • Married | 1.7 (1.1–2.6) | .014 |
| Up-to-date screening | ||
| • Married | 1.6 (1.1–2.3) | .023 |
| • Presence of FH | 0.48 (0.35–0.66) | < .001 |
| Breast cancer (n = 555) | ||
| Ever had screening | ||
| • Age ≥ 55 y | 11.5 (4.0–33.1) | < .001 |
| • Born in Canada | 2.4 (1.2–4.6) | .013 |
| Up-to-date screening | ||
| • Employed | 1.7 (1.1–2.6) | .021 |
| • Presence of FH | 0.45 (0.29–0.71) | < .001 |
| Colorectal cancer (n = 775) | ||
| Ever had screening | ||
| • Age ≥ 55 y | 7.0 (4.6–10.5) | < .001 |
| • Recent immigrant | 0.23 (0.06–0.87) | .031 |
| • Presence of FH | 1.9 (1.1–3.1) | .016 |
| Up-to-date and correct screening | ||
| • Age ≥ 55 y | 6.3 (4.4–8.9) | < .001 |
| • Smoker | 0.55 (0.32–0.97) | .038 |
| • Income ≥ $100 00 | 0.63 (0.44–0.92) | .015 |
| • High risk based on FH | 2.7 (1.4–5.2) | .002 |
FH—family history.
Data reliability
A random 5% of all study data were checked for error, revealing a 0.3% error rate.
DISCUSSION
We found that a minimum of 12% of patients in this study of primary care practices had a reported FH of 1 of 4 chronic diseases (range 12% to 36%). Agreement on FH between patient-completed health questionnaires and EMR data was only fair to moderate, and increased risk based solely on FH was not necessarily predictive of screening.
This study found that 35% of patients at increased risk based solely on FH of diabetes, 30% at increased risk of BC owing to FH, and 12% at increased risk of CRC owing to FH were not up-to-date with screening. These are some of the individuals for whom screening is likely to be most effective and they are therefore important to identify. We also found that those patients with elevated risk based solely on FH were not more likely to have had screening or to be up-to-date with screening, except in the case of CRC. Evidence is mixed as to whether being at increased risk of a disease because of FH alters screening and risk-reducing behaviour31,32; however, several studies have provided evidence of this association,33–36 particularly for CRC, for which studies have shown increased likelihood of being up-to-date with screening in those at high risk owing to FH.37–39 Some patients might be more motivated to consider screening and lifestyle changes when such recommendations are tied to a discussion of their FH risk, although a recent systematic review with meta-analysis showed that communicating DNA-based risk assessments had no significant effect on lifestyle risk factors or screening.40 Providers might be more likely to recommend screening if prompted by FH information in the EMR. Outcomes of more effective FH risk assessment might not be restricted to healthier lifestyle choices but might result in increased demand for screening and genetic counseling.41 Our study also found that being born in Canada was a significant predictor of BC screening, and being a recent immigrant was negatively associated with CRC screening, indicating the need for strategies to reach immigrant populations.
The good patient response rate to the mailed FH questionnaire indicates that collecting FH by this means might be feasible in primary care, although the high response might have been influenced by participation in a research study. The possibility that such a method of FH collection could be time efficient10 and yield a high response rate is encouraging.
Prevalence of patients with an FH
The proportion of patients with a positive FH for each of these 4 chronic diseases revealed that FH risk needs to be considered by primary care providers, along with other risk factors, when making screening recommendations. The proportion of patients at elevated risk of diabetes, CAD, BC, and CRC based solely on FH was similar to previously published studies using patient self-report.9,42–46 Prevalence varies depending on many factors, including the risk algorithm used and the population studied.47
Interrater agreement score
The level of concordance between FH from EMR data and FH from the health questionnaire was fair to moderate. Several studies have indicated that patient chart data provide less complete cancer FH information than self-reported FH.14,15,48,49 This speaks to the need for better recording of FH in the chart and the need for clinician validation of self-reported FH. Similar results to ours have been shown for diabetes, with a higher proportion of patients with elevated familial diabetes risk in self-reported versus EMR data.48 A significantly higher proportion of patients in this study were at elevated familial risk of CAD according to EMR data than according to self-completed questionnaires (P < .001), which is counter to a previous study.50 Our results might have been affected by inconsistent application of familial CAD risk assessment guidelines to the EMR data.
Our fair-to-moderate concordance between EMR FH and patient-completed questionnaire FH was disappointing and does not point to a clear direction for FH collection in primary care. A 2009 systematic review of cancer FH collection tools in primary care was able to demonstrate 46% to 78% improvement in recording of FH in charts if any FH tool was used, and 75% to 100% agreement with a criterion-standard structured genetic interview, leading the authors to suggest that any “systematic tools may add significant family health information compared with current primary care practice.”6 A 9-item FH screening questionnaire designed to identify people at increased risk of BC, ovarian cancer, CRC, prostate cancer, melanoma, ischemic heart disease, or type 2 diabetes has recently been validated and performs well for identifying primary care patients at increased disease risk owing to FH.44
Future directions
Qureshi and colleagues have proposed the concept of a “minimum family history dataset”6 to enable identification of those at increased risk, leading to more targeted inquiries and possibly enhanced screening or genetics referral. This includes FH information on both sides of the family, all first-degree relatives, ethnicity, and age of diagnosis of affected relatives.6 It is important that this minimum data set is recorded in the EMR and that it is in a consistent location to enable easy query and automated clinical decision support algorithms. This is particularly important, as it has been shown that health care providers might not incorporate FH information provided by patients into the EMR, thereby limiting its clinical usefulness.51 Tools capturing the minimum FH data set required to assist primary care providers in chronic disease prevention and screening are being developed to facilitate the capture of this information. These tools need to be developed, applied, and evaluated in the primary care setting to be effective and useful to primary care providers. The patient self-completed FH questionnaire used in the BETTER trial18,52 is one such tool. It has since been revised and is being used to capture FH data in the BETTER 2 program (an extension of the BETTER project into community practices including those in rural and remote settings and with aboriginal populations) and to direct risk-appropriate screening.53 Additional projects are exploring the use of EMR patient risk data, as identified in this study, to generate patient reminders about screening and to alert providers to higher-risk patients for consideration of individualized screening strategies. Further adaptations of FH tools, including electronic formats with automated clinical decision support, need to continue to be evaluated in the primary care setting to identify those resources that could best capture FH in a manner that informs primary care providers, enables adoption, and improves patient outcomes.51,54,55
Limitations
This is the first Canadian study of the prevalence of FH of chronic diseases and concordance of different methods of FH collection in primary care. It is important to acknowledge that we were looking at only one risk factor (FH) to highlight its role in risk stratification and screening. Clearly these chronic diseases are multifactorial in cause and FH is only one factor to be integrated into risk assessment and management algorithms.19 Participating patients likely exhibited the healthy volunteer effect, meaning they might have been more willing than usual patients to complete the FH health questionnaire and complete it accurately. In addition, participating patients were more likely to be female, be employed, have completed college or university, be non-smokers, and be born in Canada. This does not reflect the Canadian population, so results might not be generalizable. There were challenges in finding FH data in the EMR, such that we could only reliably determine the presence or absence of FH, not the details needed for risk assessment on the basis of FH. In this study, small numbers of individuals at high and moderate risk of each condition owing to FH made it difficult to assess predictors of screening. As all missing or unknown data were assumed to reflect an absence of FH or screening history, it is possible that some positive FHs and screening behaviour were missed.
Conclusion
This study highlighted the prevalence of positive FH for several common chronic conditions in primary care and identified a gap in screening those at elevated risk based solely on FH. Risk assessment with individualized screening and management is possible as a result of FH information, but challenges about the accuracy and completeness of both EMR and self-reported FH remain. Work is needed to determine how to facilitate the adoption of FH tools into primary care practice and the integration of FH into the EMR with automated clinical support algorithms. As well, research is needed on the value of FH risk communication as a motivator for appropriate screening.
Acknowledgments
This research was funded through a financial contribution from the Canadian Partnership Against Cancer and Health Canada, and was supported by the Heart and Stroke Foundation of Ontario (grant no. PG 10-0479), the Comprehensive Research Experience for Medical Students program, and the Department of Family Medicine in the Sinai Health System. Dr Grunfeld is supported by a clinician scientist award from the Ontario Institute for Cancer Research with funds from the Ontario Ministry of Research and Innovation. The opinions, results, and conclusions reported are those of the authors and are independent from the funding sources, and no endorsement by Ontario Institute for Cancer Research or the Ontario Ministry of Research and Innovation is intended or should be inferred.
EDITOR’S KEY POINTS
Family history (FH) reflects genetic factors influencing health but is often incomplete or not documented in patients’ medical records. Patient-completed FH questionnaires are gaining attention, with evidence that they are a reasonably complete and accurate way of collecting FH data. To be clinically useful, FH must facilitate appropriate screening.
The prevalence of positive FH was assessed for several common chronic conditions in primary care, and a gap in screening was identified for those at elevated risk based solely on FH. Risk assessment with individualized screening and management is possible using FH information, but challenges about the accuracy and completeness of both electronic medical record and self-reported FH remain.
Work is needed to determine how to facilitate the adoption of FH tools into primary care practice and the integration of FH into the electronic medical record with automated clinical support algorithms. As well, research is needed on the value of FH risk communication as a motivator for appropriate screening.
POINTS DE REPÈRE DU RÉDACTEUR
Les antécédents familiaux (AF) permettent de connaître les facteurs génétiques qui influencent la santé, mais souvent, ils sont incomplets ou ne sont pas documentée dans les dossiers médicaux des patients. Les questionnaires sur les AF auxquels répondent les patients suscitent de plus en plus d’intérêt parce qu’ils semblent être une façon de recueillir des données complètes et précises à ce sujet. Toutefois, pour être utiles en clinique, les AF doivent favoriser un dépistage approprié.
On a évalué la prévalence des AF positifs pour diverses conditions chroniques dans un contexte de soins primaires, ce qui a permis de révéler la présence d’un défaut de dépistage pour les patients présentant un risque élevé basé uniquement les AF. En utilisant l’information tirée des AF, il est possible évaluer le risque et d’intervenir, mais certains problèmes persistent quant à la précision et à l’exhaustivité des dossiers médicaux électroniques et des AF fournis par le patient.
Il reste encore à trouver une façon de faciliter l’adoption de mesures pour que les AF fassent partie intégrante du milieu des soins primaires et qu’ils soient consignés dans les dossiers médicaux électroniques à l’aide d’algorithmes cliniques automatisés. Il faudra également d’autres études pour savoir s’il vaut la peine d’identifier les risques pour inciter les soignants à effectuer les dépistages appropriés.
Footnotes
This article has been peer reviewed.
Cet article a fait l’objet d’une révision par des pairs.
Contributors
Drs Carroll, Campbell-Scherer, and Manca, Mr Meaney, and Drs Moineddin and Grunfeld contributed to the design of the study. Dr Carroll, Ms Permaul, Dr Myers, Mr Meaney, and Dr Moineddin contributed to the analysis. All authors contributed to writing and editing of the manuscript and have agreed to this version for submission.
Competing interests
None declared
References
- 1.Valdez R, Yoon PW, Qureshi N, Green RF, Khoury MJ. Family history in public health practice: a genomic tool for disease prevention and health promotion. Annu Rev Public Health. 2010;31:69–87. doi: 10.1146/annurev.publhealth.012809.103621. [DOI] [PubMed] [Google Scholar]
- 2.Pyeritz RE. The family history: the first genetic test, and still useful after all those years? Genet Med. 2012;14(1):3–9. doi: 10.1038/gim.0b013e3182310bcf. Epub 2011 Oct 7. [DOI] [PubMed] [Google Scholar]
- 3.Khoury MJ, Feero WG, Valdez R. Family history and personal genomics as tools for improving health in an era of evidence-based medicine. Am J Prev Med. 2010;39(2):184–8. doi: 10.1016/j.amepre.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 4.Doerr M, Teng K. Family history: still relevant in the genomics era. Cleve Clin J Med. 2012;79(5):331–6. doi: 10.3949/ccjm.79a.11065. [DOI] [PubMed] [Google Scholar]
- 5.Fuller M, Myers M, Webb T, Tabangin M, Prows C. Primary care providers’ responses to patient-generated family history. J Genet Couns. 2010;19(1):84–96. doi: 10.1007/s10897-009-9264-6. Epub 2009 Oct 24. [DOI] [PubMed] [Google Scholar]
- 6.Qureshi N, Carroll JC, Wilson B, Santaguida P, Allanson J, Brouwers M, et al. The current state of cancer family history collection tools in primary care: a systematic review. Genet Med. 2009;11(7):495–506. doi: 10.1097/GIM.0b013e3181a7e8e0. [DOI] [PubMed] [Google Scholar]
- 7.Acheson LS, Wiesner GL, Zyzanski SJ, Goodwin MA, Stange KC. Family history-taking in community family practice: implications for genetic screening. Genet Med. 2000;2(3):180–5. doi: 10.1097/00125817-200005000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Powell KP, Christianson CA, Hahn SE, Dave G, Evans LR, Blanton SH, et al. Collection of family health history for assessment of chronic disease risk in primary care. N C Med J. 2013;74(4):279–86. [PubMed] [Google Scholar]
- 9.O’Neill SM, Rubinstein WS, Wang C, Yoon PW, Acheson LS, Rothrock N, et al. Familial risk for common diseases in primary care: the Family Healthware Impact Trial. Am J Prev Med. 2009;36(6):506–14. doi: 10.1016/j.amepre.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Reid GT, Walter FM, Brisbane JM, Emery JD. Family history questionnaires designed for clinical use: a systematic review. Public Health Genomics. 2009;12(2):73–83. doi: 10.1159/000160667. Epub 2008 Oct 2. [DOI] [PubMed] [Google Scholar]
- 11.Facio FM, Feero WG, Linn A, Oden N, Manickam K, Biesecker LG. Validation of My Family Health Portrait for six common heritable conditions. Genet Med. 2010;12(6):370–5. doi: 10.1097/GIM.0b013e3181e15bd5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orlando LA, Buchanan AH, Hahn SE, Christianson CA, Powell KP, Skinner CS, et al. Development and validation of a primary care-based family health history and decision support program (MeTree) N C Med J. 2013;74(4):287–96. [PMC free article] [PubMed] [Google Scholar]
- 13.Doerr M, Edelman E, Gabitzsch E, Eng C, Teng K. Formative evaluation of clinician experience with integrating family history-based clinical decision support into clinical practice. J Pers Med. 2014;4(2):115–36. doi: 10.3390/jpm4020115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plat AW, Kroon AA, Van Schayck CP, De Leeuw PW, Stoffers HE. Obtaining the family history for common, multifactorial diseases by family physicians. A descriptive systematic review. Eur J Gen Pract. 2009;15(4):231–42. doi: 10.3109/13814780903447572. [DOI] [PubMed] [Google Scholar]
- 15.Murff HJ, Greevy RA, Syngal S. The comprehensiveness of family cancer history assessments in primary care. Community Genet. 2007;10(3):174–80. doi: 10.1159/000101759. [DOI] [PubMed] [Google Scholar]
- 16.Berg AO, Baird MA, Botkin JR, Driscoll DA, Fishman PA, Guarino PD, et al. National Institutes of Health State-of-the-Science Conference Statement: family history and improving health: August 24–26, 2009. NIH Consens State Sci Statements. 2009;26(1):1–19. [PubMed] [Google Scholar]
- 17.Yoon PW, Scheuner MT, Khoury MJ. Research priorities for evaluating family history in the prevention of common chronic diseases. Am J Prev Med. 2003;24(2):128–35. doi: 10.1016/s0749-3797(02)00585-8. [DOI] [PubMed] [Google Scholar]
- 18.Grunfeld E, Manca D, Moineddin R, Thorpe KE, Hoch JS, Campbell-Scherer D, et al. Improving chronic disease prevention and screening in primary care: results of the BETTER pragmatic cluster randomized controlled trial. BMC Fam Pract. 2013;14:175. doi: 10.1186/1471-2296-14-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campbell-Scherer D, Rogers J, Manca D, Lang-Robertson K, Bell S, Salvalaggio G, et al. Guideline harmonization and implementation plan for the BETTER trial: building on existing tools to improve chronic disease prevention and screening in family practice. CMAJ Open. 2014;2(1):E1–10. doi: 10.9778/cmajo.20130040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carroll JC, Blaine S, Permaul J, Dicks E, Warner E, Esplen MJ, et al. Efficacy of an educational intervention on family physicians’ risk assessment and management of colorectal cancer. J Community Genet. 2014;5(4):303–11. doi: 10.1007/s12687-014-0185-1. Epub 2014 Apr 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Canadian Diabetes Association 2008 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes. 2008;32(Suppl 1):S1–201. doi: 10.1016/j.jcjd.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 22.Genest J, McPherson R, Frohlich J, Anderson T, Campbell N, Carpentier A, et al. 2009 Canadian Cardiovascular Society/Canadian guidelines for the diagnosis and treatment of dyslipidemia and prevention of cardiovascular disease in the adult: 2009 recommendations. Can J Cardiol. 2009;25(10):567–79. doi: 10.1016/s0828-282x(09)70715-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Canadian Task Force on Preventive Health Care. Tonelli M, Connor Gorber S, Joffres M, Dickinson J, Singh H, et al. Recommendations on screening for breast cancer in average-risk women aged 40–74 years. CMAJ. 2011;183(17):1991–2001. doi: 10.1503/cmaj.110334. Erratum in: CMAJ 2011;183(18):2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leddin D, Hunt R, Champion M, Cockeram A, Flook N, Gould M, et al. Canadian Association of Gastroenterology and the Canadian Digestive Health Foundation: guidelines on colon cancer screening. Can J Gastroenterol. 2004;18(2):93–9. doi: 10.1155/2004/983459. [DOI] [PubMed] [Google Scholar]
- 25.Leddin DJ, Enns R, Hilsden R, Plourde V, Rabeneck L, Sadowski DC, et al. Canadian Association of Gastroenterology position statement on screening individuals at average risk for developing colorectal cancer: 2010. Can J Gastroenterol. 2010;24(12):705–14. doi: 10.1155/2010/683171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scheuner MT, Whitworth WC, McGruder H, Yoon PW, Khoury MJ. Expanding the definition of a positive family history for early-onset coronary heart disease. Genet Med. 2006;8(8):491–501. doi: 10.1097/01.gim.0000232582.91028.03. [DOI] [PubMed] [Google Scholar]
- 27.Hasanaj Q, Wilson BJ, Little J, Montazeri Z, Carroll JC, CIHR Emerging Team in Genomics in Screening. Family history: impact on coronary heart disease risk assessment beyond guideline-defined factors. Public Health Genomics. 2013;16(5):208–14. doi: 10.1159/000353460. Epub 2013 Jul 25. [DOI] [PubMed] [Google Scholar]
- 28.Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol. 2007;165(6):710–8. doi: 10.1093/aje/kwk052. Epub 2006 Dec 20. [DOI] [PubMed] [Google Scholar]
- 29.Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49(12):1373–9. doi: 10.1016/s0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 30.Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Fam Med. 2005;37(5):360–3. [PubMed] [Google Scholar]
- 31.Wilson BJ, Qureshi N, Santaguida P, Little J, Carroll JC, Allanson J, et al. Systematic review: family history in risk assessment for common diseases. Ann Intern Med. 2009;151(12):878–85. doi: 10.7326/0003-4819-151-12-200912150-00177. [DOI] [PubMed] [Google Scholar]
- 32.Claassen L, Henneman L, Janssens AC, Wijdenes-Pijl M, Qureshi N, Walter FM, et al. Using family history information to promote healthy lifestyles and prevent diseases; a discussion of the evidence. BMC Public Health. 2010;10:248. doi: 10.1186/1471-2458-10-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Audrain-McGovern J, Hughes C, Patterson F. Effecting behavior change: awareness of family history. Am J Prev Med. 2003;24(2):183–9. doi: 10.1016/s0749-3797(02)00592-5. [DOI] [PubMed] [Google Scholar]
- 34.Baptiste-Roberts K, Gary TL, Beckles GL, Gregg EW, Owens M, Porterfield D, et al. Family history of diabetes, awareness of risk factors, and health behaviors among African Americans. Am J Public Health. 2007;97(5):907–12. doi: 10.2105/AJPH.2005.077032. Epub 2007 Mar 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qureshi N, Kai J. Informing patients of familial diabetes mellitus risk: how do they respond? A cross-sectional survey. BMC Health Serv Res. 2008;8:37. doi: 10.1186/1472-6963-8-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruffin MT, 4th, Nease DE, Jr, Sen A, Pace WD, Wang C, Acheson LS, et al. Effect of preventive messages tailored to family history on health behaviors: the Family Healthware Impact Trial. Ann Fam Med. 2011;9(1):3–11. doi: 10.1370/afm.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steinwachs D, Allen JD, Barlow WE, Duncan RP, Egede LE, Friedman LS, et al. Enhancing use and quality of colorectal cancer screening. NIH Consens State Sci Statements. 2010;27(1):1–31. [PubMed] [Google Scholar]
- 38.Felsen CB, Piasecki A, Ferrante JM, Ohman-Strickland PA, Crabtree BF. Colorectal cancer screening among primary care patients: does risk affect screening behavior? J Community Health. 2011;36(4):605–11. doi: 10.1007/s10900-010-9348-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carney PA, O’Malley JP, Gough A, Buckley DI, Wallace J, Fagnan LJ, et al. Association between documented family history of cancer and screening for breast and colorectal cancer. Prev Med. 2013;57(5):679–84. doi: 10.1016/j.ypmed.2013.08.031. Epub 2013 Sep 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hollands GJ, French DP, Griffin SJ, Prevost AT, Sutton S, King S, et al. The impact of communicating genetic risks of disease on risk-reducing health behavior: systematic review with meta-analysis. BMJ. 2016;352:i1102. doi: 10.1136/bmj.i1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Orlando LA, Wu RR, Beadles C, Himmel T, Buchanan AH, Powell KP, et al. Implementing family health history risk stratification in primary care: impact of guideline criteria on populations and resource demand. Am J Med Genet C Semin Med Genet. 2014;166C(1):24–33. doi: 10.1002/ajmg.c.31388. Epub 2014 Mar 10. [DOI] [PubMed] [Google Scholar]
- 42.Au MG, Cornett SJ, Nick TG, Wallace J, Wang Y, Warren NS, et al. Familial risk for chronic disease and intent to share family history with a health care provider among urban Appalachian women, southwestern Ohio, 2007. Prev Chronic Dis. 2010;7(1):A07. Epub 2009 Dec 15. [PMC free article] [PubMed] [Google Scholar]
- 43.Scheuner MT, McNeel TS, Freedman AN. Population prevalence of familial cancer and common hereditary cancer syndromes. The 2005 California Health Interview Survey. Genet Med. 2010;12(11):726–35. doi: 10.1097/GIM.0b013e3181f30e9e. [DOI] [PubMed] [Google Scholar]
- 44.Emery JD, Reid G, Prevost AT, Ravine D, Walter FM. Development and validation of a family history screening questionnaire in Australian primary care. Ann Fam Med. 2014;12(3):241–9. doi: 10.1370/afm.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walter FM, Prevost AT, Birt L, Grehan N, Restarick K, Morris HC, et al. Development and evaluation of a brief self-completed family history tool for common chronic disease prevention in primary care. Br J Gen Pract. 2013;63(611):e393–400. doi: 10.3399/bjgp13X668186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scheuner MT, Wang SJ, Raffel LJ, Larabell SK, Rotter JI. Family history: a comprehensive genetic risk assessment method for the chronic conditions of adult-hood. Am J Med Genet. 1997;71(3):315–24. doi: 10.1002/(sici)1096-8628(19970822)71:3<315::aid-ajmg12>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 47.Yoon PW, Scheuner MT, Jorgensen C, Khoury MJ. Developing Family Healthware, a family history screening tool to prevent common chronic diseases. Prev Chronic Dis. 2009;6(1):A33. Epub 2008 Dec 15. [PMC free article] [PubMed] [Google Scholar]
- 48.Frezzo TM, Rubinstein WS, Dunham D, Ormond KE. The genetic family history as a risk assessment tool in internal medicine. Genet Med. 2003;5(2):84–91. doi: 10.1097/01.GIM.0000055197.23822.5E. [DOI] [PubMed] [Google Scholar]
- 49.Ferrante JM, Ohman-Strickland P, Hahn KA, Hudson SV, Shaw EK, Crosson JC, et al. Self-report versus medical records for assessing cancer-preventive services delivery. Cancer Epidemiol Biomarkers Prev. 2008;17(11):2987–94. doi: 10.1158/1055-9965.EPI-08-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qureshi N, Armstrong S, Dhiman P, Saukko P, Middlemass J, Evans PH, et al. Effect of adding systematic family history enquiry to cardiovascular disease risk assessment in primary care: a matched-pair, cluster randomized trial. Ann Intern Med. 2012;156(4):253–62. doi: 10.7326/0003-4819-156-4-201202210-00002. [DOI] [PubMed] [Google Scholar]
- 51.Feero WG. Connecting the dots between patient-completed family health history and the electronic health record. J Gen Intern Med. 2013;28(12):1547–8. doi: 10.1007/s11606-013-2544-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.The BETTER Program. BETTER health survey. First visit. Edmonton, AB: The BETTER Program; 2014. Available from: https://static1.squarespace.com/static/546d05b0e4b08897ae0800fb/t/55dba638e4b096ec230dfdda/1440458296328/BETTER+Health+Survey_First+Visit_cc.pdf. Accessed 2016 Dec 14. [Google Scholar]
- 53.Manca DP, Campbell-Scherer D, Aubrey-Bassler K, Kandola K, Aguilar C, Baxter J, et al. Developing clinical decision tools to implement chronic disease prevention and screening in primary care: the BETTER 2 program (building on existing tools to improve chronic disease prevention and screening in primary care) Implement Sci. 2015;10:107. doi: 10.1186/s13012-015-0299-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murray MF, Giovanni MA, Klinger E, George E, Marinacci L, Getty G, et al. Comparing electronic health record portals to obtain patient-entered family health history in primary care. J Gen Intern Med. 2013;28(12):1558–64. doi: 10.1007/s11606-013-2442-0. Epub 2013 Apr 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Welch BM, Dere W, Schiffman JD. Family health history: the case for better tools. JAMA. 2015;313(17):1711–2. doi: 10.1001/jama.2015.2417. [DOI] [PubMed] [Google Scholar]