Abstract
Fibrinogen-like protein 2 (Fgl2), a member of the fibrinogen family, can be expressed as a membrane-associated protein with coagulation activity or in a secreted form possessing unique immune suppressive functions. The biological importance of Fgl2 is evident within viral-induced fibrin depositing inflammatory diseases and malignancies and provides a compelling rationale for Fgl2 expression to not only be considered as a disease biomarker but also as a therapeutic target. This article will provide a comprehensive review of the currently known biological properties of Fgl2 and clarifies future scientific directives.
Keywords: secreted immune checkpoint regulator, membrane-bound procoagulant, fibrin deposit, inflammatory diseases, immune suppression, Tregs, DC maturation, liver injury, glioblastoma
Introduction
Although Fgl2 has an embryonic developmental role, Fgl2 expression is usually confined to T cells, endothelial, and tumor cells. Fgl2 expression can be induced in a variety of situations but especially within the monocyte-macrophage lineage. Fgl2 expression is triggered by at least two distinctive signaling pathway cascades - one initiated by viral proteins and a second initiated by immune cytokines. The outcome and severity of viral hepatitis, although initiated by the infection, is directly related to the activity of membrane-associated Fgl2 in macrophages which then leads to the deposit of fibrin and the development of necrosis within the liver. In the case of tumor-associated, often secreted Fgl2, normal haemostatic immune suppressive mechanisms are appropriated by the tumor to evade immune recognition and clearance – similar to how tumors can also induce expression of PD-L1 on tumor and stromal cells to engage the inhibitory immune checkpoint molecule PD-1 on T cells. Soluble Fgl2 plays a multimodal role on the immune system and can act as an effector of Tregs, can suppresses Th1 cytokine IL-2 and IFN-γ production while enhancing Th2 cytokines such as IL-4 and IL-10, and can down-regulate antigen presentation and maturation of dendritic cell by binding to FcγRIIB, down-regulating CD80 and MHC II expression and by blocking NF-κB translocation. Furthermore, Fgl2 assumes a functional role within malignancies to enhance tumor cell proliferation, promote the coagulation cascade, and induce angiogenesis. Collectively, Fgl2 is suspected to play a key immune regulatory role in pathogenic infections and cancer progression. Thus, there is strong rationale for the therapeutic targeting of Fgl2.
1. Structure and Expression
1.1 FGL2 structure
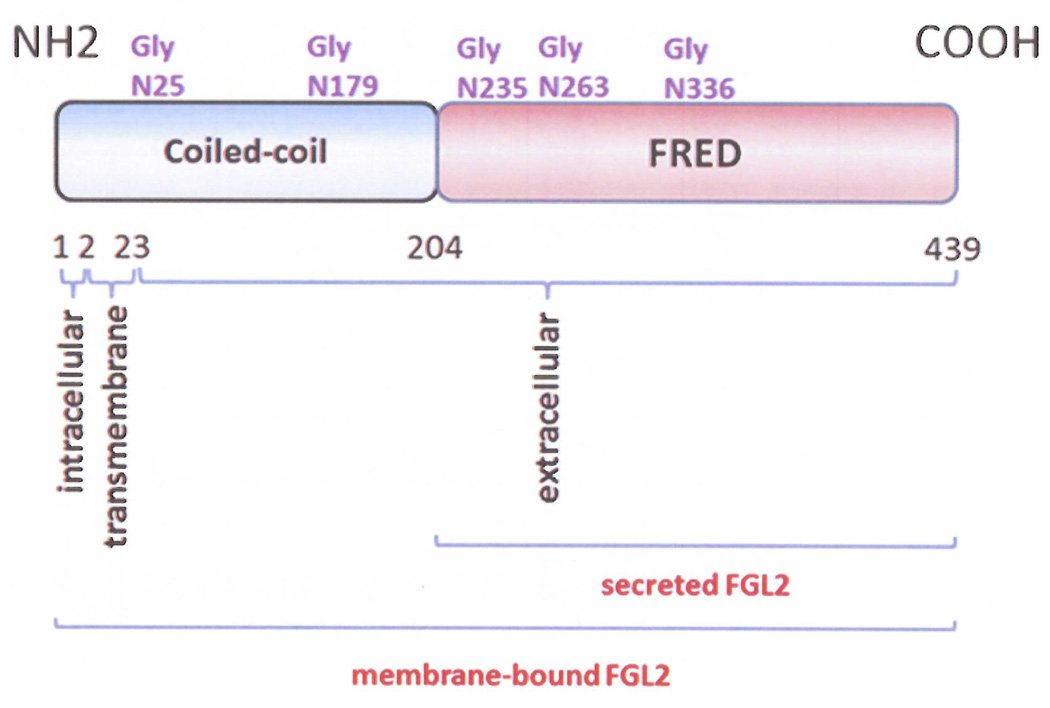
Fgl2 has been identified as a member of the fibrinogen super family, with 36% homology to fibrinogen β and γ subunits (1). Human and murine FGL2 are located on chromosome 7 and 5, respectively, and share 80% homology (2). The FGL2 gene has two exons, with mRNA transcripts of 1.5 and 5.0 kb, separated by one 2.2kb intron. Exon I consists of the first 204 amino acids, which includes an additional 7 amino acids in human. The amino terminus of the Fgl2 protein contains conserved cysteine residues (3), which can assemble into a mature Fib complex and α-helical structure to enable coiled-coil formation (2). Exon II includes the remaining 234 amino acids and encodes a type II membrane protein. The two amino acids at the amino terminus localize to the cytoplasm, and the amino acid 3–23 within the transmembrane domain. The extracellular domain contains 416 amino acids at the carboxyl terminus and also contains a 229 amino acid conserved sequence known as the fibrinogen-related domain (FRED). Serine 91, 142 and 423 are predicted to be protease active sites (4, 5); the R/G sites at amino acid 49 and 399 could function as the thrombin cleavage sites; and the R/T at 266 could act as a Factor Xa-sensitive site [2]. The membrane-bound form of Fgl2 contains both the N-terminal domain 1 and C-terminal domain 2. Domain 1, within the membrane-bound Fgl2 form, accounts for the coagulation activity – especially serine 89, whereas the secreted form of Fgl2 only contains the C-terminal FRED domain, which is responsible for the immune regulatory effect (Figure 1) (6). When monoclonal antibodies are used against the Fgl2 carboxyl terminus, the Fgl2 protein displays a molecular mass of 64kD in reducing SDS-PAGE conditions, and 250–300kD in non-reducing SDS-PAGE conditions, indicating that Fgl2 naturally forms tetramers via disulfide bonds (7).
Figure 1.
The molecular structure of Fgl2. Exon 1 is shown in blue and exon 2 in red.
1.2 FGL2 expression
Fgl2 knockout neonatal mice have higher death rates due to embryonic heart contractile dysfunction and rhythm abnormalities (8). The Fgl2−/− mice that are born do appear normal at a young age (6–8 weeks) but have lower body weights, smaller kidney size and enlarged spleens compared to their wild-type littermates (9). Upon further maturation, the mice develop glomerulonephritis and have high levels of albumin found in the blood and urine (7, 9). These data indicate that Fgl2 is expressed in multiple organs and plays a role during development. However, postnatal constitutive expression of Fgl2 is most commonly detected in human and mouse T lymphocytes (2, 7). Although Fgl2 is detected in the supernatants from T lymphocytes, it is not expressed on the surface of either CD4+ or CD8+ T lymphocytes (7), arguing against both membrane-bound and secreted Fgl2 expression. However, during pathogenic infection, membrane-bound Fgl2 expression is increased on both endothelial cells and macrophages (2, 7). Yet, this up-regulated expression is not observed in the liver after a viral infection in BTLA (B and T lymphocyte attenuator) knockout mice (10, 11). The induced Fgl2 expression co-localized to epithelial cells, CD68+ macrophages, CD11c+ dendritic cells (DCs), CD31+ endothelial cells and occasional CD3+ T cells (11). High levels of Fgl2 have also been detected in the livers of hepatitis B infected patients (12). In contrast, the secreted form of Fgl2 is frequently produced by Treg cells which play an immunosuppressive role on T cell proliferation and DC maturation (13). Fgl2 upregulation is detected in memory T lymphocytes (CD3+/CD45R0+) and during in vitro culture, but not usually in naïve T lymphocytes (CD3+/CD45RA+) (7). Finally, with IFN-γ and IL-2 stimulation, Fgl2 transcriptional and translational levels are increased by 10–100 folds in HUVEC and THP-1 cell lines (14), suggesting that Fgl2 may contribute to tumor progression.
2. Fgl2 signaling regulation
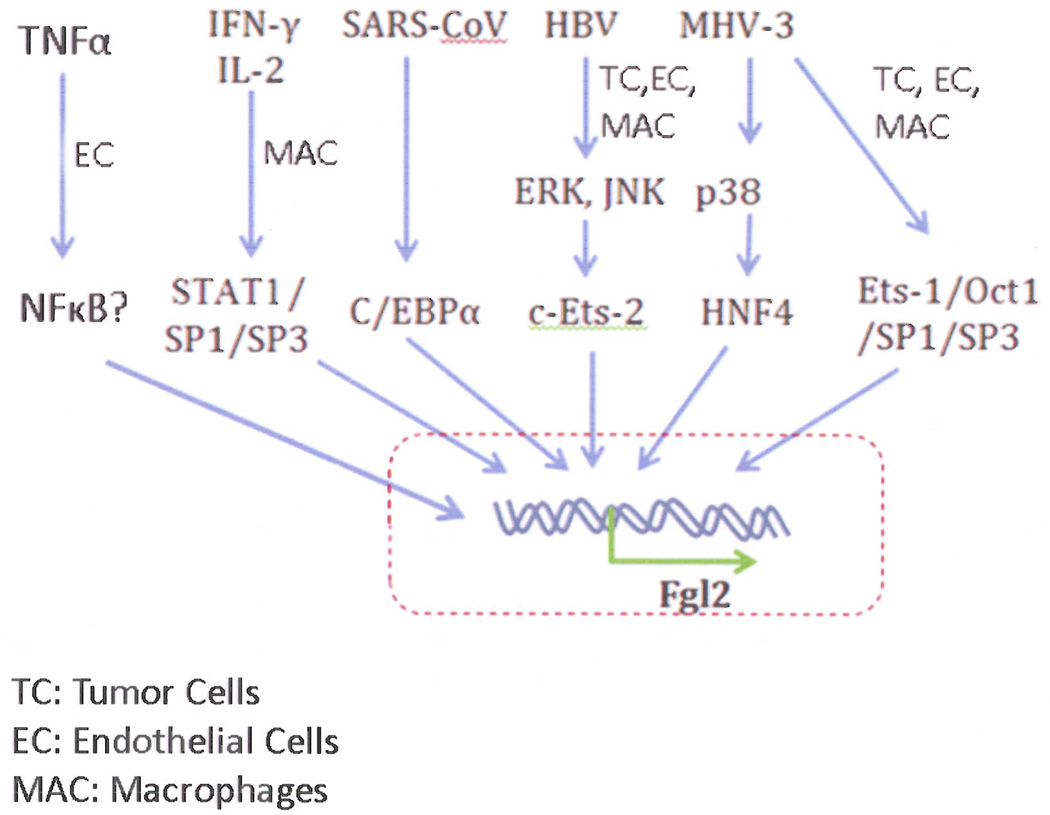
Since the pathogenic role of Fgl2 is not entirely understood, data are scarce regarding the specifics of the activation pathway. As a multifunctional protein, Fgl2 transcription regulation depends on its function in different cells and during developmental stages. A comprehensive assessment of basal Fgl2 promoter activity in murine vascular endothelial cells defined a minimal 119bp region responsible for constitutive Fgl2 transcription. A complex positive regulatory domain, spanning a 39-bp sequence from −87 to −49 (relative to the transcription start site), represents a functional cis-DNA regulatory element that interacts with Oct-1, and Ets-1, Sp1/Sp3 (15). Constitutive expression of Fgl2 transcripts at low levels are seen in cytotoxic T cells, endothelial, intestinal and trophoblast cells (16, 17), while specific factors (such as viral infection and cytokines) are required to induce high levels of Fgl2 expression in other cell types including monocytes/macrophages. For example, IFN-γ induces macrophage expression of Fgl2 whereas TNF-α endothelial cell Fgl2-transcription (18). The induction of Fgl2 transcription in macrophages involves a STAT1-dependent pathway by forming a Sp1/Sp3-STAT1/PU.1 transcriptional complex. Other factors such as murine hepatitis virus (MHV-3) have been reported to contribute to murine Fgl2 transcription through p38 mitogen-activated protein kinase (MAPK) activation (19) and interactions with the transcription factor HNF4 α(Figure 2) (20, 21).
Figure 2.
Summary of operational signaling pathways that induce expression of Fgl2.
Su et al. (14) reported that high human Fgl2 levels were expressed in both cancer cells and in interstitial inflammatory cells such as macrophages and vascular endothelial cells. A strong regulatory region from −712 to −568 (relative to the transcription start site) was shown to be responsible for Fgl2 gene transcription through c-Ets-2 and MAPK signal pathways in response to hepatitis B virus (HBV) protein (22). Whereas the hepatitis B core regulated c-Ets-2 by the ERK pathway, the HBV X protein regulated c-Ets-2 by the JNK pathway. The MAPK cascades are central signaling pathways that regulate a wide variety of stimulated cellular processes, including proliferation, differentiation, apoptosis and stress response. ERK, p38 and JNKs are three core elements in the MAPK pathway that phosphorylate other protein kinases and transcription factors. In chronic obstructive pulmonary disease, increased Fgl2 expression was co-localized with up-regulated p-JNK within the activated alveolar macrophage (22). Furthermore, knockdown of Fgl2 in human hepatocellular carcinoma cells was accompanied by decreased phosphorylation of ERK and JNK, whereas overexpression of Fgl2 induced phosphorylation of p38-MAPK and ERK, involving protease-activated receptor activation (23).
Fgl2 was shown to be expressed at high levels in the lungs of severe acute respiratory syndrome (SARS) patients which had a physiological role in the immune coagulation of these patients (24). Molecular analysis of the SARS associated coronavirus (SARS-coV) identified 13 open reading frames (25). The N protein of SARS-coV mediated regulatory region from −817 to −467 (relative to the transcription start site) was shown to be responsible for Fgl2 expression but required C/EBP-α (22).
Collectively, these data suggest that there are at least two pathways, one initiated by viral proteins and a second initiated by cytokines, which are involved in Fgl2 gene transcription and subsequent protein expression in vivo. These might occur simultaneously or sequentially during infection. Thus, the existence of complex cell type-specific molecular cascades regulating Fgl2 expression is consistent with the notion that Fgl2 is an important biological mediator.
3. The role of Fgl2 in immune suppression
3.1 FGL2 suppresses T cell proliferation
Because proliferating T lymphocytes secrete less Fgl2 in comparison to fresh peripheral blood mononuclear cells, Marazzi et al. suggested for the first time that Fgl2 plays a role in regulating the function of T lymphocytes (7). Kohno et al. further found that Fgl2 was absent in T cell leukemia and lymphoma patients (26), implying that the soluble Fgl2 is involved in T lymphocyte activation. To test this hypothesis, soluble Fgl2 protein was incubated with T lymphocytes in the presence of anti-CD3 mAb and anti-CD28 mAb or ConA and was found to completely inhibit T cell proliferation in a dose dependent manner (13). Furthermore, clinical studies of hepatitis C and hepatitis B patients have shown that increasing levels of Fgl2 are associated with reduced CD86 expression and impaired T cell activation (16, 27). Chan et al. also demonstrated that only an antibody targeting the C-terminal FRED domain was capable of successfully abolishing the Fgl2 inhibitory effect on T cell proliferation, whereas an antibody against the N-terminal domain failed to do so, proving that the FRED-containing domain at the C-terminus is responsible for immune suppression (13).
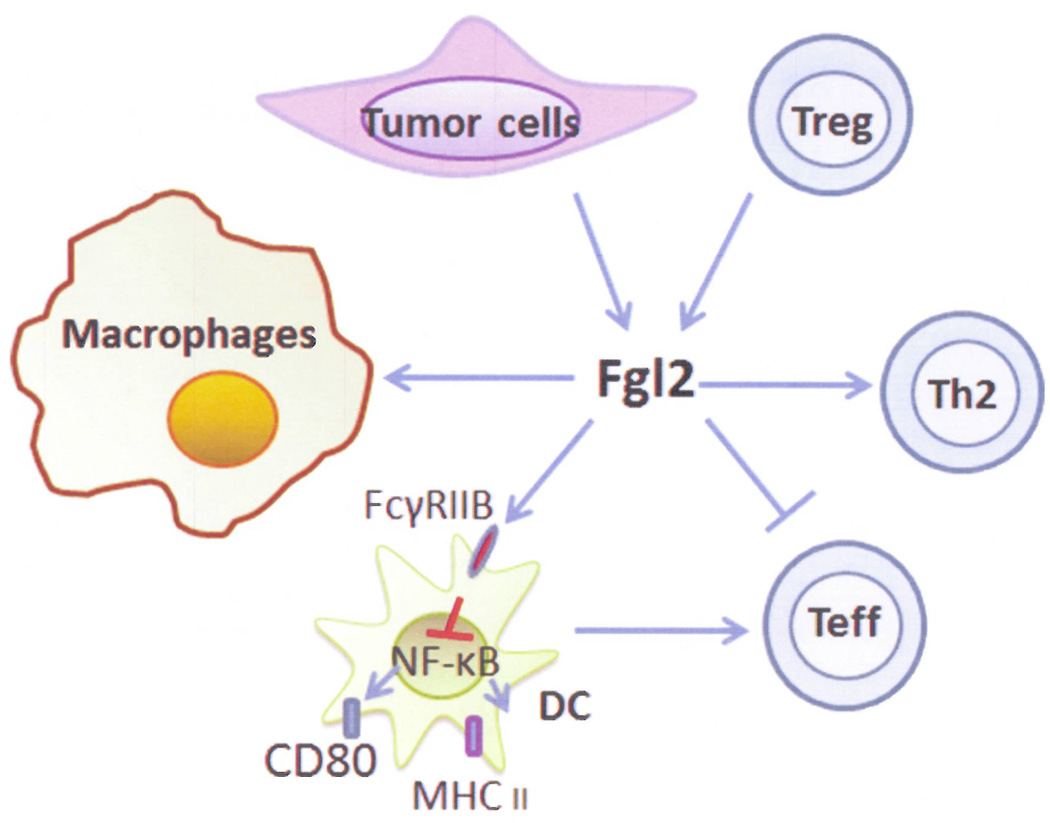
To ascertain how Fgl2 inhibits T cell proliferation, the cytokine profile and the induction of apoptosis in T cells were examined. Soluble Fgl2 failed to affect T cell survival or induce T cell apoptosis, revealing that the inhibitory activity of Fgl2 was independent of direct cytotoxicity (13). IL-2 is known to induce IFN-γ production, which boosts T lymphocytes proliferation and differentiation (28–30). In contrast, IL-4 and IL-10 down-regulate IL-2 induced T lymphocyte activation (31–33). As expected, in the presence of CD3/CD28 mAb or ConA stimulation, soluble Fgl2 protein suppressed the production of the immune activating cytokines IL-2 and IFN-γ, while promoting the production of inhibitory cytokines such as IL-4 and IL-10 (13). This data is consistent with the analysis of the fgl2−/− mice, which had increased levels of Th1 cytokine such as IFN-γ and reduced levels of Th2 cytokine IL-4 (9). Cumulatively, these data suggest that the changes in cytokine profile induced by Fgl2 might account for the inhibitory effect on T lymphocyte proliferation (Figure 3).
Figure 3.
Summary of postulated Fgl-2 tumor and immune interactions.
3.2 FGL2 is an effective molecule of Tregs
In 2003, Fontenol et al. showed that the CD4+CD25+FoxP3+ regulatory T cell population (Tregs) exert immune suppressive activity in many diseases and maintain self-tolerance to prevent autoimmunity (34). Tregs suppress immune responses through direct contact, cytotoxicity and immunosuppressive effector molecules (35). In subsequent studies, Fgl2 mRNA was detected in FoxP3+ Tregs (34, 36) and Gavin et al. confirmed several genes could be amplified by FoxP3, including Fgl2, CD73, CD39 TRAIL and CTLA-4 (37). Fgl2 was also found to be co-expressed FoxP3 in cardiac and liver allograft models, implying their roles in tolerant liver and heart allografts (38).Both Williams et al. and Zheng et al. have suggested that Fgl2 acts as a Treg effector molecule to suppress T cell activities in a FoxP3-dependent manner (39, 40). To clarify this association, Fgl2 transcription was compared between CD4+CD25+ T cells and CD4+CD25− T cells. In agreement with previous studies, Fgl2 showed a 6-fold increase in CD4+CD25+ T cells (9). Surprisingly, an increase in the number of Treg cells were observed from fgl2−/− mice compared to fgl2+/+ mice, which possibly served as a positive feedback to compensate for the loss of Fgl2 (9). One known function of Tregs is to inhibit the proliferation of effector immune cells. To assess whether Fgl2 contributed to such an inhibitory effect, Tregs from fgl2+/+ and fgl2−/− mice were co-incubated with stimulated CD4+CD25− T cells at different ratios. Intriguingly, the loss of fgl2 partially abolished the Treg inhibitory activity on immune effector cells. Supplementing this finding was that blockade of Fgl2 by a monoclonal antibody also impaired the Tregs inhibitory activity. Cumulatively, these observations support the notion that Fgl2 serves as an effector molecule of Tregs (9).
During pathogen infection, Tregs inhibit effector T cell cytolytic functions (41). Shalev et al. compared MHV-3 susceptible BALB/cJ mice with resistant A/J mice to reveal the role of Fgl2 in Treg-mediated immune suppression. Before infection, higher levels of Fgl2 expression and Tregs were observed from BALB/cJ mice relative to A/J mice. After infection, BALB/cJ mice showed a dramatic increase in the number of liver infiltrating Tregs as well as Fgl2 expression, which could serve as prognostic markers for disease progression. Blocking Fgl2 with a monoclonal antibody reduced MHV-3 infected liver damage, extended survival time and accordingly suppressed viral activities. Furthermore, fgl2−/− mice, which showed resistance to MHV-3 infection, turned susceptible to MHV-3 infection when Tregs from the fgl2+/+ background were adoptively transferred (42). Of note, Fgl2 has been shown to play a key role in abrogating immune responses against a variety of infections including hepatitis C virus (HCV) (43), HIV (44), and SARS (24, 45) suggesting this is a generalized and ubiquitous mechanism. Thus, the levels of soluble Fgl2 in the plasma of patients could potentially be measured as a prognostic marker for disease progression or moreover blocking Fgl2 could supplement the treatment of viral infectious diseases.
3.3 FGL2 inhibits DC maturation
Not only does Fgl2 have a biological role in T cell activity and responses, Chan et al. argued that Fgl2 also influence DCs (13). Interestingly, fgl2−/− mice have 30% more DCs in the spleens and bone marrow (9). Upon closer examination, soluble fgl2 had no effect on DC viability or expression of MHC class I or CD86. However, CD80 and MHC class II molecules, which play crucial roles in antigen presentation and T cell activation, were remarkably reduced (13). After LPS stimulation, DCs from fgl2−/− mice showed higher expression of CD80 and MHCII compared to fgl2+/+ littermates (9). Fgl2+/+ mice reconstituted with fgl2−/− bone marrow exhibited an increase in proliferating T cells and up-regulated expression of MHCII and CD80, representing the phenotype of fgl2−/− mice (9). DCs that were exposed to soluble Fgl2 failed to induce naïve T cell proliferation even after LPS stimulation; whereas Fgl2 untreated DCs activated T cell proliferation as expected (13). Thus, soluble Fgl2 suppresses LPS-induced DC maturation.
Liu et al. have purified the Fgl2-Fc recombinant protein to identify its receptor and found that Fgl2 binds to Raw cells (a murine macrophage cell line), bone marrow -derived DCs and A20 (a murine B cell lymphoma), but not A20IIA1.6 cells (a murine B cell lymphoma) or EL4 cells (murine T cell). After studying the receptors that are displayed on these cell lines and using various receptor knockout mice, they concluded that Fgl2 bound to both FcγRIIB and FcγRIII on antigen presenting cells. They also reported a reduction of CD40, CD80, CD86 and MHC II expression on DCs from FcγRIIB+/+ mice, but not FcγRIIB−/− mice despite these mice having FcγRIII expression, implying that Fgl2-induced DC inhibition was through binding to FcγRIIB. Furthermore, Fgl2 binding to FcγRIIB positive B cells induced apoptosis, which was not observed in FcγIIB deficient B cells, revealing Fgl2-induced B cell apoptosis was through binding to FcγRIIB (46). FcγRIIB is a single chain inhibitory receptor that contains an intracellular ITIM domain (47). Several studies have demonstrated that the inhibitory effect of FcγRIIB is often dependent on ITIM phosphorylation, which in turn recruits SHIP to activate phosphatidylinositol (3,4,5)-trisphosphate (PIP3), triggering the downstream cascade (48–50). However, ITIM-independent mechanisms of FcγRIIB signaling that induce cell apoptosis have also been reported (51). In the case of Fgl2-induced inhibition of DC and B cell functions, FcγRIIB phosphorylation was not observed, suggesting that the signaling is triggered in an ITIM-independent manner (46). As an alternative, soluble Fgl2 could block NF-κB translocation into nucleus, resulting in the down-regulation of CD80 and MHC II expression on DCs (13) and likely their maturation (52, 53). Finally, data is emerging that shows that Fgl2 regulates adaptive immunity via Th1 and Th2 cytokines. Liu et al. reported Fgl2 was required for IFN-γ and TNF-α induced hepatic apoptosis (54). Specifically, Fgl2 transcription was activated by IFN-γ and IL-2 (54, 55). Cumulatively, these data indicate that Fgl2 has multiple immune suppressive roles. Given that Fgl2 inhibits immune responses against virus infection, Fgl2 expression could serve as a prognostic marker of pathogen progression.
4. FGL2 induced diseases
4.1 Role of Fgl2 in viral-induced inflammation
Fgl2 expression is often associated with virus-induced inflammation in normal organs especially the liver. In a murine hepatitis model (MHV-3), initial studies revealed that the severity of liver injury was associated with the procoagulant activity (PCA) of macrophages (56, 57). Parr et al. reported that MHV-3 infection dramatically up-regulated murine fgl2, which then contributed to PCA (58), revealing a crucial role of fgl2 in MHV-3 infection-mediated hepatitis. Follow up studies demonstrated that the kinetics of fgl2 induction occurred 12–24 hours after MHV-3 infection within macrophage-enriched tissues (10, 17, 59). However, the PCA of fgl2 was detected only in livers in which Fgl2 was expressed on Kupffer and endothelial cells. The resulting deposit of fibrin in liver sinusoids caused the liver necrosis (17). In a complementary analysis, Fgl2/fibroleukin knockout mice failed to induce fibrin deposit and liver necrosis after MHV-3 infection, proving that Fgl2 expression was the initial factor driving liver necrosis in viral hepatitis (12).
The frequency of IFN-γ producing CD8+ and CD4+ T cells and the titers of total and neutralizing anti-viral antibodies were increased in Fgl2−/− mice infected with lymphocytic choriomeningitis virus relative to wild-type mice (60). This observation may be secondary to an association of Fgl2 and the negative regulators of T cell activation PD-L1, PD-L2, and BTLA (61). Fgl2 has been shown to co-localize with PD-L1 and PD-L2 in the liver sections from patients suffering hepatitis B virus (HBV)-related acute-on-chronic liver failure, suggesting these three proteins are possible biomarkers in the diagnosis (55, 62). Interestingly, PD-1 seems to lessen Fgl2-mediated fibrinogen deposit within the liver of MHV-3 infected mice. In PD-1-deficient mice, the MHV-3 infection resulted in higher mortality rates compared to wild type littermates. Furthermore, in wild type mice, PD-1 and Fgl2 did not co-localize within tissues; however the induction of Fgl2 in PD-1 deficient mice was dependent on IFN-γ and TNF-α expression (63) - consistent with previous reports (54, 55). Thus, PD-1 expression inhibits IFN-γ and TNF-α expression (64), which are inducers of Fgl2 expression.
A more in-depth study of patients with viral hepatitis patients showed positive staining of Fgl2 expression in macrophages, which was associated with fibrin deposit in necrotic liver tissues, indicating that the PCA of human Fgl2 accounts for the pathogenesis of viral hepatitis (16). In HBV-related acute-on-chronic liver failure patients, Fgl2 was enhanced specifically in macrophages, bile ducts and capillaries in necrotic liver tissues with fibrinogen deposit, while the normal liver tissues were almost absent of Fgl2 expression (62). Moreover, a dramatic induction of Fgl2 mRNA was observed within chronic HBV infected patients (12). Fgl2 expression on peripheral blood mononuclear cells has also been shown to be associated with pathogenesis of viral infected hepatitis (65). Consensus opinion at this time is that the development of viral hepatitis is initiated by the hepatic viral infection which then induces and activates the Fgl2 in macrophages which then leads to deposit of fibrin and the development of necrosis in liver tissues (16). Ultimately, the severity of liver necrosis is not determined by the viral load, but rather on the induction of Fgl2 and PCA (56, 57, 66, 67).
Fgl2 has been implicated to be associated with other types of viral induced inflammation. One human study showed that Fgl2 levels in the plasma of chronic HCV infected patients correlated with the severity of liver fibrosis (65). Another study on hepatic ischemia and reperfusion reported that Fgl2 binding to FcγRIIB receptor on sinusoidal endothelial cells accounted for the disease (68). Fgl2 expression was also found to correlate with the degree of pancreatitis-associated liver injury in a rat model (69). Immunohistochemical staining has also shown that high levels of Fgl2 expression in the respiratory tract were linked to the viral load of SARS (70). Finally, in a second murine model (A/J) that is virally infected with MHV-1, Fgl2 expression was found in the lungs (71).
In 2000, Levy et al. proposed that blocking Fgl2 antibodies could potentially be used to reduce liver injury in patients (16) based on murine studies (66). Alternatively, Fgl2 antisense plasmid DNA can be delivered hydrodynamically to shut down Fgl2 expression as was the case in a murine MHV-3 infected liver injury model (72). Significantly, this was shown to reduce fibrin deposition and improve survival times compared to animals receiving control DNA treatment (72). To further enhance the therapeutic effects, the same group, using the same antisense DNA delivery strategy, also targeted tumor necrosis factor receptor (TNFR) – an upstream inducer of Fgl2 expression. This combination resulted in almost complete abolishment of fibrin deposition and further prolongation of survival (73). Although there are not yet clinical anti-Fgl2 strategies, Fgl2 is nonetheless a promising target for hepatitis treatment and can serve as a potential biomarker in the diagnosis of hepatic pathogenesis.
4.2 Fgl2 contributes to xenograft rejection
Acute vascular xenograft rejection (AVR) often results in the failure of xenotransplantation between species (74). One of the main features of AVR is fibrin deposition. Fgl2 induction has been reported during kidney, cardiac and liver xenotransplantation rejection (75–77) and likely plays a critical role in AVR. More specifically, Fgl2 was shown to be induced on vascular endothelial cells both in vitro and in vivo during a pig-to-baboon kidney xenograft (49). Furthermore, no thrombosis or AVR developed in heart transplants from fgl2−/− mice relative to fgl2+/− mice into recipient Lewis rats (76), revealing that fgl2 contributes to AVR-associated thrombosis. In one mouse heterotopic cardiac transplant study, the use of blocking anti-fgl2 neutralizing antibodies reduced allorejection and prolonged survival time (77). In human renal transplantation subjects, biopsies revealed that fgl2 transcription levels were extremely high in renal tubule cells, infiltrating immune cells and endothelial cells, which was associated with fibrin deposition during allograft rejection. Thus, targeting Fgl2 could also be a therapeutic approach for treating human allograft rejection (77) and monitoring tolerance during and after transplantation (38).
4.3 Fgl2 expression is associated with abortion
The prothrombinase activity of Fgl2 has also been observed in a murine abortion model (43). Fgl2 expression was suggested to account for T-cell dependent spontaneous abortion in mice, as well as unexpected miscarriages in women (78, 79). By using fgl2−/− mice, Clark et al. indicated that fgl2+/− and fgl2−/− mice showed much lower abortion rates compared with the wild type littermates (80). Further, abortions triggered by LPS occurred only in fgl2+/+ mice, but not in fgl2−/− mice (80). Blocking Fgl2 can reduce occult losses during pregnancy (81); however whether this is an operational mechanism for spontaneous abortions in humans is unknown.
4.4 Fgl2 in other diseases
Because Fgl2 is expressed in many organs, it is beginning to be broadly studied in a variety of different diseases. For example, Fgl2 has been shown to play a role in the transition from acute to asymptomatic stages after HIV infection (44) and in MAPK signaling-mediated Fgl2 induction of activated lung-infiltrating macrophages in chronic obstructive pulmonary disease (82).
5. Fgl2 in tumors
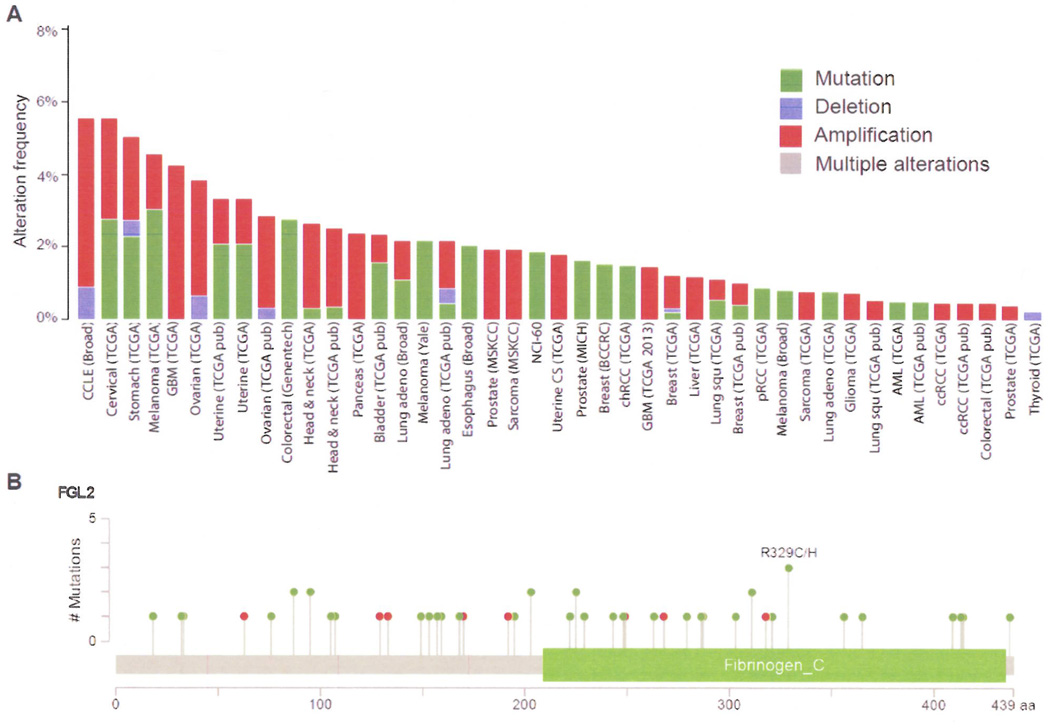
Based on the documented propensity of tumors to promote clot formation and regulate immune responses by tissue factor, tissue-type plasminogen activator, urokinase-type plasminogen activator, TGF-β1, IL-6, and TNF-α, etc (83, 84), Fgl2 expression within tumors was suspected. Bioinformatics analysis of copy number, mutational status, and mRNA levels (http://www.cbioportal.org/public-portal), reveals that fgl2 mRNA is expressed in a wide variety of human tumors. This is supplemented with The Cancer Genome Atlas (TCGA) data in which Fgl2 mRNA is detected in cervical, pancreatic, ovarian, uterine, esophageal, melanoma, glioblastoma, head & neck, colorectal, bladder, lung, prostate, sarcoma, breast, liver, and renal malignancies and acute myelogeneous leukemia. The alteration frequency of the Fgl2 gene for all cancer sets in the cBioPortal (Figure 4) was 1.24 ± 0.01% (mean ± SD), which is comparable to other immune regulators and pro-coagulation factors such as FOXP3 (1.20 ± 0.02%), TGF-β1 (1.16 ± 0.02%), TNF-α (1.17 ± 0.02%), IL-6 (2.00 ± 0.02%), tissue factor (0.96 ± 0.01%), and VEGFA (1.57 ± 0.02%). Furthermore, Fgl2 has showed a tendency towards co-occurrence with FOXP3 (odds Ratio: 4.62963) especially within glioblastoma (GBM TCGA 2013) when conducting mutual exclusivity and co-occurrence analysis. Within malignancies, the overwhelming majority of Fgl2 alterations are either amplifications or mutations. For the latter, 61 mutations were identified in the cBioPortal of which 15 had a high or medium functional impact score which assigns a probability that a specific amino acid mutation would alter protein function. Approximately 20% of the Fgl2 mutations (12 out of 61) resulted in a low level gain copy-number status and 2% (1 out of 61) resulted in a heterozygous deleted copy-number.
Figure 4.
Cross-cancer alteration summary for FGL2 (A) and mutation sites (B) in cancers as analyzed from the cBioPortal for Cancer Genomics. Mutation type: red (nonsense), green (missence).
Fgl2 protein expression levels relative to normal tissue is dependent on tumor type. For example, Fgl2 is highly up-regulated in solid tumors such as liver, renal, colon, breast, lung, gastric, esophageal, and cervical cancers (3, 14, 43). In contrast, Fgl2 is down-regulated acute and chronic adult T-cell leukemia/lymphoma (14, 26). Within the tumors, Fgl2 is expressed in tumor cells, immune cells (macrophages, NK cells, and CD8+ T lymphocytes), and the vascular endothelium cells of the microvasculature. This expression of Fgl2 can be modulated especially with immunotherapies. For example, Fgl2 has been shown to be up-regulated in the evading renal cell carcinoma after DC vaccine treatment (85) and with IFN-γ and IL-2 stimulation (14). The prognostic impact of Fgl2 protein expression, including its association with other co-variant markers such as FoxP3, PD-1 and PD-L1, in various malignancies has not yet been determined and is an area of future investigation.
The functional role of Fgl2 within malignancies has been shown to include the enhancement of tumor cell proliferation, the promotion of the coagulation cascade, induction of angiogenesis, and the promotion of immune suppression. Using knockdown of a high Fgl2-expressing HCCLM6 cell line, Liu et al observed a 2~3 time fold reduction on in vitro tumor cell proliferation and in vivo growth (86). Cell cycle analysis showed that the Fgl2 knockdown caused G1 arrest but not tumor apoptosis. Since Fgl2 is a prothrombinase that cleaves prothrombin into thrombin – the latter of which is a potent mitogen that can increase the growth of tumor cells (42), this cumulative data would indicate that the proliferation effect of Fgl2 on tumor cells is associated with its ability to generate thrombin. Additionally, in the study of Liu et al, the density of tumor blood vessels and the neovascularization incubation period was delayed in the Fgl2 knockdown HCCLM6 cells implanted in vivo, a key observation given that angiogenesis is required for invasive tumor growth and metastasis. The expression of Fgl2 in tumor tissues has also been shown to be associated with fibrin deposition in tumor tissues which provides a scaffold for supporting vessel formation and stimulating endothelial cell proliferation and migration (14, 87). Both vascular endothelial growth factor (VEGF) and interleukin-8 (IL-8) have been shown to be important activators of tumor-associated angiogenesis. Interestingly, Fgl2 expression was found to significantly correlate with VEGF and IL-8 expression in tumor tissues. Within the context of the Fgl2 knockdown experiments in HCCLM6 cells, both VEGF and IL-8 were reduced. However, since thrombin also can up-regulate the expression of many other angiogenesis-related genes, such as TF, bFGF, VEGF receptors, and MMPs, which can create a number of pleiotropic responses contributing to increased tumor angiogenesis (88), it is likely the Fgl2 facilitates tumor angiogenesis by a multiplicity of thrombin-mediated mechanisms.
Conclusion
Although Fgl2 induced thrombin generation can induce the recruitment of inflammatory cells to the tumor site; secreted Fgl2 probably mediates an overall immune suppressive effect on adaptive immunity by down modulating T cell effector function, inhibiting DC maturation, and inducing apoptosis of B cells [48]. Immune suppression has been acknowledged to facilitate the development of cancer and progression. Since Fgl2 has been shown to have an immune suppressive regulatory role in pathogen infection, it is likely to have one as well on cancer progression – especially since viruses have been shown to have an etiological role in multiple human malignancies. Ultimately suppressing Fgl2 expression in tumor cells or within the tumor microenvironment may constitute a potential therapeutic strategy by reversing tumor-mediated immune suppression and subsequently enhancing anti-tumor immune effector responses. The distinct association of FoxP3 and Fgl2 within glioblastoma suggests a particularly attractive possibility to promote immune-mediated rejection of this cancer by blocking Fgl2 activity. Furthermore, due to the increase of Fgl2 expression in pathological tissues and a secreted serum isoform, Fgl2 has the potential to act as a diagnostic tool – for early detection of malignancies, tumor progression, allograft rejection, and viral therapy failure. Currently efforts are underway to devise therapeutics that can target Fgl2 that could have clinical utility in the treatment of hepatitis, transplant rejection and malignancies.
Acknowledgments
The authors would like to thank Dr. David M Wildrick for editing this manuscript.
Funding
This work is partially supported by NIH/NCI RO1 CA120985 and CA1208113.
Footnotes
Declaration of Interest
The authors report no conflicts of interest.
References
- 1.Koyama T, Hall LR, Haser WG, Tonegawa S, Saito H. Structure of a cytotoxic T-lymphocyte-specific gene shows a strong homology to fibrinogen beta and gamma chains. Proceedings of the National Academy of Sciences of the United States of America. 1987;84(6):1609–1613. doi: 10.1073/pnas.84.6.1609. PubMed PMID: 3550794; PubMed Central PMCID: PMC304485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruegg C, Pytela R. Sequence of a human transcript expressed in T-lymphocytes and encoding a fibrinogen-like protein. Gene. 1995;160(2):257–262. doi: 10.1016/0378-1119(95)00240-7. PubMed PMID: 7642106. [DOI] [PubMed] [Google Scholar]
- 3.Yuwaraj S, Ding J, Liu M, Marsden PA, Levy GA. Genomic characterization, localization, and functional expression of FGL2, the human gene encoding fibroleukin: a novel human procoagulant. Genomics. 2001;71(3):330–338. doi: 10.1006/geno.2000.6444. PubMed PMID: 11170750. [DOI] [PubMed] [Google Scholar]
- 4.Rawlings ND, Barrett AJ. Families of cysteine peptidases. Methods in enzymology. 1994;244:461–486. doi: 10.1016/0076-6879(94)44034-4. PubMed PMID: 7845226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrett AJ, Rawlings ND. Families and clans of serine peptidases. Archives of biochemistry and biophysics. 1995;318(2):247–250. doi: 10.1006/abbi.1995.1227. PubMed PMID: 7733651. [DOI] [PubMed] [Google Scholar]
- 6.Liu H, Yang PS, Zhu T, Manuel J, Zhang J, He W, et al. Characterization of fibrinogen-like protein 2 (FGL2): monomeric FGL2 has enhanced immunosuppressive activity in comparison to oligomeric FGL2. Int J Biochem Cell Biol. 2012;45(2):408–418. doi: 10.1016/j.biocel.2012.10.014. Epub 2012/11/07. doi: S1357-2725(12)00360-3 [pii] 10.1016/j.biocel.2012.10.014. PubMed PMID: 23127799. [DOI] [PubMed] [Google Scholar]
- 7.Marazzi S, Blum S, Hartmann R, Gundersen D, Schreyer M, Argraves S, et al. Characterization of human fibroleukin, a fibrinogen-like protein secreted by T lymphocytes. Journal of immunology. 1998;161(1):138–147. PubMed PMID: 9647217. [PubMed] [Google Scholar]
- 8.Mu J, Qu D, Bartczak A, Phillips MJ, Manuel J, He W, et al. Fgl2 deficiency causes neonatal death and cardiac dysfunction during embryonic and postnatal development in mice. Physiological genomics. 2007;31(1):53–62. doi: 10.1152/physiolgenomics.00026.2007. PubMed PMID: 17550996. [DOI] [PubMed] [Google Scholar]
- 9.Shalev I, Liu H, Koscik C, Bartczak A, Javadi M, Wong KM, et al. Targeted deletion of fgl2 leads to impaired regulatory T cell activity and development of autoimmune glomerulonephritis. Journal of immunology. 2008;180(1):249–260. doi: 10.4049/jimmunol.180.1.249. PubMed PMID: 18097026. [DOI] [PubMed] [Google Scholar]
- 10.Ning Q, Brown D, Parodo J, Cattral M, Gorczynski R, Cole E, et al. Ribavirin inhibits viral-induced macrophage production of TNF, IL-1, the procoagulant fgl2 prothrombinase and preserves Th1 cytokine production but inhibits Th2 cytokine response. Journal of immunology. 1998;160(7):3487–3493. PubMed PMID: 9531310. [PubMed] [Google Scholar]
- 11.Yang C, Chen Y, Guo G, Li H, Cao D, Xu H, et al. Expression of B and T lymphocyte attenuator (BTLA) in macrophages contributes to the fulminant hepatitis caused by murine hepatitis virus strain-3. Gut. 2013;62(8):1204–1213. doi: 10.1136/gutjnl-2012-302239. PubMed PMID: 22637698. [DOI] [PubMed] [Google Scholar]
- 12.Marsden PA, Ning Q, Fung LS, Luo X, Chen Y, Mendicino M, et al. The Fgl2/fibroleukin prothrombinase contributes to immunologically mediated thrombosis in experimental and human viral hepatitis. The Journal of clinical investigation. 2003;112(1):58–66. doi: 10.1172/JCI18114. PubMed PMID: 12840059; PubMed Central PMCID: PMC162293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan CW, Kay LS, Khadaroo RG, Chan MW, Lakatoo S, Young KJ, et al. Soluble fibrinogen-like protein 2/fibroleukin exhibits immunosuppressive properties: suppressing T cell proliferation and inhibiting maturation of bone marrow-derived dendritic cells. Journal of immunology. 2003;170(8):4036–4044. doi: 10.4049/jimmunol.170.8.4036. PubMed PMID: 12682232. [DOI] [PubMed] [Google Scholar]
- 14.Su K, Chen F, Yan WM, Zeng QL, Xu L, Xi D, et al. Fibrinogen-like protein 2/fibroleukin prothrombinase contributes to tumor hypercoagulability via IL-2 and IFN-gamma. World journal of gastroenterology : WJG. 2008;14(39):5980–5989. doi: 10.3748/wjg.14.5980. PubMed PMID: 18932275; PubMed Central PMCID: PMC2760190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu M, Leibowitz JL, Clark DA, Mendicino M, Ning Q, Ding JW, et al. Gene transcription of fgl2 in endothelial cells is controlled by Ets-1 and Oct-1 and requires the presence of both Sp1 and Sp3. European journal of biochemistry / FEBS. 2003;270(10):2274–2286. doi: 10.1046/j.1432-1033.2003.03595.x. PubMed PMID: 12752447. [DOI] [PubMed] [Google Scholar]
- 16.Levy GA, Liu M, Ding J, Yuwaraj S, Leibowitz J, Marsden PA, et al. Molecular and functional analysis of the human prothrombinase gene (HFGL2) and its role in viral hepatitis. The American journal of pathology. 2000;156(4):1217–1225. doi: 10.1016/S0002-9440(10)64992-9. PubMed PMID: 10751347; PubMed Central PMCID: PMC1876871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ding JW, Ning Q, Liu MF, Lai A, Leibowitz J, Peltekian KM, et al. Fulminant hepatic failure in murine hepatitis virus strain 3 infection: tissue-specific expression of a novel fgl2 prothrombinase. Journal of virology. 1997;71(12):9223–9230. doi: 10.1128/jvi.71.12.9223-9230.1997. PubMed PMID: 9371581; PubMed Central PMCID: PMC230225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu H, Yang PS, Zhu T, Manuel J, Zhang J, He W, et al. Characterization of fibrinogen-like protein 2 (FGL2): monomeric FGL2 has enhanced immunosuppressive activity in comparison to oligomeric FGL2. The international journal of biochemistry & cell biology. 2013;45(2):408–418. doi: 10.1016/j.biocel.2012.10.014. PubMed PMID: 23127799. [DOI] [PubMed] [Google Scholar]
- 19.McGilvray ID, Lu Z, Wei AC, Dackiw AP, Marshall JC, Kapus A, et al. Murine hepatitis virus strain 3 induces the macrophage prothrombinase fgl-2 through p38 mitogen-activated protein kinase activation. The Journal of biological chemistry. 1998;273(48):32222–32229. doi: 10.1074/jbc.273.48.32222. PubMed PMID: 9822700. [DOI] [PubMed] [Google Scholar]
- 20.Ning Q, Lakatoo S, Liu M, Yang W, Wang Z, Phillips MJ, et al. Induction of prothrombinase fgl2 by the nucleocapsid protein of virulent mouse hepatitis virus is dependent on host hepatic nuclear factor-4 alpha. The Journal of biological chemistry. 2003;278(18):15541–15549. doi: 10.1074/jbc.M212806200. PubMed PMID: 12594208. [DOI] [PubMed] [Google Scholar]
- 21.Ning Q, Liu M, Kongkham P, Lai MM, Marsden PA, Tseng J, et al. The nucleocapsid protein of murine hepatitis virus type 3 induces transcription of the novel fgl2 prothrombinase gene. The Journal of biological chemistry. 1999;274(15):9930–9936. doi: 10.1074/jbc.274.15.9930. PubMed PMID: 10187767. [DOI] [PubMed] [Google Scholar]
- 22.Han M, Yan W, Guo W, Xi D, Zhou Y, Li W, et al. Hepatitis B virus-induced hFGL2 transcription is dependent on c-Ets-2 and MAPK signal pathway. The Journal of biological chemistry. 2008;283(47):32715–32729. doi: 10.1074/jbc.M806769200. PubMed PMID: 18801734. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Xu L, Zeng Q, Wang J, Wang M, Xi D, et al. Downregulation of FGL2/prothrombinase delays HCCLM6 xenograft tumour growth and decreases tumour angiogenesis. Liver international : official journal of the International Association for the Study of the Liver. 2012;32(10):1585–1595. doi: 10.1111/j.1478-3231.2012.02865.x. PubMed PMID: 22925132. [DOI] [PubMed] [Google Scholar]
- 24.Robertson M. Fgl2: link between hepatitis B and SARS? Drug discovery today. 2003;8(17):768–770. doi: 10.1016/S1359-6446(03)02836-8. PubMed PMID: 12946632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drosten C, Gunther S, Preiser W, van der Werf S, Brodt HR, Becker S, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. The New England journal of medicine. 2003;348(20):1967–1976. doi: 10.1056/NEJMoa030747. PubMed PMID: 12690091. [DOI] [PubMed] [Google Scholar]
- 26.Kohno T, Moriuchi R, Katamine S, Yamada Y, Tomonaga M, Matsuyama T. Identification of genes associated with the progression of adult T cell leukemia (ATL) Japanese journal of cancer research : Gann. 2000;91(11):1103–1110. doi: 10.1111/j.1349-7006.2000.tb00892.x. PubMed PMID: 11092974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Auffermann-Gretzinger S, Keeffe EB, Levy S. Impaired dendritic cell maturation in patients with chronic, but not resolved, hepatitis C virus infection. Blood. 2001;97(10):3171–3176. doi: 10.1182/blood.v97.10.3171. PubMed PMID: 11342445. [DOI] [PubMed] [Google Scholar]
- 28.Hecht TT, Longo DL, Matis LA. The relationship between immune interferon production and proliferation in antigen-specific, MHC-restricted T cell lines and clones. Journal of immunology. 1983;131(3):1049–1055. PubMed PMID: 6193170. [PubMed] [Google Scholar]
- 29.Everson MP, Spalding DM, Koopman WJ. Enhancement of IL-2-induced T cell proliferation by a novel factoRs) present in murine spleen dendritic cell-T cell culture supernatants. Journal of immunology. 1989;142(4):1183–1194. PubMed PMID: 2644351. [PubMed] [Google Scholar]
- 30.Maraskovsky E, Chen WF, Shortman K. IL-2 and IFN-gamma are two necessary lymphokines in the development of cytolytic T cells. Journal of immunology. 1989;143(4):1210–1214. PubMed PMID: 2501391. [PubMed] [Google Scholar]
- 31.Wagner F, Fischer N, Lersch C, Hart R, Dancygier H. Interleukin 4 inhibits the interleukin 2-induced production of its functional antagonist, interferon gamma. Immunology letters. 1989;21(3):237–241. doi: 10.1016/0165-2478(89)90110-7. PubMed PMID: 2504666. [DOI] [PubMed] [Google Scholar]
- 32.Taga K, Tosato G. IL-10 inhibits human T cell proliferation and IL-2 production. Journal of immunology. 1992;148(4):1143–1148. PubMed PMID: 1737931. [PubMed] [Google Scholar]
- 33.Han X, Itoh K, Balch CM, Pellis NR. Recombinant interleukin 4 (RIL4) inhibits interleukin 2-induced activation of peripheral blood lymphocytes. Lymphokine research. 1988;7(3):227–235. PubMed PMID: 3263554. [PubMed] [Google Scholar]
- 34.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22(3):329–341. doi: 10.1016/j.immuni.2005.01.016. PubMed PMID: 15780990. [DOI] [PubMed] [Google Scholar]
- 35.Miyara M, Sakaguchi S. Natural regulatory T cells: mechanisms of suppression. Trends in molecular medicine. 2007;13(3):108–116. doi: 10.1016/j.molmed.2007.01.003. PubMed PMID: 17257897. [DOI] [PubMed] [Google Scholar]
- 36.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nature immunology. 2005;6(11):1142–1151. doi: 10.1038/ni1263. PubMed PMID: 16227984. [DOI] [PubMed] [Google Scholar]
- 37.Gavin MA, Rasmussen JP, Fontenot JD, Vasta V, Manganiello VC, Beavo JA, et al. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445(7129):771–775. doi: 10.1038/nature05543. PubMed PMID: 17220874. [DOI] [PubMed] [Google Scholar]
- 38.Xie L, Ichimaru N, Morita M, Chen J, Zhu P, Wang J, et al. Identification of a novel biomarker gene set with sensitivity and specificity for distinguishing between allograft rejection and tolerance. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2012;18(4):444–454. doi: 10.1002/lt.22480. PubMed PMID: 22162188. [DOI] [PubMed] [Google Scholar]
- 39.Williams LM, Rudensky AY. Maintenance of the Foxp3-dependent developmental program in mature regulatory T cells requires continued expression of Foxp3. Nature immunology. 2007;8(3):277–284. doi: 10.1038/ni1437. PubMed PMID: 17220892. [DOI] [PubMed] [Google Scholar]
- 40.Zheng Y, Josefowicz SZ, Kas A, Chu TT, Gavin MA, Rudensky AY. Genome-wide analysis of Foxp3 target genes in developing and mature regulatory T cells. Nature. 2007;445(7130):936–940. doi: 10.1038/nature05563. PubMed PMID: 17237761. [DOI] [PubMed] [Google Scholar]
- 41.Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nature immunology. 2005;6(4):353–360. doi: 10.1038/ni1181. PubMed PMID: 15785761. [DOI] [PubMed] [Google Scholar]
- 42.Shalev I, Wong KM, Foerster K, Zhu Y, Chan C, Maknojia A, et al. The novel CD4+CD25+ regulatory T cell effector molecule fibrinogen-like protein 2 contributes to the outcome of murine fulminant viral hepatitis. Hepatology. 2009;49(2):387–397. doi: 10.1002/hep.22684. PubMed PMID: 19085958. [DOI] [PubMed] [Google Scholar]
- 43.Shalev I, Selzner N, Helmy A, Foerster K, Adeyi OA, Grant DR, et al. The Role of FGL2 in the Pathogenesis and Treatment of Hepatitis C Virus Infection. Rambam Maimonides medical journal. 2010;1(1):e0004. doi: 10.5041/RMMJ.10004. PubMed PMID: 23908776; PubMed Central PMCID: PMC3721661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Q, Smith AJ, Schacker TW, Carlis JV, Duan L, Reilly CS, et al. Microarray analysis of lymphatic tissue reveals stage-specific, gene expression signatures in HIV-1 infection. Journal of immunology. 2009;183(3):1975–1982. doi: 10.4049/jimmunol.0803222. PubMed PMID: 19596987; PubMed Central PMCID: PMC3552354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Han M, Yan W, Huang Y, Yao H, Wang Z, Xi D, et al. The nucleocapsid protein of SARS-CoV induces transcription of hfgl2 prothrombinase gene dependent on C/EBP alpha. Journal of biochemistry. 2008;144(1):51–62. doi: 10.1093/jb/mvn042. PubMed PMID: 18390877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu H, Shalev I, Manuel J, He W, Leung E, Crookshank J, et al. The FGL2-FcgammaRIIB pathway: a novel mechanism leading to immunosuppression. European journal of immunology. 2008;38(11):3114–3126. doi: 10.1002/eji.200838338. PubMed PMID: 18991288. [DOI] [PubMed] [Google Scholar]
- 47.Muta T, Kurosaki T, Misulovin Z, Sanchez M, Nussenzweig MC, Ravetch JV. A 13-amino-acid motif in the cytoplasmic domain of Fc gamma RIIB modulates B-cell receptor signalling. Nature. 1994;368(6466):70–73. doi: 10.1038/368070a0. PubMed PMID: 8107887. [DOI] [PubMed] [Google Scholar]
- 48.Nadler MJ, Chen B, Anderson JS, Wortis HH, Neel BG. Protein-tyrosine phosphatase SHP-1 is dispensable for FcgammaRIIB-mediated inhibition of B cell antigen receptor activation. The Journal of biological chemistry. 1997;272(32):20038–20043. doi: 10.1074/jbc.272.32.20038. PubMed PMID: 9242674. [DOI] [PubMed] [Google Scholar]
- 49.Tridandapani S, Pradhan M, LaDine JR, Garber S, Anderson CL, Coggeshall KM. Protein interactions of Src homology 2 (SH2) domain-containing inositol phosphatase (SHIP): association with Shc displaces SHIP from FcgammaRIIb in B cells. Journal of immunology. 1999;162(3):1408–1414. PubMed PMID: 9973396. [PubMed] [Google Scholar]
- 50.Jacob A, Cooney D, Tridandapani S, Kelley T, Coggeshall KM. FcgammaRIIb modulation of surface immunoglobulin-induced Akt activation in murine B cells. The Journal of biological chemistry. 1999;274(19):13704–13710. doi: 10.1074/jbc.274.19.13704. PubMed PMID: 10224144. [DOI] [PubMed] [Google Scholar]
- 51.Ravetch JV, Bolland S. IgG Fc receptors. Annual review of immunology. 2001;19:275–290. doi: 10.1146/annurev.immunol.19.1.275. PubMed PMID: 11244038. [DOI] [PubMed] [Google Scholar]
- 52.Koski GK, Lyakh LA, Cohen PA, Rice NR. CD14+ monocytes as dendritic cell precursors: diverse maturation-inducing pathways lead to common activation of NF-kappab/RelB. Critical reviews in immunology. 2001;21(1–3):179–189. PubMed PMID: 11642603. [PubMed] [Google Scholar]
- 53.Neumann M, Fries H, Scheicher C, Keikavoussi P, Kolb-Maurer A, Brocker E, et al. Differential expression of Rel/NF-kappaB and octamer factors is a hallmark of the generation and maturation of dendritic cells. Blood. 2000;95(1):277–285. PubMed PMID: 10607713. [PubMed] [Google Scholar]
- 54.Liu M, Mendicino M, Ning Q, Ghanekar A, He W, McGilvray I, et al. Cytokine-induced hepatic apoptosis is dependent on FGL2/fibroleukin: the role of Sp1/Sp3 and STAT1/PU.1 composite cis elements. Journal of immunology. 2006;176(11):7028–7038. doi: 10.4049/jimmunol.176.11.7028. PubMed PMID: 16709865. [DOI] [PubMed] [Google Scholar]
- 55.Hancock WW, Szaba FM, Berggren KN, Parent MA, Mullarky IK, Pearl J, et al. Intact type 1 immunity and immune-associated coagulative responses in mice lacking IFN gamma-inducible fibrinogen-like protein 2. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(9):3005–3010. doi: 10.1073/pnas.0308369101. PubMed PMID: 14976252; PubMed Central PMCID: PMC365735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Levy GA, MacPhee PJ, Fung LS, Fisher MM, Rappaport AM. The effect of mouse hepatitis virus infection on the microcirculation of the liver. Hepatology. 1983;3(6):964–973. doi: 10.1002/hep.1840030614. PubMed PMID: 6313508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.MacPhee PJ, Dindzans VJ, Fung LS, Levy GA. Acute and chronic changes in the microcirculation of the liver in inbred strains of mice following infection with mouse hepatitis virus type 3. Hepatology. 1985;5(4):649–660. doi: 10.1002/hep.1840050422. PubMed PMID: 2991107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parr RL, Fung L, Reneker J, Myers-Mason N, Leibowitz JL, Levy G. Association of mouse fibrinogen-like protein with murine hepatitis virus-induced prothrombinase activity. Journal of virology. 1995;69(8):5033–5038. doi: 10.1128/jvi.69.8.5033-5038.1995. PubMed PMID: 7609073; PubMed Central PMCID: PMC189320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fingerote RJ, Abecassis M, Phillips MJ, Rao YS, Cole EH, Leibowitz J, et al. Loss of resistance to murine hepatitis virus strain 3 infection after treatment with corticosteroids is associated with induction of macrophage procoagulant activity. Journal of virology. 1996;70(7):4275–4282. doi: 10.1128/jvi.70.7.4275-4282.1996. PubMed PMID: 8676449; PubMed Central PMCID: PMC190359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khattar R, Luft O, Yavorska N, Shalev I, Phillips MJ, Adeyi O, et al. Targeted deletion of FGL2 leads to increased early viral replication and enhanced adaptive immunity in a murine model of acute viral hepatitis caused by LCMV WE. PloS one. 2013;8(10):e72309. doi: 10.1371/journal.pone.0072309. PubMed PMID: 24146739; PubMed Central PMCID: PMC3795679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nature reviews Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. PubMed PMID: 22437870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cao D, Xu H, Guo G, Ruan Z, Fei L, Xie Z, et al. Intrahepatic expression of programmed death-1 and its ligands in patients with HBV-related acute-on-chronic liver failure. Inflammation. 2013;36(1):110–120. doi: 10.1007/s10753-012-9525-7. PubMed PMID: 22895698. [DOI] [PubMed] [Google Scholar]
- 63.Chen Y, Wu S, Guo G, Fei L, Guo S, Yang C, et al. Programmed death (PD)-1-deficient mice are extremely sensitive to murine hepatitis virus strain-3 (MHV-3) infection. PLoS pathogens. 2011;7(7):e1001347. doi: 10.1371/journal.ppat.1001347. PubMed PMID: 21750671; PubMed Central PMCID: PMC3131267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annual review of immunology. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. PubMed PMID: 18173375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Foerster K, Helmy A, Zhu Y, Khattar R, Adeyi OA, Wong KM, et al. The novel immunoregulatory molecule FGL2: a potential biomarker for severity of chronic hepatitis C virus infection. Journal of hepatology. 2010;53(4):608–615. doi: 10.1016/j.jhep.2010.04.020. PubMed PMID: 20615566. [DOI] [PubMed] [Google Scholar]
- 66.Li C, Fung LS, Chung S, Crow A, Myers-Mason N, Phillips MJ, et al. Monoclonal antiprothrombinase (3D4.3) prevents mortality from murine hepatitis virus (MHV-3) infection. The Journal of experimental medicine. 1992;176(3):689–697. doi: 10.1084/jem.176.3.689. PubMed PMID: 1324969; PubMed Central PMCID: PMC2119354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pope M, Rotstein O, Cole E, Sinclair S, Parr R, Cruz B, et al. Pattern of disease after murine hepatitis virus strain 3 infection correlates with macrophage activation and not viral replication. Journal of virology. 1995;69(9):5252–5260. doi: 10.1128/jvi.69.9.5252-5260.1995. PubMed PMID: 7636967; PubMed Central PMCID: PMC189358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Selzner N, Liu H, Boehnert MU, Adeyi OA, Shalev I, Bartczak AM, et al. FGL2/fibroleukin mediates hepatic reperfusion injury by induction of sinusoidal endothelial cell and hepatocyte apoptosis in mice. Journal of hepatology. 2012;56(1):153–159. doi: 10.1016/j.jhep.2011.05.033. PubMed PMID: 21756857. [DOI] [PubMed] [Google Scholar]
- 69.Chen T, Ye X, Huang Z, Chen R, Zhuge X, Chen X, et al. Fgl2 prothrombinase is involved in severe acute pancreatitis-associated liver injury. Hepato-gastroenterology. 2012;59(116):1225–1229. doi: 10.5754/hge12117. PubMed PMID: 22456282. [DOI] [PubMed] [Google Scholar]
- 70.Chen WJ, Yang JY, Lin JH, Fann CS, Osyetrov V, King CC, et al. Nasopharyngeal shedding of severe acute respiratory syndrome-associated coronavirus is associated with genetic polymorphisms. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2006;42(11):1561–1569. doi: 10.1086/503843. PubMed PMID: 16652313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.De Albuquerque N, Baig E, Ma X, Zhang J, He W, Rowe A, et al. Murine hepatitis virus strain 1 produces a clinically relevant model of severe acute respiratory syndrome in A/J mice. Journal of virology. 2006;80(21):10382–10394. doi: 10.1128/JVI.00747-06. PubMed PMID: 17041219; PubMed Central PMCID: PMC1641767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhu C, Sun Y, Luo X, Yan W, Xi D, Ning Q. Novel mfgl2 antisense plasmid inhibits murine fgl2 expression and ameliorates murine hepatitis virus type 3-induced fulminant hepatitis in BALB/cJ mice. Human gene therapy. 2006;17(6):589–600. doi: 10.1089/hum.2006.17.589. PubMed PMID: 16776568. [DOI] [PubMed] [Google Scholar]
- 73.Gao S, Wang M, Ye H, Guo J, Xi D, Wang Z, et al. Dual interference with novel genes mfgl2 and mTNFR1 ameliorates murine hepatitis virus type 3-induced fulminant hepatitis in BALB/cJ mice. Human gene therapy. 2010;21(8):969–977. doi: 10.1089/hum.2009.177. PubMed PMID: 20218879. [DOI] [PubMed] [Google Scholar]
- 74.Bach FH, Winkler H, Ferran C, Hancock WW, Robson SC. Delayed xenograft rejection. Immunology today. 1996;17(8):379–384. doi: 10.1016/0167-5699(96)10024-4. PubMed PMID: 8783499. [DOI] [PubMed] [Google Scholar]
- 75.Ghanekar A, Mendicino M, Liu H, He W, Liu M, Zhong R, et al. Endothelial induction of fgl2 contributes to thrombosis during acute vascular xenograft rejection. Journal of immunology. 2004;172(9):5693–5701. doi: 10.4049/jimmunol.172.9.5693. PubMed PMID: 15100314. [DOI] [PubMed] [Google Scholar]
- 76.Mendicino M, Liu M, Ghanekar A, He W, Koscik C, Shalev I, et al. Targeted deletion of Fgl-2/fibroleukin in the donor modulates immunologic response and acute vascular rejection in cardiac xenografts. Circulation. 2005;112(2):248–256. doi: 10.1161/CIRCULATIONAHA.105.534271. PubMed PMID: 15998670. [DOI] [PubMed] [Google Scholar]
- 77.Ning Q, Sun Y, Han M, Zhang L, Zhu C, Zhang W, et al. Role of fibrinogen-like protein 2 prothrombinase/fibroleukin in experimental and human allograft rejection. Journal of immunology. 2005;174(11):7403–7411. doi: 10.4049/jimmunol.174.11.7403. PubMed PMID: 15905589. [DOI] [PubMed] [Google Scholar]
- 78.Clark DA, Chaouat G, Arck PC, Mittruecker HW, Levy GA. Cytokine-dependent abortion in CBA×DBA/2 mice is mediated by the procoagulant fgl2 prothrombinase [correction of prothombinase] Journal of immunology. 1998;160(2):545–549. PubMed PMID: 9551885. [PubMed] [Google Scholar]
- 79.Knackstedt M, Ding JW, Arck PC, Hertwig K, Coulam CB, August C, et al. Activation of the novel prothrombinase, fg12, as a basis for the pregnancy complications spontaneous abortion and pre-eclampsia. American journal of reproductive immunology. 2001;46(3):196–210. doi: 10.1034/j.1600-0897.2001.d01-3.x. PubMed PMID: 11554693. [DOI] [PubMed] [Google Scholar]
- 80.Clark DA, Foerster K, Fung L, He W, Lee L, Mendicino M, et al. The fgl2 prothrombinase/fibroleukin gene is required for lipopolysaccharide-triggered abortions and for normal mouse reproduction. Molecular human reproduction. 2004;10(2):99–108. doi: 10.1093/molehr/gah013. PubMed PMID: 14742694. [DOI] [PubMed] [Google Scholar]
- 81.Foerster K, He W, Manuel J, Bartczak A, Liu M, Markert UR, et al. LPS-induced occult loss in mice requires FGL2. American journal of reproductive immunology. 2007;58(6):524–529. doi: 10.1111/j.1600-0897.2007.00543.x. PubMed PMID: 17997751. [DOI] [PubMed] [Google Scholar]
- 82.Liu Y, Xu S, Xiao F, Xiong Y, Wang X, Gao S, et al. The FGL2/fibroleukin prothrombinase is involved in alveolar macrophage activation in COPD through the MAPK pathway. Biochemical and biophysical research communications. 2010;396(2):555–561. doi: 10.1016/j.bbrc.2010.04.145. PubMed PMID: 20438701. [DOI] [PubMed] [Google Scholar]
- 83.Young A, Chapman O, Connor C, Poole C, Rose P, Kakkar AK. Thrombosis and cancer. Nature reviews Clinical oncology. 2012;9(8):437–449. doi: 10.1038/nrclinonc.2012.106. PubMed PMID: 22777060. [DOI] [PubMed] [Google Scholar]
- 84.Kawakami Y, Yaguchi T, Sumimoto H, Kudo-Saito C, Tsukamoto N, Iwata-Kajihara T, et al. Cancer-induced immunosuppressive cascades and their reversal by molecular-targeted therapy. Ann N Y Acad Sci. 2013;1284:80–86. doi: 10.1111/nyas.12094. PubMed PMID: 23651199. [DOI] [PubMed] [Google Scholar]
- 85.Birkhauser FD, Koya RC, Neufeld C, Rampersaud EN, Lu X, Micewicz ED, et al. Dendritic cell-based immunotherapy in prevention and treatment of renal cell carcinoma: efficacy, safety, and activity of Ad-GM.CAIX in immunocompetent mouse models. J Immunother. 2013;36(2):102–111. doi: 10.1097/CJI.0b013e31827bec97. Epub 2013/02/05. PubMed PMID: 23377663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu Y, Xu L, Zeng Q, Wang J, Wang M, Xi D, et al. Downregulation of FGL2/prothrombinase delays HCCLM6 xenograft tumour growth and decreases tumour angiogenesis. Liver Int. 2012 doi: 10.1111/j.1478-3231.2012.02865.x. Epub 2012/08/29. PubMed PMID: 22925132. [DOI] [PubMed] [Google Scholar]
- 87.van Hinsbergh VW, Collen A, Koolwijk P. Role of fibrin matrix in angiogenesis. Ann N Y Acad Sci. 2001;936:426–437. doi: 10.1111/j.1749-6632.2001.tb03526.x. Epub 2001/07/20. PubMed PMID: 11460496. [DOI] [PubMed] [Google Scholar]
- 88.Maragoudakis ME, Tsopanoglou NE, Andriopoulou P. Mechanism of thrombin-induced angiogenesis. Biochem Soc Trans. 2002;30(2):173–177. doi: 10.1042/. Epub 2002/05/25. doi:10.1042/. PubMed PMID: 12023846. [DOI] [PubMed] [Google Scholar]