Abstract
More rapid skeletal maturation in African-American (AA) children is recognized and generally attributed to an increased prevalence of obesity. The objective of the present study was to evaluate the effects of population ancestry on relative skeletal maturation in healthy, non-obese children and adolescents, accounting for body composition and sexual maturation. To do this, we leveraged a multiethnic, mixed-longitudinal study with annual assessments for up to 7 years (The Bone Mineral Density in Childhood Study and its ancillary cohort) conducted at five US clinical centers. Participants included 1592 children, skeletally immature (45% females, 19% AA) who were aged 5 to 17 years at study entry. The primary outcome measure was relative skeletal maturation as assessed by hand-wrist radiograph. Additional covariates measured included anthropometrics, body composition by dual-energy X-ray absorptiometry (DXA), and Tanner stage of sexual maturation. Using mixed effects longitudinal models, without covariates, advancement in relative skeletal maturation was noted in self-reported AA girls (~0.33 years, p<0.001) and boys (~0.43 years, p<0.001). Boys and girls of all ancestry groups showed independent positive associations of height, lean mass, fat mass, and puberty with relative skeletal maturation. The effect of ancestry was attenuated but persistent after accounting for covariates: for girls, 0.12 years (ancestry by self-report, p = 0.12) or 0.29 years (ancestry by admixture, p = 0.004); and for boys, 0.20 years (ancestry by self-report, p = 0.0038) or 0.29 years (ancestry by admixture, p = 0.004). In summary, we conclude that advancement in relative skeletal maturation was associated with AA ancestry in healthy, non-obese children, independent of growth, body composition, and puberty. Further research into the mechanisms underlying this observation may provide insights into the regulation of skeletal maturation.
Keywords: skeletal maturation, growth, population ancestry, pediatric endocrinology, bone age
Introduction
Skeletal maturation is the developmental process by which the skeleton achieves the adult form through increases in bone size, shape, and density throughout childhood and adolescence. Understanding its mechanistic underpinnings may help to develop strategies to ensure children achieve optimal peak bone mass.(1) African-American (AA) children have more rapid skeletal maturation compared to their counterparts of European ancestry.(2–5) This difference is evident from infancy through adolescence, and is independent of birth size.
Isolating the relative effects of population ancestry on skeletal maturation can be challenging because numerous other known factors affecting growth, including nutritional status, body composition, and pubertal timing all exert important confounding effects.(6–9) Previous studies have suggested that advanced relative skeletal maturation in AA children compared to their non-AA counterparts may be largely attributable to differences in body composition and, most notably, greater adiposity.(8) It is possible that the hormonal milieu of obesity obscures important ancestry-specific mechanisms that affect relative skeletal maturation, and that an investigation limited to nonobese children would be more informative.
Therefore, the objective of the present study was to evaluate the effects of population ancestry (as defined by both self-report and by genetic admixture) and other clinical covariates on relative skeletal maturation in a cohort of healthy, nonobese children, and adolescents.
Subjects and Methods
Study sample
The Bone Mineral Density in Childhood Study (BMDCS) and its ancillary study together constituted a mixed-longitudinal study of over 2000 children enrolled at five centers in the United States as described.(10) Study participants, ages 5 through 19 years at enrollment, were evaluated annually for up to six visits. Inclusion and exclusion criteria were specified with a view to obtaining a healthy, multiethnic sample of children with typical development and bone health.(10) In addition, height, weight, and body mass index (BMI) at study entry needed to be between the third and 97th percentile (%ile) for age. An ancillary study to the BMDCS enrolled a cross-sectional cohort, ages 5 to 18 years, of approximately 500 children from two of the five BMDCS sites following identical study procedures. For the purposes of the present investigation, the initial and ancillary cohorts were analyzed together.
Bone age
Hand-wrist radiographs were acquired and bone age assessed using the Greulich and Pyle Atlas.(11) Ratings were performed by a single pediatric radiologist (SM) for the BMDCS, and a pediatric endocrinologist (AK) for the ancillary study. Both were blinded to the age and ancestry of the study participant, and were instructed not to interpolate between bone age categories. Relative skeletal maturation was expressed as bone age minus chronological age. Skeletal immaturity was defined as bone age <13 years for girls and bone age <15 years for boys, because girls are estimated to have achieved 95.8% of adult height at bone age 13 years, and boys 96.8% of adult height at bone age 15 years.(12) Only skeletally immature films were included in the subsequent analyses.
Self-reported population ancestry
In keeping with the previously published reference curves from this cohort,(10) individuals were categorized as either AA or non-AA, based on the parent’s report.
Physical examination
Height and weight were assessed in light clothing, with shoes removed, following standard procedures. BMI and height Z-scores were calculated.(13) Tanner stage of breast development in girls, and pubic hair development in boys and girls was assessed by a pediatric endocrinologist or skilled nurse practitioner. Testicular volume was assessed in boys using a Prader orchidometer and categorized into stages of pubertal development. Pubertal maturation assessment for breast development (for girls) and testicular volume (for boys) is referred to as “gonadal” pubertal stage.
Body composition
Whole-body DXA scans were acquired on Hologic, Inc. (Bedford, MA, USA) bone densitometers (QDR4500A, QDR4500W, Delphi A, and Apex models). Central analysis of all scans was performed by the DXA Core Laboratory (University of California, San Francisco, San Francisco, CA, USA) using Hologic software version Discovery 12.3 (baseline scans) and Hologic Apex 2.1 software. Total fat mass and lean body mass (excluding bone) were determined and corrected for intermachine differences and longitudinal drift.
Genotyping
Blood or saliva was collected at the final study visit, from which DNA was extracted. We performed high-throughput genomewide SNP genotyping, using the Illumina Infinium II OMNI Express plus Exome BeadChip technology (Illumina, San Diego, CA, USA), at CHOP’s Center for Applied Genomics, as described.(14,15)
Estimation of genetic population ancestry proportions
Based on ~100,000 pruned autosomal SNPs, we performed estimation of the genetic population ancestry components of each individual by maximum likelihood (ML) using ADMIXTURE software.(16)
Statistical analyses
All measurements with complete visit data (bone age film, anthropometrics, pubertal staging, body composition) were included; 281 measurements (6%) were excluded because BMI was ≥95th percentile for age and sex at the time they were obtained (participants were only required to have BMI <97th percentile at study entry). Thus, in total, 4622 measurements from 1592 individuals were included.
The independent effects of clinical covariates on relative skeletal maturation were assessed using mixed effects regression analysis to account for the multiple observations per subject. Included in the models were: between-subject variability (random effect), ancestry (specified as a fixed, time-invariant effect), as well as age, body composition, height, and sexual maturation (all of these were considered fixed, time-variant covariates). Nonlinear dependence on age coincident with the more rapid changes expected to occur with puberty was tested by inclusion of higher-order polynomial terms (age2 and age3) in these models. For both girls and boys, final models were chosen based on the following criteria: (1) highest-order age term included exerted a statistically significant effect on skeletal maturation; and (2) goodness of fit was optimized (as assessed by minimization of Akaike information criterion [AIC] and Bayesian information criterion [BIC]. With respect to sexual maturation, we included estimates by physical examination of gonadal and pubic hair maturation. Ancestry was modeled separately either by self-report (dichotomous variable) or by proportion of genetic African admixture (continuous variable). We performed a sensitivity analysis by excluding participants without available genotypic data to assess for any potential associated source of bias. Modeling was performed separately for girls and boys.
All analyses were conducted using R (version 3.1.3; R Project for Statistical Computing; https://www.r-project.org/), and statistical significance was taken as two-sided p value of <0.05.
Results
Sample characteristics
Characteristics of the study sample are shown in Tables 1 and 2.
Table 1.
Sample Characteristics
| First visit (n = 1592) |
All visits (n = 4622) |
|
|---|---|---|
| Sex, % female (n) | 45 (711) | 42 (1941) |
| AA ancestry, by self-report, % (n) |
19 (295) | 19 (887) |
| AA ancestry, % genetic admixture (n = 1185 subjects and 3547 measurements) | ||
| 0–33, % (n) | 80 (947) | 77 (2728) |
| 33–66, % (n) | 4 (49) | 5 (174) |
| >66, % (n) | 16 (188) | 18 (645) |
| Age (years), mean ± SD | 9.0 ± 2.7 | 10.0 ± 2.6 |
| BMI Z-score, mean ± SD | 0.22 ± 0.79 | 0.15 ± 0.83 |
| Height Z-score, mean ± SD | 0.12 ± 0.82 | 0.11 ± 0.84 |
| Gonadal stage | ||
| Prepubertal, % (n) | 67 (1060) | 54 (2501) |
| Pubertal, % (n) | 31 (490) | 41 (1887) |
| Postpubertal, % (n) | 3 (42) | 5 (234) |
| Pubic hair Tanner stage | ||
| Tanner I, % (n) | 75 (1194) | 64 (2946) |
| Tanner II–IV, % (n) | 24 (379) | 33 (1531) |
| Tanner V, % (n) | 1 (19) | 3 (145) |
Values are presented at study entry (ie, one visit per subject, left) as well as over all visits (ie, more than one visit per subject, right).
Table 2.
Bone Age Characteristics
| All participants (n = 1592) |
African Americans (n = 295) |
Non-African Americans (n = 1297) |
|
|---|---|---|---|
| Number of measurements per participant, % (n) | |||
| 1 | 32 (515) | 23 (69) | 34 (446) |
| 2–4 | 46 (728) | 56 (164) | 43 (564) |
| 5–7 | 22 (349) | 21 (62) | 22 (287) |
| Percentage of abnormal bone age measurements per participant, by number of measurements per participant, % (number abnormal/total) | |||
| 1 | 13 (68/515) | 9 (6/69) | 14 (62/446) |
| 2–4 | 9 (66/728) | 8 (13/164) | 9 (53/564) |
| 5–7 | 15 (54/349) | 11 (7/62) | 16 (47/287) |
| Percentage of participants with all abnormal bone age measurements, by number of measurements per participant, % (number abnormal/total) | |||
| 1 | 13 (68/515) | 9 (6/69) | 14 (62/446) |
| 2–4 | 3 (20/728) | 3 (5/164) | 3 (15/564) |
| 5–7 | 2 (8/348) | 2 (1/62) | 2 (7/287) |
Abnormal bone age measurements are ≥2SD away from the mean for nearest chronologic age, according to Brush Foundation reference values.
The sample consisted of 1592 study participants (711 girls, 45%) who, in total, completed 4622 study visits; 295 (19%) of subjects self-identified as AA. Just over one-half of the visits occurred in prepubertal subjects. Approximately one-third of participants had only one bone age measurement performed. Twelve percent of all bone age measurements were considered “clinically relevant”; ie, two or more standard deviations (≥2SD) away from the mean for the nearest chronologic age according to the Greulich and Pyle standards (Table 2).(11) Of note, for participants who had more than one bone age measurement taken, only 2.6% had all obtained bone age measurements ≥2SD away from the mean. Table 3 shows the prevalence of “clinically relevant” advanced or delayed relative skeletal maturation according to participant characteristics. Overall, there were more delayed (≥2SD below the mean) than advanced bone age measurements. As expected, children who were shorter (lower height Z-score) or lighter (lower BMI Z-score) had more delayed skeletal maturation.
Table 3.
Skeletal Maturation, by Age, Tanner Stage, BMI and Height, by Ancestry: Relative Skeletal Maturation (Bone Age–Chronologic Age), All Visits
| AA | Non-AA | |||||||
|---|---|---|---|---|---|---|---|---|
| Years (mean ± SD) |
Advanced (%)a |
Delayed (%)b |
n | Years (mean ± SD) |
Advanced (%)a |
Delayed (%)b |
n | |
| Girls | ||||||||
| Age | ||||||||
| 5 to <8 years | 0.52 ± 0.76 | 1 | 2 | 105 | 0.31 ± 0.78 | 0 | 4 | 471 |
| 8 to <11 years | 0.08 ± 0.91 | 1 | 2 | 174 | −0.10 ± 0.95 | 1 | 5 | 688 |
| 11 to <14 years | −0.37 ± 0.72 | 0 | 8 | 73 | −0.62 ± 0.83 | 0 | 17 | 422 |
| >14 yrs | – | – | – | – | −2.39 ± 0.25 | 0 | 100 | 8 |
| Tanner stage | ||||||||
| I | 0.24 ± 0.87 | 1 | 2 | 182 | −0.02 ± 0.93 | 0 | 6 | 964 |
| II | 0.04 ± 0.99 | 0 | 6 | 52 | −0.17 ± 0.99 | 0 | 11 | 244 |
| III | −0.01 ± 0.89 | 0 | 6 | 70 | −0.33 ± 0.93 | 0 | 11 | 264 |
| IV | −0.09 ± 0.82 | 0 | 2 | 41 | −0.57 ± 0.92 | 0 | 18 | 106 |
| V | 0.03 ± 0.63 | 0 | 0 | 7 | 0.21 ± 0.85 | 0 | 0 | 11 |
| BMI %ile | ||||||||
| <15%ile | −0.58 ± 0.79 | 0 | 7 | 27 | −0.66 ± 1.02 | 0 | 23 | 162 |
| 15 to <50%ile | 0.04 ± 0.89 | 1 | 4 | 80 | −0.32 ± 0.91 | 0 | 11 | 531 |
| 50 to <85%ile | 0.19 ± 0.80 | 0 | 2 | 164 | 0.02 ± 0.89 | 0 | 5 | 669 |
| 85 to <95%ile | 0.28 ± 0.97 | 1 | 5 | 81 | 0.24 ± 0.90 | 1 | 3 | 227 |
| Height %ile | ||||||||
| <15%ile | −0.58 ± 0.99 | 0 | 7 | 29 | −0.62 ± 0.99 | 0 | 19 | 156 |
| 15 to <50%ile | −0.28 ± 0.80 | 0 | 8 | 105 | −0.36 ± 0.89 | 0 | 12 | 615 |
| 50 to <85%ile | 0.22 ± 0.81 | 0 | 1 | 158 | 0.04 ± 0.91 | 0 | 5 | 616 |
| >85%ile | 0.87 ± 0.71 | 3 | 0 | 60 | 0.45 ± 0.81 | 0 | 1 | 202 |
| Boys | ||||||||
| Age | ||||||||
| 5 to <8 years | 0.07 ± 0.76 | 3 | 4 | 114 | −0.49 ± 0.85 | 1 | 13 | 448 |
| 8 to <11 years | −0.18 ± 1.20 | 6 | 14 | 150 | −0.6 ± 1.08 | 2 | 21 | 626 |
| 11 to <14 years | 0.08 ± 1.09 | 1 | 8 | 210 | −0.23 ± 1.10 | 0 | 12 | 837 |
| >14 yrs | −0.86 ± 0.75 | 0 | 18 | 61 | −0.92 ± 0.72 | 0 | 15 | 235 |
| Tanner stage | ||||||||
| I | −0.16 ± 1.05 | 4 | 13 | 264 | −0.58 ± 1.00 | 1 | 18 | 1091 |
| II | −0.23 ± 1.19 | 3 | 10 | 90 | −0.48 ± 1.15 | 1 | 17 | 404 |
| III | −0.02 ± 1.16 | 0 | 9 | 67 | −0.21 ± 1.02 | 0 | 7 | 228 |
| IV | 0.12 ± 0.97 | 1 | 4 | 76 | −0.24 ± 0.95 | 1 | 8 | 245 |
| V | 0.02 ± 0.85 | 0 | 5 | 38 | −0.39 ± 0.97 | 0 | 12 | 178 |
| BMI %ile | ||||||||
| <15%ile | −0.56 ± 1.04 | 3 | 23 | 35 | −0.85 ± 1.01 | 0 | 20 | 186 |
| 15 to <50%ile | −0.32 ± 1.01 | 1 | 11 | 152 | −0.59 ± 1.00 | 1 | 16 | 748 |
| 50 to <85%ile | −0.07 ± 1.05 | 2 | 9 | 261 | −0.41 ± 1.00 | 1 | 15 | 877 |
| 85 to <95%ile | 0.36 ± 1.05 | 8 | 5 | 87 | −0.13 ± 1.08 | 2 | 10 | 335 |
| Height %ile | ||||||||
| <15%ile | −1.27 ± 1.17 | 0 | 33 | 33 | −1.16 ± 1.11 | 2 | 37 | 176 |
| 15 to <50%ile | −0.62 ± 0.89 | 1 | 19 | 166 | −0.78 ± 0.93 | 0 | 22 | 839 |
| 50 to <85%ile | 0.06 ± 0.88 | 2 | 4 | 218 | −0.22 ± 0.94 | 2 | 7 | 818 |
| >85%ile | 0.65 ± 0.94 | 7 | 2 | 118 | 0.10 ± 0.97 | 1 | 5 | 313 |
Mean ± SD for relative skeletal maturation (bone age minus chronologic age, in years) are shown for all categories.
The percentage of films classified as “advanced” (greater than or equal to two standard deviations above the mean, according to the Brush Foundation standards).
The percentage of films classified as “delayed” (≤2SD below the mean, according to the Brush standards) are shown.
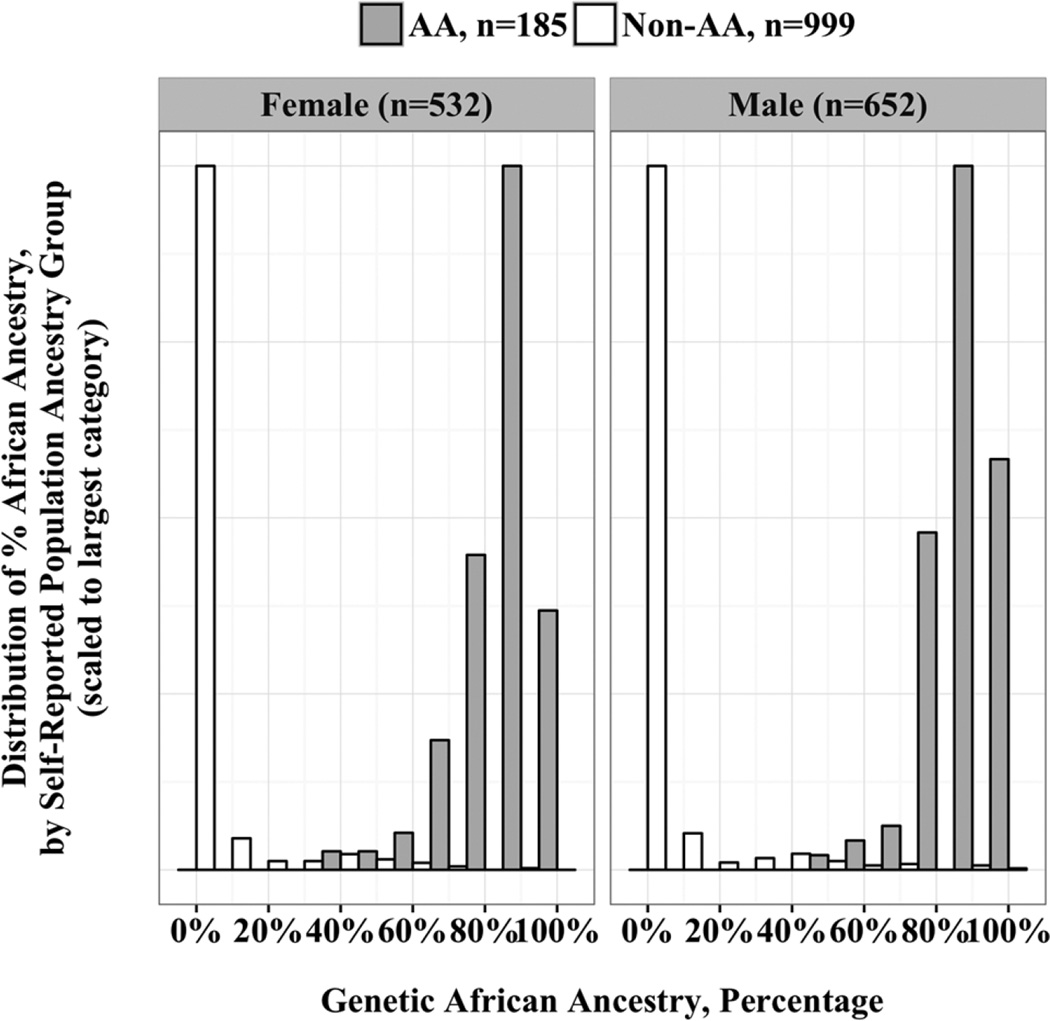
Genetic markers were used to identify the proportion of African ancestry in 1184 subjects (74% of the sample). Subjects without available genotyping data were more likely to be younger at their last study visit (10.7 versus 11.1 years, p = 0.01 for two-sample t test) and to self-identify as AA (27% versus 16%, p<0.001). In subjects self-identifying as AA, percentage of African ancestry was 88% (versus 4% in self-identified non-AAs), p<0.001 by two-sample t test. The distribution of genetic African admixture across the cohort is shown in Fig. 1. This distribution shows that in individuals who self-identify as AA, there is heterogeneity with respect to the degree of genetic African admixture. In contrast, those who self-identify as non-AA have mostly <10% genetic African admixture, although some heterogeneity is present in non-AA children as well. This variation supports the feasibility of considering proportion of genetic African ancestry as a continuous explanatory variable in statistical models.
Fig. 1.
Genetic population ancestry and self-reported population ancestry, by sex. Population ancestry can be estimated using genetic analyses. The distribution of the proportion of genetic African admixture is reported for the entire BMDCS/ancillary cohort with available DNA for genetic analyses, according to self-reported population ancestry and sex. AA = African-American; Non-AA = Non-African-American.
Relative skeletal maturation (unadjusted), population ancestry, and age
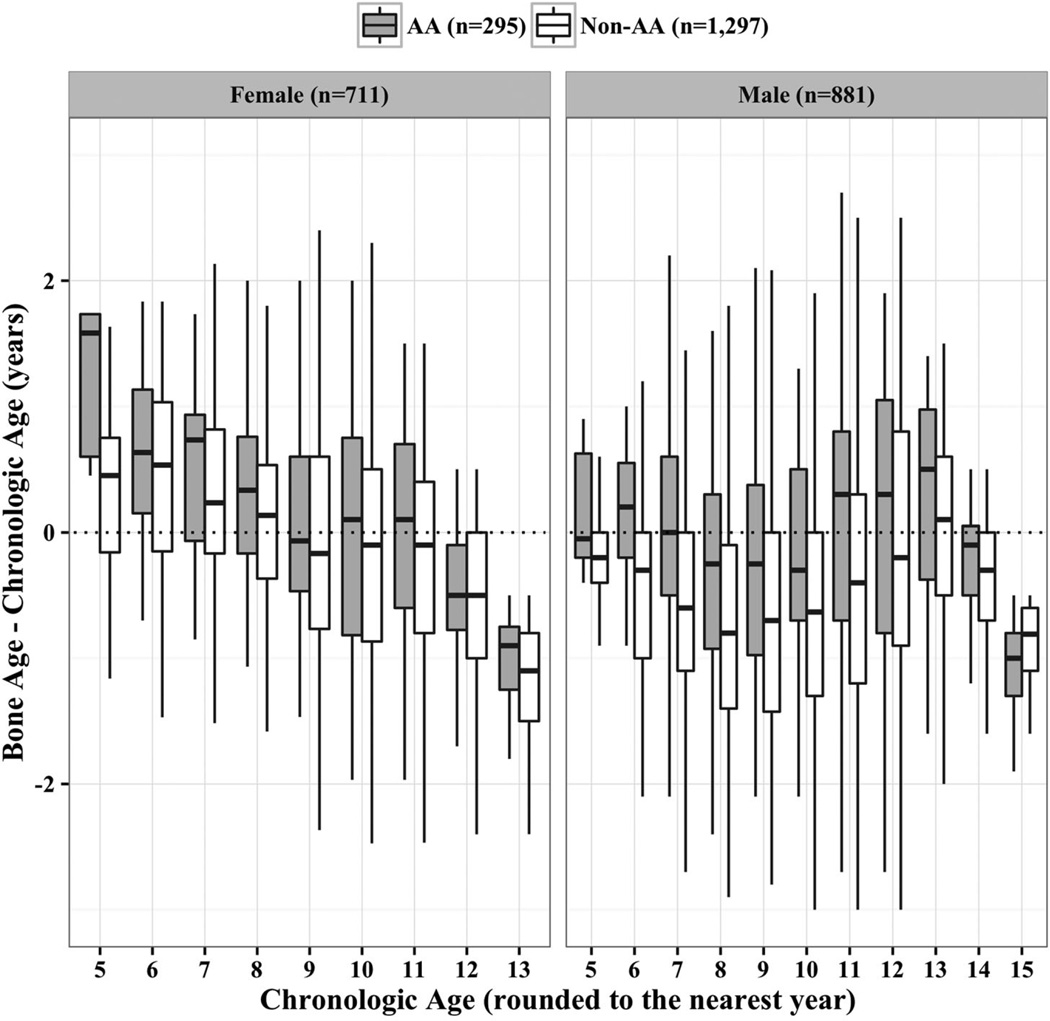
Relative skeletal maturation in AA girls was, on average, more advanced (by 0.33 years, p<0.001), as was skeletal maturation in AA boys (0.43 years, p<0.001) as compared to their non-AA counterparts in sex-specific mixed effects models (without covariates) when ancestry was assessed using self-report. The extent of relative skeletal maturation varied with age (Fig. 2), and the age-specific pattern was different for boys and girls. AA boys and girls both show relative advancement in bone age compared to non-AA boys and girls at younger ages. However, at younger chronologic ages (<9 years), both AA and non-AA girls showed average bone ages that were older than the reference values. From 9 years through around 11 years of chronologic age, girls had bone ages that were closer to reference values. In contrast, younger AA and non-AA boys (<7 years) had bone ages that approximated the reference values, but between 8 years and 11 years, both AA and non-AA boys showed bone age delay relative to the reference values. Nonlinearity at older ages, in particular in boys, was also shown. In the oldest chronologic ages (12 and 13 years in girls, and 14 and 15 years in boys), bone age delay was observed in both AA and non-AA, which is likely attributable to our excluding skeletally mature bone age films for these analyses. To determine if these ancestry differences in relative skeletal maturation were attributable to other factors, we performed mixed effects regression analyses to account for age trends, body composition, linear growth, and sexual maturation.
Fig. 2.
Self-reported population ancestry and relative skeletal maturation, by sex and chronologic age. Box plots showing the distribution of relative skeletal maturation (bone age minus chronologic age) by chronologic age (rounded to the nearest year) and self-reported population ancestry are shown for girls (on the left) and boys (on the right); the distributions for non-AA children are shown in blue, and the distributions for AA children in red. The dotted black horizontal line indicates a relative skeletal maturation of zero; ie, where the mean relative skeletal maturation would be if the observed values were similar to the reference values.
Mixed effects regression analysis of skeletal maturation
Chronologic age
We developed sex-specific mixed effects regression models that best fit the distinct, nonlinear age trends in males and females (Table 4). In girls, the best-fitting model included an age2 term; in boys, the best fitting polynomial model included an age3 term. In both boys and girls, there was a negative association with the first-order age term and relative skeletal maturation, indicating that the difference between bone age and chronological age was smaller among older children. The higher order terms in the mixed effects model for males accounted for the later increase in relative skeletal maturation.
Table 4.
Mixed Effects Regression Analysis, BMDCS, Girls and Boys, Skeletally Immature (Bone Age <13 Years in Girls, Bone Age <15 Years in Boys): Bone Age–Chronologic Age (Years), Mixed Effects Regression Analysis
| Factor | Girls | ||
| Self-report All subjects n = 711 1941 films |
Self-report Genotyped n = 532 1480 films |
Admixture Genotyped n = 532 1480 films |
|
| AA ancestry (self-report or % admixture) | 0.18* (0.07) | 0.19* (0.09) | 0.29** (0.10) |
| Age (years) | −0.34*** (0.06) | −0.33*** (0.07) | −0.33*** (0.07) |
| Age2 (years2) | −0.01*** (0.003) | −0.01*** (0.003) | −0.01*** (0.003) |
| Height (cm) | 0.06*** (0.005) | 0.05*** (0.005) | 0.05*** (0.005) |
| Fat mass (whole body, kg) | 0.03*** (0.007) | 0.03*** (0.008) | 0.03*** (0.008) |
| Gonadal stage (versus prepubertal) | |||
| Pubertal | 0.15*** (0.04) | 0.13** (0.04) | 0.13** (0.04) |
| Postpubertal | 0.26 (0.14) | 0.24 (0.15) | 0.24 (0.15) |
| Pubic hair Tanner stage (versus Tanner I) | |||
| Tanner II–IV | 0.16*** (0.04) | 0.23*** (0.04) | 0.22*** (0.04) |
| Tanner V | 0.12 (0.18) | 0.27 (0.20) | 0.25 (0.02) |
| AIC | 3553 | 2673 | 2669 |
| BIC | 3620 | 2736 | 2732 |
| Factor | Boys | ||
| Self-report All subjects n = 81 2681 films |
Self-report Genotyped n = 652 2067 films |
Admixture Genotyped n = 652 2067 films |
|
| AA ancestry (self-report or % admixture) | 0.26*** (0.07) | 0.20* (0.08) | 0.29** (0.09) |
| Age (years) | −3.34*** (0.21) | −3.33*** (0.23) | −3.33*** (0.23) |
| Age2 (years2) | 0.31*** (0.02) | 0.30*** (0.02) | 0.30*** (0.02) |
| Age3 (years3) | −0.01*** (0.0007) | −0.01*** (0.0007) | −0.01*** (0.0007) |
| Height (cm) | 0.07*** (0.003) | 0.08*** (0.005) | 0.08*** (0.005) |
| Fat mass (whole body, kg) | 0.02** (0.006) | 0.02*** (0.007) | 0.02*** (0.007) |
| Gonadal stage (versus prepubertal) | |||
| Pubertal | −0.03 (0.05) | −0.03 (0.05) | −0.03 (0.05) |
| Postpubertal | −0.09 (0.08) | −0.10 (0.09) | −0.11 (0.09) |
| Pubic hair Tanner stage (vs Tanner I) | |||
| Tanner II–IV | 0.26*** (0.05) | 0.25*** (0.05) | 0.25*** (0.05) |
| Tanner V | 0.30*** (0.09) | 0.33*** (0.10) | 0.33*** (0.10) |
| AIC | 5987 | 4583 | 4579 |
| BIC | 6064 | 4657 | 4652 |
The independent effects of ancestry, age, height, and body composition (fat mass) on skeletal maturation were evaluated. Regression coefficients are indicated; bold text indicates statistical significance with p < 0.05. Standard error of each regression coefficient is in parentheses.
Symbols indicate statistically significant coefficients:
p < 0.05;
p < 0.01;
p < 0.001.
Linear growth and body composition
Height and lean body mass were highly correlated (at study entry, r = 0.95 for girls and r = 0.96 for boys, p<0.001 for both). Therefore, to avoid collinearity in the mixed effects regression models, analyses were performed separately for models including either height or lean body mass. For both boys and girls, the models including height provided a better fit (Table 4); the models using lean body mass are included in Supporting Table 1. Both models produced similar estimates for the effects of other covariates.
In mixed effects models, taller stature (adjusting for covariates including age, Table 4) was associated with relative bone age advancement. There also was an independent positive association between fat mass and relative skeletal maturation (Table 4) (girls: 0.03 years, and boys: 0.02 years of advancement per kg of fat mass, both p<0.001). In the alternate model using lean body mass rather than height (Supporting Table 1), lean body mass was positively associated with relative skeletal maturation adjusting for covariates (girls: 0.07 years, and boys: 0.07 to 0.08 years of advancement per kg of lean mass, both p<0.001).
Pubertal maturation
Compared to being prepubertal, being in gonadal stage II to IV (Table 4) was associated with more advanced skeletal maturation in girls (0.13 to 0.15 years of skeletal advancement attributable to pubertal gonadal stage, p<0.01), adjusting for covariates. This effect of gonadal stage was not observed in boys in the model including additional higher order age term (age3), but was evident when only up to age2 terms were included (data not shown). In both boys and girls, the effect of Tanner stage for pubic hair was statistically significant (boys: 0.25 to 0.26, and girls: 0.16 to 0.23 years of advancement attributable to Tanner stage for pubic hair II to IV versus I, all p<0.001).
Relative skeletal maturation (adjusted for covariates) and population ancestry
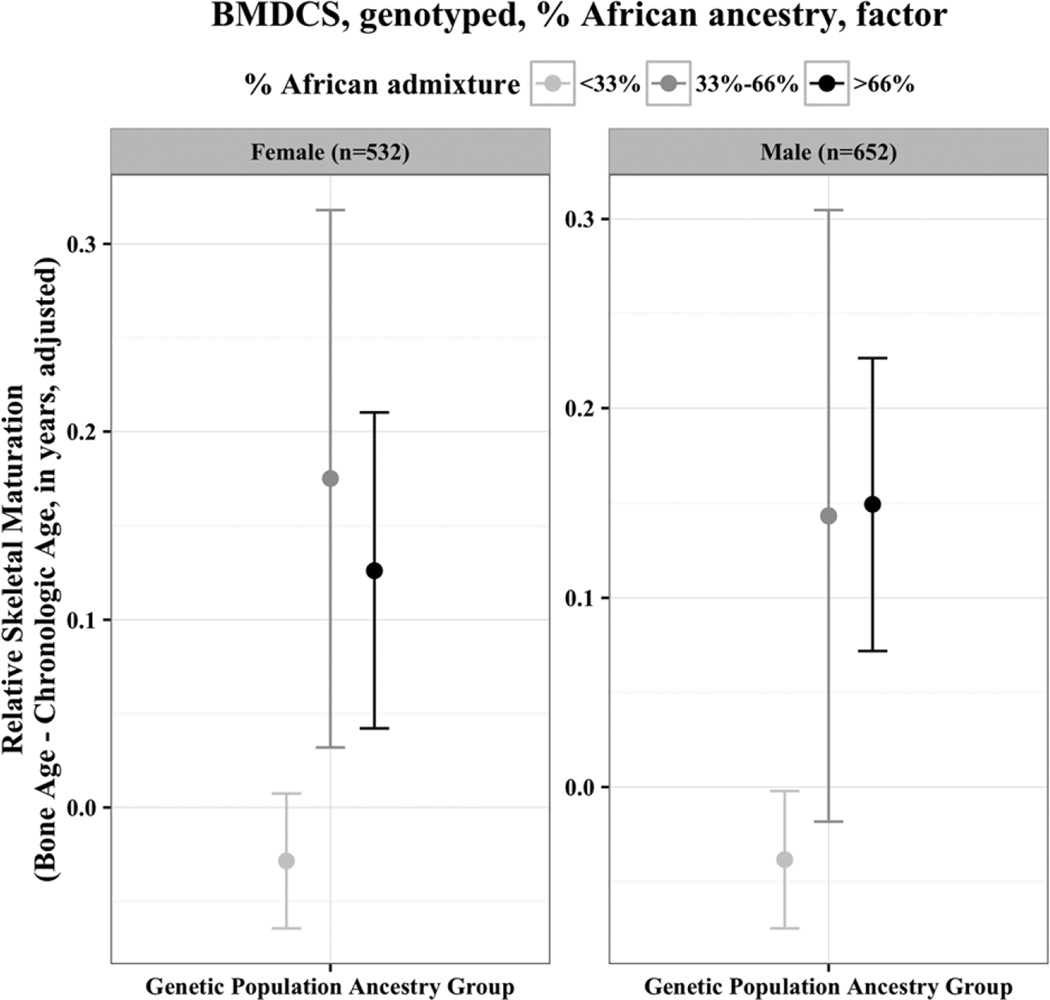
The mixed effects regression analyses (Table 4) showed that AA ancestry, whether by self-report or by genetic admixture, was associated with advanced relative skeletal maturation in both girls (0.18 to 0.29 years, p<0.05 and p<0.01) and boys (0.20 to 0.29 years, p<0.01 and p<0.001), after accounting for all covariates. The AA population ancestry effect was similar when expressed using genetic admixture (Table 4). To show visually the “dose effect” attributable to increasing amounts of genetic AA population ancestry in the mixed effects regression models, Figure 3 shows relative skeletal maturation, adjusted for covariates, according to tertiles of African admixture. The group with a higher proportion of African ancestry had greater skeletal age advancement, even after accounting for clinical covariates.
Fig. 3.
Genetic population ancestry and relative skeletal maturation, adjusted for clinical covariates. Mixed effects regression analyses of relative skeletal maturation (defined by the difference between bone age and chronologic age), here illustrating the effect of genetically defined ancestry, after accounting for other important clinical covariates. To provide a visual demonstration of the “dose effect” of African ancestry, ancestry is modeled as a factor in this figure (but as a continuous variable in the tables). Only skeletally immature (<13 years for girls, <15 years for boys) films are included. Clinical covariates included in mixed effects regression analyses were: age, body composition (lean mass, fat mass), height, and pubertal stage.
Discussion
Our findings of advanced relative skeletal maturation in children of AA ancestry compared to children mostly of European ancestry builds on previous work through the restriction of our analyses to non-obese children, thereby more clearly isolating the role of genetic ancestry from the contribution of excess adiposity. Indeed, AA children in the United States have higher obesity rates than most other ancestry groups,(6) and obesity is associated with advanced skeletal maturation. Further, we showed that the effect of AA ancestry on skeletal maturation was persistent even after accounting for growth, body composition, and pubertal timing, which are also associated with skeletal maturation, and differ between children of AA and non-AA ancestry in the United States.(7,17,18) Finally, our results were confirmed using genetic estimates of population ancestry. These findings point to a population-related genetic contribution to skeletal maturation, because degree of African admixture was associated with relative skeletal maturation in our multivariable models.
Past studies have noted differences in skeletal maturation related to African ancestry in children, though results are not uniform. Few of these previous studies accounted for differences in growth, body composition, and sexual maturation, and none, to our knowledge, used estimates of population-specific genetic admixture. For example, our findings are consistent with historical US data available from the Health Examination Survey (HES) from 1963 to 1965 for 6-year-old to 11-year-old children, and 1966 to 1970, for 12-year-old to 17-year-old adolescents.(19,20) In both BMDCS and HES, advancement in skeletal maturation in AA children is apparent, particularly in younger children, and in children of all ancestry groups, the difference between bone age and chronologic age was not consistent over chronologic ages. The persistent discrepancies between overall bone age and chronologic age in these two population studies (BMDCS and HES) illustrate likely differences between the children in BMDCS and HES compared to the cohort of the children used to generate the Greulich and Pyle reference values.(21)
In one large cross-sectional study (n = 534 children), investigators found that prior to puberty, children of AA descent had significantly more advanced bone ages.(22) The longitudinal “Birth to Twenty” cohort study of South African children provides an interesting contrast to our US sample.(23) Among black boys in South Africa, skeletal maturation began its acceleration at an older age, and then development proceeded at a similar rate as white boys, resulting in a net delay in attainment of skeletal maturity. In contrast, black girls began to mature later than white girls, but the pace of development occurred more quickly, such that the ultimate attainment of maturity was similar. Discrepancies between these findings and those of our study may be due to differences in the overall health, nutritional status, social environment, and genetic admixture between cohorts. Also, the South African study used the Tanner-Whitehouse III system for bone age assessment and different statistical modeling techniques, which could add to contrasting findings between the studies.
At least two other reports have noted a difference in other bone phenotypes in AA children relative to children of European ancestry when using genetic admixture to examine population effects. Specifically, genetic African admixture was positively associated with both bone mineral content (BMC) or bone mineral density (BMD) even after adjusting for clinical covariates in healthy children.(24,25)
Several related physiologic mechanisms have been posited to explain the observed ancestry-specific differences in skeletal maturation. First, differential rates of excess adiposity in AA children in the United States may underlie the observed ancestry-specific differences in relative skeletal maturation. In a previous study of children ages 5 to 12 years, there were no significant differences in relative skeletal maturation attributable to ancestry after statistical adjustment for lean mass and fat mass.(8) Of note, the average BMI SD score was significantly higher in AA as compared to white children in that study (2.7 ± 3.4 versus 1.7 ± 2.4, p<0.05).(8) Furthermore, their sample size (n = 252) may have been too small to detect the differences we observed (they estimated a minimum detectable difference of around 0.2 years in relative skeletal maturation, close to the effect size detected in the present study). Further, their analysis did not attempt to capture sex differences and age trends in relative skeletal maturation. Finally, we additionally excluded obese children in our study in order to evaluate differences in skeletal maturation outside the context of obesity.
There are additional ancestry-specific differences in body composition that may relate to the more rapid pace of skeletal development in AAs.(26) Higher lean body mass index (LBMI) in AAs even prior to puberty(7) may be a consequence of the more rapid pace of sexual and skeletal maturation in this population. Certainly, earlier pubertal onset in AAs(18,27) could also account for age-related differences in both lean body mass and skeletal maturation. In the present study, ancestry-specific differences in relative skeletal maturation persisted after including both body composition and indices of pubertal status in the models. Interestingly, in the “Birth to Twenty” cohort, there were no detectable ancestry-specific differences in pubertal timing.(28,29) The investigators conjecture that delayed skeletal maturation in South African black boys represents a male-specific vulnerability to adverse environmental conditions.(30) Even though obese children were excluded from analyses in the present study, the current and past nutritional status is likely to differ between BMDCS and “Birth to Twenty” cohorts and differences in skeletal assessment techniques do not easily permit direct comparisons. In addition, African ancestry is a broad term that does not capture potential genetic differences between AA and South African black children, but could be the focus of future studies on the interactions between ancestry, sex, and divergent environmental conditions to improve our understanding of skeletal maturation regulation and population variation.
It is also plausible that there may be differences in timing of adrenarche and in gonadal steroid levels either related to and/or independent of body composition and pubertal stage in AA children. Indeed, the differences in overall skeletal maturation between prepubertal girls and prepubertal boys may be related to slightly higher levels of circulating estrogen in girls during this age.(31) In one previous cross-sectional study, during puberty, estradiol levels were higher in AA boys as compared to white males(32) and could contribute to more rapid skeletal maturation.
Higher levels of adrenal androgens, including dehydroepian-drosterone-sulfate (DHEA-S), are also associated with more rapid skeletal maturation.(33) The potentiating role of weak androgens produced by premature adrenarche in obese children on skeletal advancement has also been shown.(34) Adrenal androgens may be disproportionately elevated in the non-obese but overweight AA children in the BMDCS. Indeed, in a small study of prepubertal children, AA children had higher concentrations of DHEA-S even before clinical evidence of adrenarche, and also higher levels of insulin-like growth factor 1 (IGF-1).(35)
Taken together, ancestry-specific differences in activity of one or more of the above hormonal axes could contribute to the observed differences in relative skeletal maturation between AA and non-AA children. In addition, the complex interaction of environment with hormonal milieu, especially in the setting of excess adiposity, could also play a role in more rapid maturation.(36) It remains challenging, however, to establish whether genetic ancestry is causally related to these differences, or whether skeletal maturity is correlated with other ancestry-specific patterns that have a separate, common etiology (eg, intrauterine, nutritional, activity, environmental, etc.). However, our findings of a “dose”-like effect of degree of admixture supports the hypothesis that genetic factors play some role in determining relative skeletal maturation, at least in this population.
We also observed a complex association between relative skeletal maturation and advancing chronologic age in both boys and girls (Fig. 2). A complex relationship was noted in the “Birth to Twenty” cohort as well, related to the correlation between the tempo and velocity of skeletal maturation. The residual association of skeletal maturation with chronologic age may exist because the children studied to produce the Brush Foundation standards differ from contemporary children in the United States with respect to their diversity in population ancestry, socioeconomic status, and pubertal progression.(22) Sex-specific secular trends in skeletal maturation in a US cohort of predominantly European ancestry have been reported, and may be related to changes in timing of sexual maturation.(37) Overall, the clinical relevance of sex-specific differences can be the focus of future studies, for example with respect to the potential role of changes in nutritional status and exposure to endocrine-disrupting chemicals.
The present study has several strengths and limitations. BMDCS is a carefully screened cohort of healthy children with prospectively and rigorously collected longitudinal measurements. Approximately 19% of participants were AA, and entry criteria with respect to BMI and pubertal timing increase the homogeneity of the sample. This study was designed to discern ancestry-specific differences in BMD, and this secondary analysis of bone age leverages the diversity of the cohort. Serial hormonal studies would have enriched our mechanistic understanding of the observed ancestry-specific differences in skeletal maturation but are not available; these could be included in future studies. In addition, the clinical relevance of measuring relative skeletal maturation using the existing clinical standards is frequently revisited.(38) Despite their limitations,(39) these standards remain in clinical use and represent a valuable, if imperfect, tool for evaluating growth potential in the clinic and understanding the physiology of skeletal development in the research setting. These points also highlight the need for carefully constructed, regularly updated skeletal maturation standards, along with improved understanding of the complex relationship between the myriad environmental, nutritional, anthropometric, genetic, and other factors that influence development of the pediatric skeleton.(40)
In conclusion, in a non-obese, otherwise healthy cohort in the United States, we have shown that skeletal maturation is more advanced in AA children compared to non-AA children, accounting for age, body composition, and sexual maturation. This observation is novel, because previous studies have suggested that excess adiposity and earlier pubertal timing are sufficient to explain the more advanced skeletal maturation observed in AA children. From a clinical perspective, improved insight into the factors that affect skeletal maturation enhances the utility and interpretability of bone age measurements. In addition, skeletal maturation likely has implications for the interpretation of bone health in children. One relatively small case-control study showed the association of skeletal delay and fracture risk, but this association appeared to be mediated by the association of skeletal delay with lower aBMD.(41) A previous analysis from the same cohort (ie, BMDCS) has reported that like chronologic age, absolute skeletal age, was associated with increased risk of fracture (hazard ratio 2.17; 95% CI, 1.65 to 2.85, p<0.001) in bivariate analyses, but in multivariable analyses, European ancestry was the strongest risk factor for fracture(42); the effect of relative skeletal maturation, however, was not evaluated in multivariable analyses. This is a potential direction for future research. Indeed, the 2013 Pediatric Position Development Conference from the International Society of Clinical Densitometry called for further research to delineate ethnic differences in bone age, and how to incorporate this information into the interpretation of DXA results in children.(43) The present study adds further clarity to population ancestry differences in skeletal maturation, and the age and sex-specific patterns in these differences. Also, a better appreciation of the etiology of ancestry-specific differences in pediatric skeletal maturation could inform our understanding of corresponding variations in adult BMD, including the lower rates of osteoporosis and fracture, observed in AA adults.(44)
Acknowledgments
The BMDCS study was funded by R01 HD58886 and R01 HD076321; the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) contracts (N01-HD-1-3228, N01-HD-1-3329, N01-HD-1-3330, N01-HD-1-3331, N01-HD-1-3332, N01-HD-1-3333); the CTSA program Grant 8 UL1 TR000077. Additional support was provided by: R01 HD058886 (SFAG); DK094723-01; DK102659-01; Pediatric Endocrine Society Clinical Scholars Award; Children’s Hospital of Philadelphia Metabolism, Nutrition, and Development Research Affinity Group Pilot and Feasibility Grant (SEM).
Footnotes
Additional Supporting Information may be found in the online version of this article.
Disclosures
All authors state that they have no conflicts of interest.
Authors’ roles: Study conception and design: SM, SG, and BZ. Acquisition of data: SG, BZ, HK, JL, VG, SO, KW, and JS. Data analysis: SM and AC. Interpretation of data: SM, JM, AC, DC, SR, HK, JL, VG, SO, JS, AK, SG, and BZ. Drafting manuscript: SM. Revising manuscript content: AC, JM, SR, DC, HK, JL, VG, SO, JS, KW, AK, SG, and BZ. Approving final version of manuscript: SM, AC, JM, SR, DC, HK, JL, VG, SO, JS, KW, AK, SG, and BZ. SM takes full responsibility for the integrity of the data analysis.
References
- 1.Nahhas RW, Sherwood RJ, Chumlea WC, Towne B, Duren DL. Predicting the timing of maturational spurts in skeletal age. Am J Phys Anthropol. 2013;150(1):68–75. doi: 10.1002/ajpa.22142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malina RM. Growth and physical performance of American Negro and white children. A comparative survey of differences in body size, proportions and composition, skeletal maturation, and various motor performances. Clin Pediatr. 1969;8(8):476–483. doi: 10.1177/000992286900800812. [DOI] [PubMed] [Google Scholar]
- 3.Mazess RB, Cameron JR. Skeletal growth in school children: maturation and bone mass. Am J Phys Anthropol. 1971;35(3):399–407. doi: 10.1002/ajpa.1330350319. [DOI] [PubMed] [Google Scholar]
- 4.Garn SM, Clark DC. Nutrition, growth, development, and maturation: findings from the ten-state nutrition survey of 1968–1970. Pediatrics. 1975;56(2):306–319. [PubMed] [Google Scholar]
- 5.Garn SM, Clark DC. Problems in the nutritional assessment of black individuals. Am J Public Health. 1976;66(3):262–267. doi: 10.2105/ajph.66.3.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weber DR, Moore RH, Leonard MB, Zemel BS. Fat and lean BMI reference curves in children and adolescents and their utility in identifying excess adiposity compared with BMI and percentage body fat. Am J Clin Nutr. 2013;98(1):49–56. doi: 10.3945/ajcn.112.053611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russell DL, Keil MF, Bonat SH, et al. The relation between skeletal maturation and adiposity in African American and Caucasian children. J Pediatr. 2001;139(6):844–848. doi: 10.1067/mpd.2001.119446. [DOI] [PubMed] [Google Scholar]
- 9.Kelly A, Winer KK, Kalkwarf H, et al. Age-based reference ranges for annual height velocity in US children. J Clin Endocrinol Metab. 2014;99(6):2104–2112. doi: 10.1210/jc.2013-4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zemel BS, Kalkwarf HJ, Gilsanz V, et al. Revised reference curves for bone mineral content and areal bone mineral density according to age and sex for black and non-black children: results of the bone mineral density in childhood study. J Clin Endocrinol Metab. 2011;96(10):3160–3169. doi: 10.1210/jc.2011-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greulich WW, Pyle SI. Radiographic atlas of skeletal development of the hand and wrist. 2nd. Stanford, CA: Stanford University Press; 1959. [Google Scholar]
- 12.Bayley N, Pinneau SR. Tables for predicting adult height from skeletal age: revised for use with the Greulich-Pyle hand standards. J Pediatr. 1952;40(4):423–441. doi: 10.1016/s0022-3476(52)80205-7. [DOI] [PubMed] [Google Scholar]
- 13.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat 11. 2002;(246):1–190. [PubMed] [Google Scholar]
- 14.Mitchell JA, Chesi A, Elci O, et al. Genetics of bone mass in childhood and adolescence: effects of sex and maturation interactions. J Bone Miner Res. 2015 Sep;30(9):1676–1683. doi: 10.1002/jbmr.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chesi A, Mitchell JA, Kalkwarf HJ, et al. A trans-ethnic genome-wide association study identifies gender-specific loci influencing pediatric aBMD and BMC at the distal radius. Hum Mol Genet. 2015;24(17):5053–5059. doi: 10.1093/hmg/ddv210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alexander DH, Novembre J, Lange K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009;19(9):1655–1664. doi: 10.1101/gr.094052.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Biro FM, Greenspan LC, Galvez MP, et al. Onset of breast development in a longitudinal cohort. Pediatrics. 2013;132(6):1019–1027. doi: 10.1542/peds.2012-3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herman-Giddens ME, Steffes J, Harris D, et al. Secondary sexual characteristics in boys: data from the Pediatric Research in Office Settings Network. Pediatrics. 2012;130(5):e1058–e1068. doi: 10.1542/peds.2011-3291. [DOI] [PubMed] [Google Scholar]
- 19.Roche AF, Roberts J, Hamill PV. Skeletal maturity of children 6–11 years: racial, geographic area, and socioeconomic differentials, United States. Vital Health Stat 11. 1975;(149):1–81. [PubMed] [Google Scholar]
- 20.Roche AF, Roberts J, Hamill PV. Skeletal maturity of youths 12–17 years: racial, geographic area, and socioeconomic differentials. United States, 1966–1970. Vital Health Stat 11. 1978;(167):1–98. [PubMed] [Google Scholar]
- 21.Pyle SI, Waterhouse AM, Greulich WW. Attributes of the radiographic standard of reference for the National Health Examination Survey. Am J Phys Anthropol. 1971;35(3):331–337. doi: 10.1002/ajpa.1330350306. [DOI] [PubMed] [Google Scholar]
- 22.Mora S, Boechat MI, Pietka E, Huang HK, Gilsanz V. Skeletal age determinations in children of European and African descent: applicability of the Greulich and Pyle standards. Pediatr Res. 2001;50(5):624–628. doi: 10.1203/00006450-200111000-00015. [DOI] [PubMed] [Google Scholar]
- 23.Cole TJ, Rousham EK, Hawley NL, Cameron N, Norris SA, Pettifor JM. Ethnic and sex differences in skeletal maturation among the Birth to Twenty cohort in South Africa. Arch Dis Child. 2015;100(2):138–143. doi: 10.1136/archdischild-2014-306399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Casazza K, Thomas O, Dulin-Keita A, Fernandez JR. Adiposity and genetic admixture, but not race/ethnicity, influence bone mineral content in peripubertal children. J Bone Miner Metab. 2010;28(4):424–432. doi: 10.1007/s00774-009-0143-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Medina-Gomez C, Chesi A, Heppe DH, et al. BMD loci contribute to ethnic and developmental differences in skeletal fragility across populations: assessment of evolutionary selection pressures. Mol Biol Evol. 2015 Nov;32(11):2961–2972. doi: 10.1093/molbev/msv170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wagner DR, Heyward VH. Measures of body composition in blacks and whites: a comparative review. Am J Clin Nutr. 2000;71(6):1392–1402. doi: 10.1093/ajcn/71.6.1392. [DOI] [PubMed] [Google Scholar]
- 27.Euling SY, Herman-Giddens ME, Lee PA, et al. Examination of US puberty-timing data from 1940 to 1994 for secular trends: panel findings. Pediatrics. 2008;121(Suppl 3):S172–S191. doi: 10.1542/peds.2007-1813D. [DOI] [PubMed] [Google Scholar]
- 28.Jones LL, Griffiths PL, Norris SA, Pettifor JM, Cameron N. Is puberty starting earlier in urban South Africa? Am J Hum Biol. 2009;21(3):395–397. doi: 10.1002/ajhb.20868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones LL, Griffiths PL, Norris SA, Pettifor JM, Cameron N. Age at menarche and the evidence for a positive secular trend in urban South Africa. Am J Hum Biol. 2009;21(1):130–132. doi: 10.1002/ajhb.20836. [DOI] [PubMed] [Google Scholar]
- 30.McCance RA, Widdowson EM. The determinants of growth and form. Proc R Soc Lond B Biol Sci. 1974;185(1078):1–17. doi: 10.1098/rspb.1974.0001. [DOI] [PubMed] [Google Scholar]
- 31.Cutler GB., Jr The role of estrogen in bone growth and maturation during childhood and adolescence. J Steroid Biochem Mol Biol. 1997;61(3–6):141–144. [PubMed] [Google Scholar]
- 32.Richards RJ, Svec F, Bao W, Srinivasan SR, Berenson GS. Steroid hormones during puberty: racial (black-white) differences in androstenedione and estradiol—the Bogalusa Heart Study. J Clin Endocrinol Metab. 1992;75(2):624–631. doi: 10.1210/jcem.75.2.1639961. [DOI] [PubMed] [Google Scholar]
- 33.Vandewalle S, Taes Y, Fiers T, et al. Relation of adrenal-derived steroids with bone maturation, mineral density and geometry in healthy prepubertal and early pubertal boys. Bone. 2014;69:39–46. doi: 10.1016/j.bone.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 34.Sopher AB, Jean AM, Zwany SK, et al. Bone age advancement in prepubertal children with obesity and premature adrenarche: possible potentiating factors. Obesity (Silver Spring) 2011;19(6):1259–1264. doi: 10.1038/oby.2010.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Girgis R, Abrams SA, Castracane VD, Gunn SK, Ellis KJ, Copeland KC. Ethnic differences in androgens, IGF-I and body fat in healthy prepubertal girls. J Pediatr Endocrinol Metab. 2000;13(5):497–503. doi: 10.1515/jpem.2000.13.5.497. [DOI] [PubMed] [Google Scholar]
- 36.Klein KO, Newfield RS, Hassink SG. Bone maturation along the spectrum from normal weight to obesity: a complex interplay of sex, growth factors and weight gain. J Pediatr Endocrinol Metab. 2016 Mar;29(3):311–318. doi: 10.1515/jpem-2015-0234. [DOI] [PubMed] [Google Scholar]
- 37.Duren DL, Nahhas RW, Sherwood RJ. Do secular trends in skeletal maturity occur equally in both sexes? Clin Orthop Relat Res. 2015;473(8):2559–2567. doi: 10.1007/s11999-015-4213-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cox LA. The biology of bone maturation and ageing. Acta Paediatr Suppl. 1997;423:107–108. doi: 10.1111/j.1651-2227.1997.tb18386.x. [DOI] [PubMed] [Google Scholar]
- 39.Topor LS, Feldman HA, Bauchner H, Cohen LE. Variation in methods of predicting adult height for children with idiopathic short stature. Pediatrics. 2010;126(5):938–944. doi: 10.1542/peds.2009-3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hochberg Z. Diagnosis Of Endocrine Disease: on the need for national-, racial- or ethnic-specific standards for the assessment of bone maturation. Eur J Endocrinol. 2016 Feb;174(2):R65–R70. doi: 10.1530/EJE-15-0673. [DOI] [PubMed] [Google Scholar]
- 41.Jones G, Ma D. Skeletal age deviation assessed by the Tanner-Whitehouse 2 method is associated with bone mass and fracture risk in children. Bone. 2005;36(2):352–357. doi: 10.1016/j.bone.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 42.Wren TA, Shepherd JA, Kalkwarf HJ, et al. Racial disparity in fracture risk between white and nonwhite children in the United States. J Pediatr. 2012;161(6):1035–1040. doi: 10.1016/j.jpeds.2012.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gordon CM, Leonard MB, Zemel BS International Society for Clinical Densitometry. 2013 Pediatric Position Development Conference: executive summary and reflections. J Clin Densitom. 2014;17(2):219–224. doi: 10.1016/j.jocd.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 44.Karagas MR, Lu-Yao GL, Barrett JA, Beach ML, Baron JA. Heterogeneity of hip fracture: age, race, sex, and geographic patterns of femoral neck and trochanteric fractures among the US elderly. Am J Epidemiol. 1996;143(7):677–682. [Google Scholar]