Abstract
Introduction
Friedreich ataxia (FRDA) leads to increased risk of diabetes. Less is known regarding the dynamics of glucose homeostasis in FRDA, the influence of disease features, and the utility of oral-based metrics for capturing metabolic dysfunction.
Methods
To examine these dynamics, we analyzed oral and intravenous glucose tolerance test data in 42 non-diabetic patients with FRDA.
Results
Patients showed high insulin responsiveness to glucose and low insulin sensitivity. Genetic severity predicted overall metabolic impairment: individuals with longer guanine–adenine–adenine (GAA) repeats on the shorter allele showed a lower disposition index. Genetic severity did not predict any other variables. Measures of disposition index from intravenous and oral glucose tolerance testing did not correlate well, possibly reflecting divergent responses to oral and intravenous glucose loads.
Conclusions
FRDA patients demonstrate abnormal compensatory activity for managing glucose. Genetic severity impacts the global homeostatic profile, whereas relative contributions of insulin secretion and action vary from patient to patient.
Keywords: Friedreich ataxia, diabetes mellitus, glucose homeostasis, glucose metabolism, neurodegenerative disease
Friedreich ataxia (FRDA) is a progressive neurodegenerative disease that poses a heightened risk of glucose metabolic dysfunction and diabetes mellitus. Approximately 1 per 50,000 persons in the USA have FRDA; most patients (about 97%) bear a recessive GAA triplet repeat expansion on intron 1 of both alleles, while a minority carry an expansion on 1 allele and a point mutation or deletion on the other.1,2 The disease-causing genotype leads to decreased production of frataxin, a mitochondrial protein involved in assembly and repair of iron–sulfur clusters and iron homeostasis.1,3,4 Frataxin deficiency leads to mitochondrial iron accumulation, deficient production of adenosine triphosphate, and a potential rise in free radical generation.1,2 These events lead to onset of a variety of symptoms, such as gait disturbance, loss of sensation, areflexia, and dyscoordination, beginning usually between ages 5 and 15 years, although later onset is not uncommon. The disease also causes specific comorbidities, including cardiomyopathy and scoliosis.1,2
Many cells impacted by this deficiency are located in the pancreas and, as a result, about 10%–30% of patients with FRDA develop diabetes, a substantially higher rate than the overall 4%–8% rate in the USA.3,5 Patients demonstrate impairment in both major components of glucose homeostasis: pancreatic beta-cell secretion of insulin, and cellular sensitivity and resistance to insulin action.5,6 In this analysis, we sought to determine whether the relative contributions of these 2 processes to the overall homeostatic profile differ between patients with FRDA and individuals without FRDA. We also gauged whether genetic severity plays a predictive role in the mechanics of homeostasis, as both earlier age of onset and greater severity of FRDA correlate with the expansion length of the GAA repeat on the shorter allele. Finally, we assessed how well different measurements of glucose metabolism correlate with one another, and whether or not oral glucose tolerance testing (OGTT) and intravenous glucose tolerance testing (IVGTT) represent comparable approaches to measuring glucose metabolism in patients with FRDA.
METHODS
Subjects and Study Design
The study was approved by the institutional review board at the Children’s Hospital of Philadelphia, and informed consent was obtained before participation. We used baseline OGTT measurements on 42 individuals with FRDA who were participating in a clinical trial7,8; this study is an ancillary analysis of the trial data (NCT01035671). All enrolled subjects had genetically confirmed FRDA and carried an expanded GAA repeat on both alleles (i.e., no subjects with point mutations were included). The trial excluded patients with diabetes to prevent a confounding influence of insulin therapy and anti-diabetes agents on outcomes. Individuals were defined as not having diabetes by presenting with fasting glucose <126 mg/dl. Due to its invasiveness, IVGTT was only performed in 31 of the 42 subjects; individuals were selected for IVGTT if they met screening criteria for insulin sensitivity [Oral Glucose Insulin Sensitivity (OGIS) index ≤450 ml/min/m2].7
In addition to the 42 subjects with FRDA involved in this study, 10 healthy control subjects (non-diabetes, non-FRDA) were identified from a parallel study of IV glucose tolerance.9,10 We utilized their data to conduct comparisons with the IVGTT data generated from the FRDA cohort.
Laboratory Testing
Subjects underwent a standard 75-g 120-minute OGTT after an overnight fast. Glucose and insulin levels were collected at t = 0, 30, 60, 90, and 120 minutes. In subjects undergoing a 180-minute IVGTT, samples for fasting insulin and glucose were obtained at t = −15, −10, and −5 minutes. At t = 0, subjects received an IV infusion of 50% glucose dosed at 0.3 g/kg over 1 minute. At t = 20, 0.025 U/kg of insulin (1 unit per 1 ml of solution) was injected over 30 seconds. Blood samples for insulin and glucose testing were collected at t = 2, 3, 4, 5, 6, 8, 10, 12, 14, 16, 18, 20, 22, 25, 30, 40, 50, 70, 100, 140, and 180 minutes after the glucose injection. Insulin resistance was defined as a fasting blood glucose between 5.5 and 7 mmol/L (100 and 126 mg/dl) or a 2-hour post-OGTT plasma glucose level between 7.8 and 11.1 mmol/L (140 and 200 mg/dl).
Measurements of Disease Severity
Indices of FRDA severity included age at symptom onset, disease duration, body mass index (BMI), number of GAA repeats on the shorter and longer alleles, and frataxin protein levels from whole blood and buccal cells.1,11
Measurements of Glucose Metabolism
We adopted a subset of measures to provide multiple outlooks on the 2 dynamics of glucose handling (Table 1). The following 9 oral-based parameters are well-established measurements of insulin secretion and insulin sensitivity/resistance:
Homeostatic model assessments of insulin resistance (HOMA-IR) and beta-cell function (HOMA-β): describes glucose regulation in terms of a feedback loop using steady-state basal plasma glucose and insulin concentrations, from which degree of insulin resistance and beta-cell deficiency can be predicted.12
Insulinogenic index (IGI): measures insulin secretion based on 30-minute incremental changes in insulin and glucose after oral glucose loading.13
Ratio of area under the insulin curve to area under the glucose curve (AUC-i/g): measures insulin response to glucose during oral glucose testing.14
Stumvoll first and second phases (Stumvoll PH1 and Stumvoll PH2): represents insulin secretions from 2 different intervals of oral glucose testing—the first 10 minutes after glucose loading (PH1), and the final hour of OGTT when insulin levels theoretically plateau (PH2).14
Metabolic clearance rate (MCR): measures insulin sensitivity using glucose infusion rate, average glucose concentration, insulin concentration, and body mass index (BMI).14
Matsuda index (MI): measures whole-body (hepatic and peripheral tissue) insulin sensitivity and is derived from fasting (basal) insulin and glucose levels and mean levels after oral glucose loading.15
Gutt insulin sensitivity index: measures insulin sensitivity from OGTT using fasting glucose, 2-hour glucose, and body weight.16
Table 1.
Categorization of metabolic variables.
| Test | Interpretation | Measure | Name |
|---|---|---|---|
| IVGTT | Sensitivity | IVGTT-IR | IVGTT insulin resistance |
| SI | Insulin sensitivity | ||
| Secretion | IVGTT-β | IVGTT-beta-cell functioning | |
| AIRg | Acute insulin response to glucose | ||
| Composite | DI | Disposition index | |
| OGTT | Sensitivity | HOMA-IR | Homeostatic model of insulin resistance |
| MI | Matsuda index | ||
| MCR | Metabolic clearance rate | ||
| Gutt | Gutt insulin sensitivity index | ||
| Secretion | HOMA-β | Homeostatic model of beta-cell function | |
| Stumvoll PH1 | Stumvoll phase 1 | ||
| Stumvoll PH2 | Stumvoll phase 2 | ||
| IGI | Insulinogenic index | ||
| AUC-i/g | Ratio of area under insulin curve to area under glucose curve | ||
| Composite | IGI × MI | Product of IGI and MI | |
| AUC × MI | Product of AUC-i/g and MI | ||
| IGI/FI | Quotient of IGI and fasting insulin |
Three OGTT-based versions of disposition index were calculated in a manner consistent with the literature17,18:
The product of IGI and MI (IGI × MI).
The product of AUC-insulin/glucose and MI (AUC × MI).
The quotient of IGI and fasting insulin (IGI/FI).
Parameters calculated from IVGTT were as follows:
Beta-cell functioning (IVGTT-β).
Insulin resistance (IVGTT-IR).
Insulin sensitivity (SI).
Acute insulin response to glucose (AIRg).
Disposition index (DI).
All of these parameters were calculated using MinMod Millennium software (copyright MINI-MOD, Inc., Pasadena, California).
Statistical Analysis
Data analysis was conducted using STATA version 11.2 (StataCorp LP, College Station, Texas). A 2-sample mean comparison test was used to compare age, BMI, AIRg, SI, and DI between the cohort and the control group. Spearman rank correlations were used to explore possible associations among measures of insulin secretion (HOMA-β, IVGTT-β, Stumvoll PH1, Stumvoll PH2, IGI, AIRg), measures of insulin resistance (HOMA-IR, IVGTT-IR, MI, MCR, SI), and measures of disposition index (AIRg × SI, IGI × MI, IGI/FI, AUC × MI). Rank correlation analysis of DI utilized all 3 OGTT-based versions of the index, whereas only IGI × MI was adopted for linear regression of relationships between metabolic and disease variables.17,19
Regression modeling evaluated relationships among these and other metabolic variables (Gutt index, AUC-i/g), specifically testing whether measures derived from IVGTT (AIRg, SI, and DI) predicted any of the oral-based measures. Regressions were also used to test whether age, BMI, GAA repeat lengths (on each allele), age of onset, disease duration, and frataxin levels (in blood and in cheek cells) predicted any of the metabolic measures. Variables were tested using single linear regression and multivariable regression (shorter GAA with age, age of onset, disease duration, and BMI; longer GAA with age; and BMI with age). Given the small sample size, only 2 independent variables were included in models of multivariable linear regression. For all calculations involving GAA repeat length, the length of the shorter allele was used unless otherwise explicitly indicated.
The threshold for statistical significance was set to 0.01 to account for multiple comparisons. All analyses were treated as largely exploratory, where meaning would be determined by the patterns of results from multiple approaches rather than single analyses.
RESULTS
Demographic Data
The 42-subject cohort contained adults without diabetes (47% men, 18–58 years of age, mean age 32.7 ± 11.7 years). Mean age of FRDA symptom onset was 16.7 ± 9.6 years. Mean GAA length on the shorter allele was 536 ± 236 repeats (range 41–901). All subjects were homozygous for the expanded repeat. Average BMI was 23.9 ± 5.3 kg/m2; 5 subjects (12%) were obese (BMI ≥ 30 kg/m2), and 15 subjects (36%) had pre-diabetes, as defined by impaired fasting glucose (100–125 mg/dl).
Abnormal Glucose Homeostasis in Non-Diabetes FRDA
We examined IVGTT parameters by comparing those subjects who underwent IVGTT against a group of healthy non-diabetic individuals without FRDA (Table 2). The cohort and control groups were similar in age and BMI, and both groups were approximately evenly divided between men and women. FRDA patients showed decreased levels of SI and increased levels of AIRg compared with controls, suggesting that patients with FRDA but without diabetes simultaneously release more insulin and resist the action of more insulin than do healthy non-FRDA individuals without diabetes.
Table 2.
Comparison of demographic and IVGTT data between FRDA cohort and control group.
| Measure | Patients | Controls | T score | P-value |
|---|---|---|---|---|
| N | 31 | 10 | — | — |
| Gender, women (%) | 16 (52%) | 5 (50%) | — | — |
| Mean age, years (± SD) | 32.8 (12.5) | 26.1 (5.7) | 1.640 | 0.109 |
| Mean BMI, kg/m2 (± SD) | 24.0 (5.6) | 25.3 (8.0) | −0.543 | 0.591 |
| DI (± SD) | 1.56E3 (207) | 1.34E3 (335) | 0.539 | 0.593 |
| SI (± SD) | 2.51 (0.317) | 5.03 (0.762) | −3.58 | <0.001 |
| AIRg (± SD) | 833 (123) | 244 (33.6) | 2.69 | 0.011 |
DI, disposition index; SI, insulin sensitivity (×10−5 min/pmol/L); AIRg, acute insulin response to glucose (pmol/L).
Relationships between IVGTT and OGTT Metrics of Glucose Metabolism in FRDA
Robust regression of IVGTT-derived measures of insulin sensitivity and insulin responsiveness with oral-based measures revealed significant relationships between measures (Table 3). With respect to insulin sensitivity, IVGTT-derived SI predicted the OGTT-derived ratio AUC-i/g, with greater values of AUC-i/g (indicative of greater insulin response) corresponding to lower values of SI (indicative of greater insulin resistance). This relationship agrees with the concept of insulin sensitivity as a compensatory adjustment based on the amount of insulin secreted in response to glucose. With respect to insulin secretion, IVGTT-derived AIRg predicted IGI, HOMA-β, HOMA-IR, and AUC-i/g. For each comparison, an increase in AIRg predicted increases in the OGTT measurement. These findings demonstrate the potential use of oral-based metrics as substitutes for IVGTT-derived measures of insulin secretion and insulin action in patients with FRDA. For both aspects of glucose handling, OGTT and IVGTT capture dysfunction similarly in patients, the former being less invasive to perform clinically.
Table 3.
Linear regression analysis of OGTT measures with SI and AIRg.
| OGTT parameter |
Independent variable |
Coefficient ± SE | P-value |
|---|---|---|---|
| Gutt index | SI | 0.064 ± 0.046 | 0.168 |
| MI | SI | 0.263 ± 0.125 | 0.044 |
| HOMA-IR | SI | −0.025 ± 0.013 | 0.068 |
| AUC-i/g | SI | −0.078 ± 0.025 | 0.004 |
| IGI | AIRg | 101 ± 34 | 0.006 |
| Stumvoll PH1 | AIRg | 0.135 ± 0.051 | 0.013 |
| Stumvoll PH2 | AIRg | 0.593 ± 0.225 | 0.013 |
| HOMA-β | AIRg | 1.74 ± 0.548 | 0.004 |
| HOMA-IR | AIRg | 16.3 ± 3.81 | <0.001 |
| AUC-i/g | AIRg | 2.04E-4 ± 5.53E-4 | 0.001 |
MI, Matsuda index; HOMA-IR, homeostatic assessment of insulin resistance; AUC-i/g, area under the insulin curve/area under the glucose curve; IGI, insulinogenic index; Stumvoll PH1, Stumvoll phases 1; Stumvoll PH2, Stumvoll phase 2; HOMA-β, homeostatic assessment of beta-cell function; SI, insulin sensitivity; AIRg, acute insulin response to glucose.
Spearman rank correlations demonstrated moderate to strong associations between various measures of insulin secretion response (Table 4) and insulin sensitivity/resistance (Table 5). For the measurements of insulin action (and, conversely, insulin resistance), there was an especially strong negative relationship between HOMA-IR and MI. This was not unexpected, because the formulae for the 2 values are closely linked algebraically. For measures of insulin secretion and beta-cell function, both Stumvoll PH1 and PH2 were strongly associated with AIRg and IGI. There was also a strong positive relationship between AIRg and IGI, which supports the use of IGI as the OGTT counterpart to AIRg.
Table 4.
Spearman rank correlations of measures of insulin secretion responses.
| Parameter | Correlate | N | Spearman rho |
Test of independence |
|---|---|---|---|---|
| HOMA-β | IVGTT-β | 27 | 0.41 | 0.03 |
| Stumvoll PH1 | 42 | 0.57 | <0.01 | |
| Stumvoll PH2 | 42 | 0.59 | <0.01 | |
| IGI | 42 | 0.22 | 0.17 | |
| AIRg | 31 | 0.42 | 0.02 | |
| IVGTT-β | Stumvoll PH1 | 27 | 0.47 | 0.01 |
| Stumvoll PH2 | 27 | 0.46 | 0.02 | |
| IGI | 27 | 0.22 | 0.26 | |
| AIRg | 27 | 0.49 | 0.01 | |
| Stumvoll PH1 | Stumvoll PH2 | 42 | >0.99 | <0.01 |
| IGI | 42 | 0.79 | <0.01 | |
| AIRg | 31 | 0.82 | <0.01 | |
| Stumvoll PH2 | IGI | 42 | 0.76 | <0.01 |
| AIRg | 31 | 0.82 | <0.01 | |
| AIRg | IGI | 31 | 0.86 | <0.01 |
IVGTT-β, intravenous glucose tolerance test of beta-cell function; IGI, insulinogenic index; HOMA-β, homeostatic assessment of beta-cell function; AIRg, acute insulin response to glucose; Stumvoll PH1, Stumvoll phase 1; Stumvoll PH2, Stumvoll phase 2.
Table 5.
Spearman rank correlations of measures of insulin sensitivity/resistance.
| Parameter | Correlate | N | Spearman rho |
Test of independence |
|---|---|---|---|---|
| HOMA-IR | IVGTT-IR | 27 | 0.54 | <0.01 |
| MI | 42 | −0.90 | <0.01 | |
| MCR | 42 | −0.28 | 0.08 | |
| SI | 31 | −0.48 | 0.01 | |
| IVGTT-IR | MI | 27 | −0.45 | 0.02 |
| MCR | 27 | −0.19 | 0.34 | |
| SI | 27 | −0.46 | 0.02 | |
| MI | MCR | 42 | 0.47 | <0.01 |
| SI | 31 | 0.50 | <0.01 | |
| SI | MCR | 31 | 0.16 | 0.39 |
IVGTT-IR, intravenous glucose tolerance test of insulin resistance; MI, Matsuda index; MCR, metabolic clearance rate; HOMA-IR, homeostatic assessment of insulin resistance; SI, insulin sensitivity.
In correlating different measures of disposition index, all 3 OGTT-based indices were strongly correlated with one another (Table 6). None of the pairwise correlations with IVGTT-based DI were significant, despite previous reports of modest relationships in healthy children.19 This suggests that, although IVGTT and OGTT both capture metabolic dysfunction in FRDA, the degree of global dysfunction differs sufficiently between the 2 approaches, such that IVGTT-DI and OGTT-DI are not comparable at an individual patient level.
Table 6.
Spearman rank correlations of measures of disposition index.
| Parameter | Correlate | N | Spearman rho |
Test of independence |
|---|---|---|---|---|
| IVGTT DI | IGI × MI | 31 | 0.26 | 0.16 |
| IGI/FI | 31 | 0.18 | 0.34 | |
| AUC × MI | 31 | 0.22 | 0.24 | |
| IGI × MI | IGI/FI | 42 | 0.92 | <0.01 |
| AUC × MI | 42 | 0.79 | <0.01 | |
| IGI/FI | AUC×MI | 42 | 0.80 | <0.01 |
MI, Matsuda index; IGI, insulinogenic index; FI, fasting insulin; IGI, insulinogenic index; AUC, area under the insulin curve/area under the glucose curve; DI (disposition index) = SI × AIRg; AIRg, acute insulin response to glucose; SI, insulin sensitivity.
Influence of Disease Features on Glucose Handling in FRDA
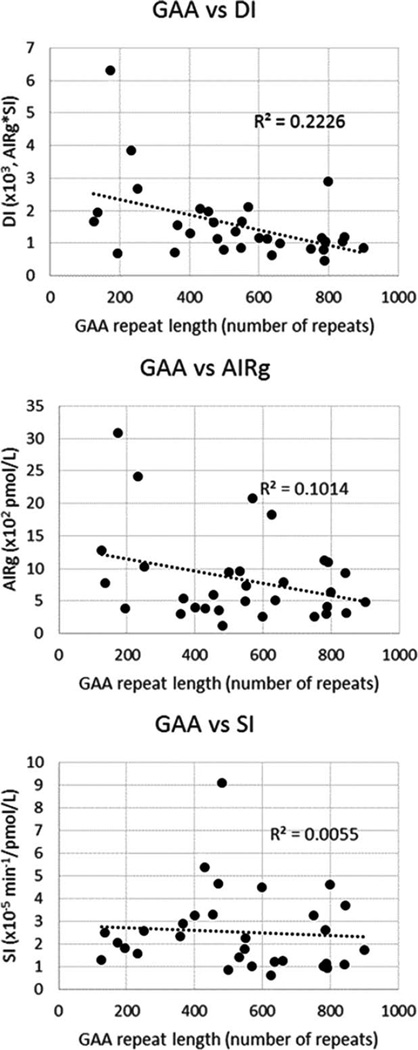
Using single linear regression, we examined the influence of features in FRDA on glucose metabolism (Table 7). Given the lack of correlation between IVGTT-DI and OGTT-DI, the effects of GAA length on each of these variables were of particular interest. As there is a selection bias between age and GAA repeat length (i.e., longer length leads to earlier onset), we also examined multivariable linear regression with age and GAA length as independent variables. The length of the shorter GAA repeat predicted DI while showing a marginal trend with AIRg and OGTT-DI (IGI × MI), but not with SI. Compared to correlations with AIRg and SI, the correlation of GAA with DI was more robust, with an increasing number of GAA repeats on the shorter allele (greater genetic severity) corresponding to lower DI scores (greater metabolic impairment) (Fig. 1). Length of the GAA repeat did not predict any results from OGTT (data not shown). Accounting for age, GAA repeat length showed no significant trends and only a marginal trend with DI (P = 0.027, R2 = 0.22; data not shown). Age also did not predict any metabolic measure. In multivariable models, neither age nor GAA showed significant relationships with any metabolic measures of insulin secretion and insulin sensitivity.
Table 7.
Linear regression analysis of metabolic measures with disease features.
| Lab | N | R2 | Variable | Coefficient ± SE | P-value |
|---|---|---|---|---|---|
| DI | 31 | 0.22 | GAA | −2.36 ± 0.817 | 0.007 |
| DI | 31 | 0.07 | AOO | 28.1 ± 18.7 | 0.143 |
| OGTT-DI | 42 | 0.09 | GAA | −0.004 ± 0.002 | 0.060 |
| OGTT-DI | 42 | 0.14 | AOO | 0.127 ± 0.049 | 0.013 |
| AIRg | 31 | 0.13 | GAA | −1.06 ± 0.516 | 0.050 |
| SI | 31 | <0.01 | GAA | −3.89E-4 ± 0.001 | 0.786 |
OGTT-DI (oral glucose tolerance test disposition index) = IGI × MI. DI, disposition index; SI, insulin sensitivity; AIRg, acute insulin response to glucose.
FIGURE 1.
Correlations of IVGTT measures with GAA repeat length. The correlation between GAA and DI (R2 = 0.22) is more robust compared with the correlations of GAA with AIRg (R2 = 0.10) and SI (R2 = 0.01), supporting the concept that genetic severity influences the overall disposition index, whereas the individual components of disposition (AIRg, insulin response; SI, insulin sensitivity) change in a manner that is relatively independent of repeat length. DI, disposition index; IVGTT, intravenous glucose tolerance test; SI, insulin sensitivity (×10−5 min/pmol/L); AIRg, acute insulin response to glucose, a measure of beta-cell function (pmol/L).
Other variables replacing shorter GAA repeat length did not predict any metabolic variables (data not shown). Such variables included GAA repeat length on the longer allele, BMI, and disease duration. This result did not change with inclusion of a second independent variable (i.e., age or shorter GAA repeat length). By itself and in combination with age, GAA repeat length on the longer allele did not predict any metabolic variable, and regression coefficients were similar to or less than those in models using the shorter allele. Age predicted none of the metabolic measurements, possibly reflecting the limited age range of the cohort.
Age of FRDA onset marginally predicted OGTT-DI but not IVGTT-DI, with a more robust statistical trend observed in the former. Age of onset also marginally predicted AIRg but not SI. Models accounting for age revealed the opposite pattern: age of onset marginally predicted SI but not AIRg. Age of onset did not predict any other measures, including when shorter GAA repeat length was added to the model (data not shown). Frataxin protein levels in buccal cells and blood were also tested and did not predict any measures of glucose handling (data not shown); these protein measures may not adequately correlate with frataxin levels in pancreatic beta-cells. Although frataxin protein levels correlate with GAA repeat length,1,20 the trends in most of these models were not substantially more or less robust compared with the regressions based on repeat length.
DISCUSSION
In this study, GAA repeat length, the major measure of genetic severity in FRDA, predicted DI, an established IVGTT composite measure of insulin responsiveness to glucose and insulin sensitivity. Although partially predicated on age, the relationship indicates that, with increased genetic severity, patients with FRDA have a greater risk of abnormal glucose homeostasis in the form of both insulin resistance and glucose intolerance. The absence of an age-independent relationship between GAA and AIRg (insulin response/release) or SI (insulin sensitivity/ action) suggests that FRDA genetic severity and pathology operate on the global presentation of systematic dysfunction in glucose handling, and not on either underlying component alone.21,22 Individuals with FRDA are predisposed to develop glucose-related metabolic problems compared with the general population and will rely on differing levels of insulin release and sensitivity. Glucose loading requires patients with FRDA to reach a balance of contributions between these dual processes; genetic severity does not dictate each person’s specific and ultimate metabolic balance, as revealed across a multitude of linear regressions that modeled metabolic variables with length of GAA repeat. Instead, genetic severity helps to dictate the overall composite achieved by this balance: the disposition index. This result contrasts with previously reported findings,23,24 although the difference may reflect cohort selection or variance in age, particularly as our cohort only consisted of adults characterized for inclusion in a clinical trial.
We found that, compared with controls, patients with FRDA released more insulin than normal and were more resistant to the effects of insulin than normal. Greater insulin responsiveness may represent a compensatory reaction to lower insulin sensitivity (and greater resistance) in patients with FRDA. The opposite directions of these differences explain the lack of significant difference in DI. The product of these opposing differences is a “normal” DI among patients compared with controls, consistent with a compensated state in FRDA patients. Combining this result with the results from linear regression, patients display a normal disposition index, but the index will nonetheless vary in part by the level of genetic severity, a variable that also predicts age of onset and FRDA disease severity.
This study has demonstrated the difficult challenge of choosing simple oral-based measures comparable to the IV-based parameters of AIRg, SI, and DI. The IVGTT provides greater precision in measurement and is an important research tool for studying glucose metabolic functioning, but the procedure is unrealistic for studies of large cohorts or for widespread application to regular clinical evaluations. Oral-based assessments of glucose handling are better suited for collecting data on a large population and for normal clinical assessments, but their reproducibility remains to be definitively shown, especially with regard to capturing insulin secretion, insulin action, and disposition index. Nevertheless, this analysis has provided clear evidence supporting the use of oral-based metrics for clinical management of metabolic functions in FRDA. Many of the parameters of insulin secretion and insulin action correlated well with each other. In particular, both AIRg and SI predicted the oral-based ratio AUC-i/g, which raises the question of whether AUC-i/g offers an optimal and informative substitute for IV-based metrics of insulin response and sensitivity, given the invasiveness of IVGTT. Further analysis in a larger cohort is needed to better describe the clinical value of AUC-i/g for studying and treating patients with FRDA.
Our findings also show a noteworthy difference between IVGTT-DI and OGTT-DI. Unlike the strong correlations between IV- and oral-based measures of insulin secretion and insulin action, none of the 3 OGTT formulations of DI correlated with IVGTT-based DI. This suggests that, although IV and oral approaches correspond well when measuring specific events in homeostasis (namely, insulin release and insulin action on cells), the compensatory system comprised of the 2 is an exception; oral and intravenous measures of the overall metabolic system do not always correlate. In addition, genetic severity appears to be more closely tied to the IV approach, predicting IVGTT-DI in single linear regression and marginally when accounting for age (whereas GAA marginally predicts OGTT-DI only when age is not taken into account). One possible explanation for the divergence is an effect of incretins on DI measurements, as incretins are released from the digestive tract and therefore can only be measured when testing glucose tolerance after oral intake (IV glucose treatment bypasses the gastrointestinal system).25 Future studies can use these findings to better characterize the relationship between repeat length and incretin levels, as well as to determine the extent to which incretins influence glucose metabolism and FRDA disease progression.
This study was limited to patients screened for a clinical trial, thus creating selection bias, especially with regard to age. In this cohort, age did not predict the metabolic variables tested in linear regression, possibly reflecting the restriction of the cohort to adult subjects. Previous results suggest that age is a major factor in cohorts with a broader age range, and the influence of age on glucose handling is especially pertinent in childhood.11 Younger patients with FRDA generally display greater disease severity, and their glucose intolerance may reflect beta-cell failure to a higher degree, because insulin resistance is more commonly associated with adulthood. The absence of pediatric patients from our cohort is therefore meaningful, as the mean GAA repeat length of the cohort was shorter than in most all-inclusive natural history studies of FRDA.11 In addition, our study did not include patients with diabetes or with point mutations. Furthermore, only those individuals with lower insulin sensitivity levels were selected to undergo IVGTT. Thus, a selection bias persisted in the group. Finally, because of the invasiveness of IVGTT, the size of the control group was relatively small.
Despite these limitations, this study elucidates the influence of disease features and genotype on the dynamics of glucose homeostasis in FRDA. Future studies can build on these results by evaluating potential mechanistic pathways behind these associations, with assessments of how other factors, such as hemoglobin A1c, fasting glucose and insulin levels, and genetic modifiers of metabolic functions, impact glucose homeostasis in patients with FRDA.
Acknowledgments
This work was supported by grants from the Muscular Dystrophy Association, the Friedreich’s Ataxia Research Alliance, and Penwest Pharmaceuticals.
This study would not have been possible without the support and participation of the patients with Friedreich ataxia in the study cohort, as well as the clinical and research teams responsible for collection of information.
Abbreviations
- AIRg
acute insulin response to glucose
- AUC-i/g (or AUC)
area under the insulin curve/area under the glucose curve
- BMI
body mass index
- DI
disposition index (derived from IVGTT unless indicated as “OGTT-DI”)
- FI
fasting insulin
- FRDA
Friedreich ataxia
- GAA
guanine–adenine–adenine triplet repeat
- HOMA-IR
homeostatic assessment of insulin resistance
- HOMA-β
homeostatic assessment of beta-cell function
- IGI
insulinogenic index
- IV
intravenous
- IVGTT
intravenous glucose tolerance test
- IVGTT-IR
IVGTT test of insulin resistance
- IVGTT-β
IVGTT test of beta-cell function
- MCR
metabolic clearance rate
- MI
Matsuda index
- OGIS index
Oral Glucose Insulin Sensitivity index
- OGTT
oral glucose tolerance test
- SI
insulin sensitivity
- Stumvoll PH1/PH2
Stumvoll phase 1/phase 2
Footnotes
Disclosure: T.S. was an employee at Penwest at the time of the study. He is no longer employed within the field.
REFERENCES
- 1.Deutsch EC, Santani AB, Perlman SL, Farmer JM, Stolle CA, Marusich MF, et al. A rapid, noninvasive immunoassay for frataxin: utility in assessment of FA. Mol Genet Metab. 2010;101:238–245. doi: 10.1016/j.ymgme.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pandolfo M. Friedreich ataxia: the clinical picture. J Neurol. 2009;256:3–8. doi: 10.1007/s00415-009-1002-3. [DOI] [PubMed] [Google Scholar]
- 3.Ristow M. Neurodegenerative disorders associated with diabetes mellitus. J Mol Med (Berl) 2004;82:510–529. doi: 10.1007/s00109-004-0552-1. [DOI] [PubMed] [Google Scholar]
- 4.Nachbauer W, Wanschitz J, Steinkellner H, Eigentler A, Sturm B, Hufler K, et al. Correlation of frataxin content in blood and skeletal muscle endorses frataxin as a biomarker in Friedreich ataxia. Mov Disord. 2011;26:1935–1938. doi: 10.1002/mds.23789. [DOI] [PubMed] [Google Scholar]
- 5.Schoenle EJ, Boltshauser EJ, Baekkeskov S, Landin Olsson M, Torresani T, von Felten A. Preclinical and manifest diabetes mellitus in young patients with Friedreich’s ataxia: no evidence of immune process behind the islet cell destruction. Diabetologia. 1989;32:378–381. doi: 10.1007/BF00277262. [DOI] [PubMed] [Google Scholar]
- 6.Khan RJ, Andermann E, Fantus IG. Glucose intolerance in Friedreich’s ataxia: association with insulin resistance and decreased insulin binding. Metabolism. 1986;35:1017–1023. doi: 10.1016/0026-0495(86)90037-5. [DOI] [PubMed] [Google Scholar]
- 7.Lynch DR, Willi SM, Wilson RB, Cotticelli MG, Brigatti KW, Deutsch EC, et al. A0001 in Friedreich ataxia: biochemical characterization and effects in a clinical trial. Mov Disord. 2012;27:1026–1033. doi: 10.1002/mds.25058. [DOI] [PubMed] [Google Scholar]
- 8.Greeley NR, Regner S, Willi S, Lynch DR. Cross-sectional analysis of glucose metabolism in Friedreich ataxia. J Neurol Sci. 2014;342:29–35. doi: 10.1016/j.jns.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 9.Teff KL, Townsend RR. Prolonged mild hyperglycemia induces vagally mediated compensatory increase in c-peptide secretion in humans. J Clin Endocrinol Metab. 2004;89:5606–5613. doi: 10.1210/jc.2003-032094. [DOI] [PubMed] [Google Scholar]
- 10.Rickels MR, Naji A, Teff KL. Insulin sensitivity, glucose effectiveness, and free fatty acid dynamics after human islet transplantation for type 1 diabetes. J Clin Endocrinol Metab. 2006;91:2138–2144. doi: 10.1210/jc.2005-2519. [DOI] [PubMed] [Google Scholar]
- 11.Lynch DR, Farmer JM, Tsou AY, Perlman S, Subramony SH, Gomez CM, et al. Measuring Friedreich ataxia: complementary features of examination and performance measures. Neurology. 2006;66:1711–1716. doi: 10.1212/01.wnl.0000218155.46739.90. [DOI] [PubMed] [Google Scholar]
- 12.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 13.Phillips DI, Clark PM, Hales CN, Osmond C. Understanding oral glucose tolerance: comparison of glucose or insulin measurements during the oral glucose tolerance test with specific measurements of insulin resistance and insulin secretion. Diabet Med. 1994;11:286–292. doi: 10.1111/j.1464-5491.1994.tb00273.x. [DOI] [PubMed] [Google Scholar]
- 14.Stumvoll M, van Haeften T, Fritsche A, Gerich J. Oral glucose tolerance test indexes for insulin sensitivity and secretion based on various availabilities of sampling times. Diabetes Care. 2001;24:796–797. doi: 10.2337/diacare.24.4.796. [DOI] [PubMed] [Google Scholar]
- 15.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 16.Gutt M, Davis CL, Spitzer SB, Llabre MM, Kumar M, Czarnecki EM, et al. Validation of the insulin sensitivity index (ISI(0,120)): comparison with other measures. Diabetes Res Clin Pract. 2000;47:177–184. doi: 10.1016/s0168-8227(99)00116-3. [DOI] [PubMed] [Google Scholar]
- 17.Retnakaran R, Shen S, Hanley AJ, Vuksan V, Hamilton JK, Zinman B. Hyperbolic relationship between insulin secretion and sensitivity on oral glucose tolerance test. Obesity (Silver Spring) 2008;16:1901–1907. doi: 10.1038/oby.2008.307. [DOI] [PubMed] [Google Scholar]
- 18.Kahn SE, Prigeon RL, McCulloch DK, Boyko EJ, Bergman RN, Schwartz MW, et al. Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes. 1993;42:1663–1672. doi: 10.2337/diab.42.11.1663. [DOI] [PubMed] [Google Scholar]
- 19.Retnakaran R, Qi Y, Goran MI, Hamilton JK. Evaluation of proposed oral disposition index measures in relation to the actual disposition index. Diabet Med. 2009;26:1198–1203. doi: 10.1111/j.1464-5491.2009.02841.x. [DOI] [PubMed] [Google Scholar]
- 20.Dürr A, Cossee M, Agid Y, Campuzano V, Mignard C, Penet C, et al. Clinical and genetic abnormalities in patients with Friedreich’s ataxia. N Engl J Med. 1996;335:1169–1175. doi: 10.1056/NEJM199610173351601. [DOI] [PubMed] [Google Scholar]
- 21.Cnop M, Igoillo-Esteve M, Rai M, Begu A, Serroukh Y, Depondt C, et al. Central role and mechanisms of b-cell dysfunction and death in Friedreich ataxia–associated diabetes. Ann Neurol. 2012;72:971–982. doi: 10.1002/ana.23698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ristow M, Mulder H, Pomplun D, Schulz TJ, Müller-Schmehl K, Krause A, et al. Frataxin deficiency in pancreatic islets causes diabetes due to loss of b-cell mass. J Clin Invest. 2003;112:527–534. doi: 10.1172/JCI18107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Povel CM, Feskens EJ, Imholz S, Blaak EE, Boer JMA, Dollé ME. Glucose levels and genetic variants across transcriptional pathways: interaction effects with BMI. Int J Obes (Lond) 2010;34:840–845. doi: 10.1038/ijo.2009.302. [DOI] [PubMed] [Google Scholar]
- 24.Igoillo-Esteve M, Gurgul-Convey E, Hu A, Romagueira Bichara Dos Santos L, Abdulkarim B, Chintawar S, et al. Unveiling a common mechanism of apoptosis in b-cells and neurons in Friedreich’s ataxia. Hum Mol Genet. 2015;24:2274–2286. doi: 10.1093/hmg/ddu745. [DOI] [PubMed] [Google Scholar]
- 25.Limb C, Tamborlane WV, Sherwin RS, Pederson R, Caprio S. Acute incretin response to oral glucose is associated with stimulation of gastric inhibitory polypeptide, not glucagon-like peptide in young subjects. Pediatr Res. 1997;41:364–367. doi: 10.1203/00006450-199703000-00010. [DOI] [PubMed] [Google Scholar]