Abstract
Immune monitoring in the tumor microenvironment allows for important insights into immune mechanisms of response and resistance to various cancer treatments; however clinical challenges exist using current strategies. Significant questions remain regarding monitoring of archival versus fresh tissue, assessment of static versus dynamic markers, evaluation of limited tissue samples, and the translation of insights gained from immunologically “hot” tumors such as melanoma to other “cold” tumor microenvironments prevalent in other cancer types. Current and emerging immune monitoring strategies will be examined herein, and genomic-based assays complementing these techniques will also be discussed. Finally, host genomic and external environmental factors influencing anti-tumor immune responses will be considered, including the role of the gut microbiome. Though optimal immune monitoring techniques are in evolution, great promise exists in recent advances that will help guide patient selection as far as type, sequence, and combination of therapeutic regimens to enhance anti-tumor immunity and clinical responses.
Introduction
The field of cancer treatment has seen unprecedented advances over the past decade through the use of immunotherapy, targeted therapy, and combination regimens. In addition to this, there is growing awareness of the role of anti-tumor immunity in mediating responses to each of these strategies, and an increasing need to be able to understand immune responses to optimize therapeutic regimens and combination strategies. Although current immune monitoring strategies pose clinical challenges (Figure 1), advances in approaches and techniques are improving our ability to better understand immune responses in the tumor microenvironment. In addition to this, improvements in genomic profiling have allowed for a deeper understanding of the influence of mutational burden and other genomic factors on anti-tumor immunity. Continued progress in immune monitoring strategies will help us better understand who will benefit from therapy and will help guide rational choice of treatment – as well as proper timing, sequence, and combinations of therapeutic regimens.
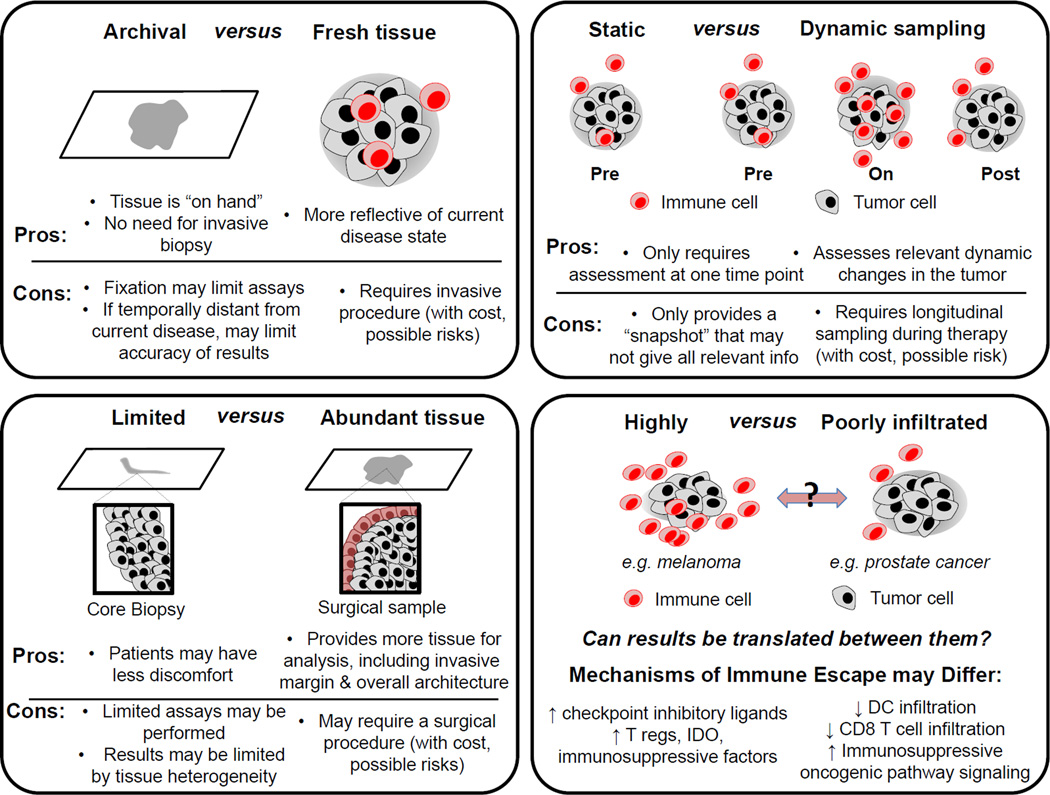
Figure 1. Challenges in performing translational research using patient cancer samples.
Current immune monitoring strategies are limited by method and source of tissue collection, each with its own strengths and weaknesses. As illustrated in this figure, there are advantages and disadvantages, for example, of working with archival versus fresh specimens (top left), limited versus abundant tissue (bottom left), and static versus dynamic samples (top right). In addition, immune heterogeneity between tumor types must be considered, in particular between highly immune cell infiltrated or ‘hot’ tumors (e.g. melanoma) and poorly infiltrated or ‘cold’ tumors (e.g. prostate cancer); therefore immune monitoring strategies should account for different immune mechanisms depending on tumor type being studied.
Clinical Challenges of Immune Monitoring
Archival versus fresh tissue
With the increasing use of immunomodulatory agents in clinical practice, there is a growing interest in assessing anti-tumor immune responses via tissue- and blood-based assays. However complexities exist in this analysis (Figure 1), particularly when considering use of archival versus fresh tissue. First, cryopreservation has been shown to alter certain immune cell subsets and cytokine profiles [1] as well as gene expression profiles [2] when assessing immune cell function in tumors and blood by flow cytometry, rendering this information less reliable compared to fresh tissue. Similarly, comparison of whole exome sequencing (WES) – important for determination of mutational burden and neoantigen prediction - in archival versus paired fresh tissue shows that genomic variants are lost by using formalin fixed paraffin embedded (FFPE) tissue [3]. In addition to inherent challenges introduced by preservation techniques, the dynamic properties of the immune system and that archival tissue is often collected in advance of treatment of interest may make data obtained from archival tissue less relevant. This is particularly pertinent with the use of immune checkpoint inhibitors in clinical trials and in standard of care treatment, where assessment of programmed death receptor-1 ligand (PD-L1) is often mandated and may be used to guide treatment decisions. However, this assessment is often done on archival tissue from a primary lesion or a metastatic focus temporally distinct from the current disease state. This may in part explain why clinical studies have produced varying results regarding utility of PD-L1 as a predictive biomarker for selection of patients [4–7], in which archival tissue was often used for PD-L1 determination. Rather, attempts should be made to obtain fresh tissue for analysis for immune and genomic analyses, also in light of clonal evolution of tumor cells and host anti-tumor responses observed during the course of therapy [8]. We as a group strive to perform analysis on fresh tissue and blood given these issues, though these factors should be taken into account in analyses of archival or cryopreserved samples.
Assessment of static versus dynamic markers
The immune system is a network of dynamic players that are interdependent and is therefore difficult to capture in a single snapshot. PD-L1, for example, is upregulated by T cell infiltration and IFN-γ secretion. Therefore, while assessment of PD-L1 may be negative at one time point, immune stimulatory agents that cause tumor infiltration of T cells with resultant IFN-γ such as ipilimumab may convert a ‘PD-L1 negative’ to a ‘PD-L1 positive’ tumor that may be more amenable to successful anti-PD-1/PD-L1 axis targeted therapy. Accordingly, there is a need for longitudinal tissue- and blood-based studies in order to better understand the complex interactions between host immunity, tumor molecular features, and response to therapeutic agents (Figure 2) [9]. In addition to dynamic PD-L1 assessment, clinical studies of immune checkpoint inhibitors, have highlighted the importance of assessment of early on-treatment immune signatures in predicting responses to therapy, as seen with CD8 T cells and ICOS positive CD4 T cells after ipilimumab treatment and CD3 and CD8 T cells after anti-PD1 treatment [10, 11]. As we move forward in this era of personalized medicine, it is critical to implement analysis of dynamic changes during the course of therapy that will help guide treatment choice, sequence, and potential combination regimens. This approach should certainly be adopted in clinical trials of novel agents and combination strategies, and should also be considered in monitoring responses on standard of care therapy.
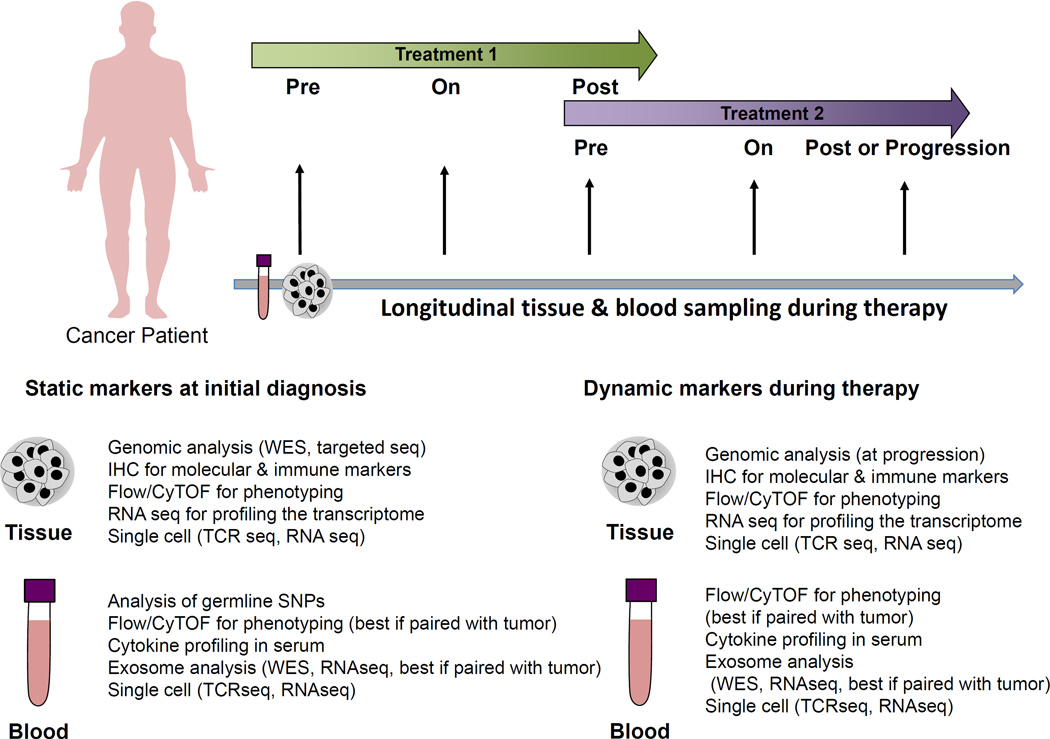
Figure 2. Proposed longitudinal studies to inform translational cancer research.
Paired tumor tissue and blood samples pre-treatment, early on-treatment, and post-treatment maximize our understanding of treatment response and mechanism of action. Additionally, assessment of samples at the time of progression can offer insight into resistance mechanisms and subsequent therapeutic options. Microbiome analysis is emerging as a potential immune monitoring strategy but its use in this regard is currently highly experimental.
Assessment of limited versus abundant tissue
Another important consideration in monitoring anti-tumor immune responses is the amount of tissue available for analysis. Analysis of limited tissue samples (such as from core biopsies) poses challenges with regard to prioritization of assays, and also limits assessment of important features – such as enumeration of tumor infiltrating lymphocytes (TIL) in the invasive margin versus center of the tumor (Figure 1). The significance of this is seen in colorectal cancer, where assessment of TIL subsets at center of the tumor as well as invasive margin from whole tissue sections increases accuracy of prediction of disease free and overall survival outcomes compared to single region analysis [12]. Another shortcoming of assessing limited tissue samples is the issue of tumor heterogeneity, as significant genomic and immune heterogeneity has been demonstrated between tumor sites and even within a single tumor site [13–15]. To address this, efforts should be made to obtain ample tissue for analysis at baseline and during the course of therapy. Pre-surgical trials, also known as neoadjuvant “window” trials, offer a unique opportunity to collect sufficient tissue for genomic and immune analysis in the context of therapy, and may allow for concurrent informed analysis of markers in blood. Such an approach was used in a bladder cancer pre-surgical trial to identify ICOS expression on CD4 T cells as a biomarker of response to ipilimumab [16, 17].
Melanoma versus other tumor types
We have learned a great deal about the role of anti-tumor immunity in shaping responses to therapy in melanoma, and these concepts are now being extended to other tumor types. However it is not clear that clinical observations made in immunologically “hot” tumors that have high numbers of immune infiltrating cells such as melanoma may be translated to more prevalent tumor types with lower frequencies of tumor-immune infiltrating immune cells [16]. As such, assessment of multiple markers within these tumors (including assessment of genomic and immune parameters) should be considered to understand the immune landscape of other tumor types as compared to melanoma and to provide information to guide therapeutic strategies. Research in this area has demonstrated different mechanisms of tumor immune escape between these “hot” and “cold” tumors [18]. While more heavily immune cell infiltrated tumors may escape anti-tumor immune responses by up-regulation of checkpoint inhibitory ligands and secretion of immunosuppressive factors such as T regulatory cells and IDO, more scarcely immune cell infiltrated tumors show escape through impaired recruitment of dendritic cells to the tumor microenvironment, and lack of effector T cell recruitment via reduced chemokine expression [18]. These immunologically “cold” tumors also demonstrate dysregulated oncogenic signaling pathways such as PI3 kinase/PTEN and p53 which may contribute to immune evasion [19, 20]. Further comprehensive immune monitoring is needed to advance our understanding of underlying immune pathophysiology and treatment responses across the full range of tumors.
Current and Emerging Immune Monitoring Strategies
Immunohistochemistry
Perhaps the most prevalent current strategy to assess immune responses within solid tumors involves enumeration of TIL via conventional staining with hematoxylin and eosin on paraffin embedded tissue – though this approach is admittedly quite limited. The use of singlet stain immunohistochemistry (IHC) for markers such as CD8 and PD-L1 is becoming more pervasive, though the predictive utility of PD-L1 assessment varies across studies [4–7] with several different antibodies and thresholds in use [21]. Markers such as these are being incorporated into algorithms such as the ‘Immunoscore’, which was developed in colorectal cancer to improve the prognostic yield of current AJCC/UICC TNM staging. The Immunoscore employs measurement of CD3 and CD8 at both tumor center and invasive margin based on findings that this improves prognostic accuracy, and utilizes digital pathology to minimize inter-observer variability and to provide specific quantitative cell density [12]. It was demonstrated to be prognostic beyond TNM staging alone in multivariable analysis of colorectal cancer patients and is currently being validated by a worldwide taskforce led by the Society for Immunotherapy of Cancer for incorporation into clinical practice. [22]. However, this approach was developed in primary colorectal cancer tumors that were surgically resected, thus may not be broadly applicable in the setting of metastatic disease. In addition, use of the Immunoscore in limited tissue samples (such as core biopsies) will not be optimal given the small amount of tissue available and lack of an invasive margin available for assessment. While single stain IHC is being optimized for clinical applicability, there are a growing number of platforms using multiplex IHC [23] that allow for multiple markers to be assessed on a single tissue section [23, 24]. Multiplex tissue imaging is critical to understanding not only the relative abundance of immune cells, but also spatial relationship of cells in the microenvironment and immune cell functionality [23, 25]. To date, this technology has been limited by technical issues such as cross-reactivity between stains and difficulty with interpretation of color combinations, among others [23]. However, these limitations are being addressed and are also circumvented by techniques incorporating sequential multiplex IHC [25]. Pushing the envelope even further, novel mass spectrometry based approaches on FFPE sections are being developed, and further enhance analyses that may be performed on single sections of tissue in the context of treatment with up to 100 markers addressed [24, 26]. This mass spectrometry based multiplex approach was recently used to simultaneously image 32 different proteins and protein modifications at subcellular resolution in human breast tissue [24].
Flow Cytometry/CyTOF
In addition to IHC-based techniques, flow cytometry may be performed to gain insight into the phenotype of infiltrating immune cells (and may help inform markers to study in peripheral blood). Though prognostic signatures to therapy have been described [11, 27, 28], phenotypic analysis of conventional markers in peripheral blood alone does not provide sufficient information about the tumor immune microenvironment and should be discouraged. Rather, paired tissue- and blood-based phenotyping should be performed, and ideally should involve conventional markers as well as novel markers to gain insight into mechanisms of therapeutic response and resistance.
In addition to conventional flow cytometry, novel methods are gaining use in characterizing immune responses in tumor and blood – such as Cytometry by Time-of-Flight (CyTOF) [29]. This technology utilizes a mass-spectometry approach with antibodies labeled with rare metals [29], with current capabilities of labeling cells with up to 40 separate markers [29, 30]. It has been used recently, for example, to show by simultaneous profiling of 16 surface and 15 intracellular proteins (in 15 million cells) to show that leukemic blasts’ surface phenotype is not reflective of the intracellular state [31], and also to identify that cell cycle differences in leukemia stem cells mediate differential responses to therapy [32]. While CyTOF is somewhat limited in tissue-based studies due to a requirement for a significant amount of substrate, its ability to allow in depth profiling of immune cells makes it advantageous when complex phenotyping is desired. This approach is being augmented by efforts to develop frameworks to couple in depth immune profiling technologies such as CyTOF and multiplex IHC with genomic, expression, and proteomic information to allow a comprehensive and real time understanding of the dynamic immune system [33].
Relationship Between Tissue and Blood Based Markers
Though unpaired assessment of phenotypic markers in blood provides limited information, significant insights may be gained by paired analysis of longitudinal tumor and blood samples during the course of treatment. As a group we do this routinely, and use information gained from tissue-based analysis to inform markers to interrogate in peripheral blood. As an example, we performed tissue- and blood-based analyses in patients on immune checkpoint blockade, and demonstrated that expression of inducible T cell co-stimulator (ICOS) on CD4+ T cells may be used as a peripheral blood pharmacodynamic marker of biologic activity of ipilimumab [11].
Genomics in Immune Monitoring
Tissue DNA-Based Assays
In addition to assessing immune markers within the microenvironment, there is increasing use of genomic profiling from tumor tissue and mounting evidence regarding the influence of genomic mutations on anti-tumor immunity [19, 20, 34]. This includes total mutational load derived from WES or targeted gene sequencing [35], which has been shown to correlate to improved treatment responses to immunotherapy [36–38]. However this is not perfectly predictive, and there is significant overlap in mutational load between those who respond to therapy and those who do not [36]. Other genomic markers such as mismatch repair (MMR) deficiency have been shown to be biomarkers of response to PD-1 based immunotherapy [39, 40]. Efforts are now also underway to characterize epigenetic alterations in tumor and immune cells that correlate with response to immunotherapy [41].
Another means of genomic characterization in the tumor microenvironment involves the use of T cell receptor sequencing (TCRseq). Monitoring changes in T cell clonality using TCRseq in longitudinal tumor biopsies during the course of treatment has been described [42, 43], and differences in T cell clonality in responders versus non-responders to therapy have been noted [44]. Like other DNA-based technologies this assay can be performed in FFPE, providing opportunities to query this in archival tissue. T cell repertoires in tumors may also be compared to those in peripheral blood from matched time points, when available. We as a group are incorporating TCRseq into many of our translational studies and novel clinical trials incorporating longitudinal blood- and tissue-based studies.
Neoantigen Prediction
Beyond using tumor genomics as a predictive variable of response, WES is also being combined with neoantigen prediction algorithms to define personalized immune targets for therapy. Algorithms can help predict putative neoepitopes in tumors by combining algorithms that model formation of neoepitopes from non-synonymous somatic mutations, proteasomal processing, HLA binding, and likelihood of neoepitopes being identified by T cell receptors [45, 46]. This is a complex pipeline with varying technologies published for each step, and optimization is needed to efficiently identify immunogenic neoantigens from WES data. Additional filters may be added to more accurately identify these neoepitopes – such as incorporating mRNA expression data to only select genes that are likely to be transcribed, peptide elution and mass spectrometry data to identify peptides that are actually expressed on the surface of the tumor cell, and MHC multimer or functional assays to select immunogenic complexes [47]. Identified neoepitopes can subsequently be used to identify personalized immune targets for adoptive cell therapy or personalized cancer vaccines [45, 48–51], and have even be used to monitor immune responses during therapy [52]. Limitations exist however, as existing algorithms are biased towards MHC Class I peptides leading to inaccuracies and limited understanding of the complete antigenic pool.
RNA-based assays
In addition to DNA-based assays to interrogate tumor mutational landscapes and mutational burden, RNA-based assays may be used to query transcriptomic profiles in the tumor microenvironment. Importantly this captures cancer cells as well as stroma (including signatures from infiltrating immune cells). RNA sequencing (RNAseq) can be used, though is somewhat costly and requires significant bioinformatic input for analysis. However, more targeted approaches are available such as NanoString nCounter that allows the added benefit of the ability to perform gene expression technology in FFPE tissue [53]. These technologies have been applied, for example, to understand the differential effects of anti-CTLA4 and anti-PD1 therapy on immune cell gene expression and function [54].
Single cell technology
Single cell technologies have highlighted the extensive heterogeneity that exists both within tumor as well as among even specific subsets of immune cells. This heterogeneity is especially important to study as we recognize the significance of individual or subset of clones to treatment resistance and tumor recurrence. Akin to CyTOF for immune markers, single cell sequencing technologies are being optimized to perform single cell WES, whole mRNA transcriptome sequencing [55], and also targeted sequencing of DNA regions or mRNA transcripts [30]. Single cell sequencing can be performed on tumor cells themselves, and also on infiltrating immune cells. Technology now exists to perform single cell TCR sequencing [56], and further linking of the TCR sequence to single cell gene expression profiling allows more accurate capturing of T cell subsets, as cells with same TCR can be functionally distinct [56]. This coupling has been used to identify allergen-specific anergic CD4+ T cell subsets that may mediate responses to successful allergen immunotherapy [57]. Other examples of coupling of single cell technologies include RNA-seq of individual macrophages with fluorescent labeling of bacterial pathogens [58] to understand heterogeneity of host immune responses to bacterial invasion, and pairing immunophenotypic with cell signaling information via antibodies against phosphorylated proteins to understand heterogeneity in AML responses to therapy [32]. As these technologies are being optimized, it is becoming possible to perform noninvasive deep single cell profiling to allow a better appreciation of in vivo cell function and immune and tumor heterogeneity [30]. Equally important are advances in bioinformatics and platforms required to synthesize the data generated from these technologies [33].
Another emerging technology involves the analysis of tumor-derived exosomes, with the potential to gain insight into several aspects of the tumor microenvironment via a non-invasive, blood-based approach. Exosomes are 50–100 nm membrane vesicles secreted by tumor and immune cells for short and long distance intercellular communication and mediate exchange of protein and genetic material between cells [59], and are thought to play an important role in mechanisms of therapeutic response and resistance [59, 60]. Importantly, several components of exosomes can be studied, including DNA, RNA, miRNA, and proteins [60]. Recent evidence suggests that longitudinally monitoring RNA expression profiles in circulating exosomes can be used to assess changes in immune pathway genes during immunotherapy, and that differential patterns of expression between responders and non-responders may be observed [59, 61].
Impact of Host Genomics and External Environment on Immune System
Host genomic factors
In addition to studying tumor-intrinsic features, we must consider the influence of host genomics and our external environment in shaping anti-tumor immunity. There is a growing appreciation of the influence of single nucleotide polymorphisms (SNPs) on host immune responses [62], and also evidence that non-heritable factors may significantly influence immunity [63]. In cancer immunotherapy, several studies have explored effect of SNPs on responses [64–66] as well as toxicity [67] to immunotherapies. Results vary, however, and large studies with functional validation are needed to identify SNPs and other host genomic factors associated with response to therapy.
Microbiome
Another rapidly emerging area of investigation that must be considered in the context of anti-tumor immune responses is the microbiome (Figure 3). The microbiome refers to the entire community of bacteria (and their genomes) within an organism, and the number of bacteria within a human outnumbers the number of human cells by at least 10:1. There is a growing role of the microbiome in health and disease, and evidence that the gut microbiome may shape anti-tumor immune responses as well as responses to immune checkpoint blockade and other immunotherapies [68–70]. Recently, it was shown that optimal anti-tumor response to ipilimumab and anti-PD-L1 therapies are dependent on Bacteroides species B. thetaiotaomicron and B. fragilis and Bifidobacterium spp; however these were largely based on results from murine studies thus the role of these bacteria in patients needs to be studied further. Accordingly, as we move forward with immune monitoring techniques, we must strongly consider assessment of the host microbiome to better understand its influence. Ultimately, such studies may lead to enhanced mechanistic insight and actionable strategies to help overcome therapeutic resistance.
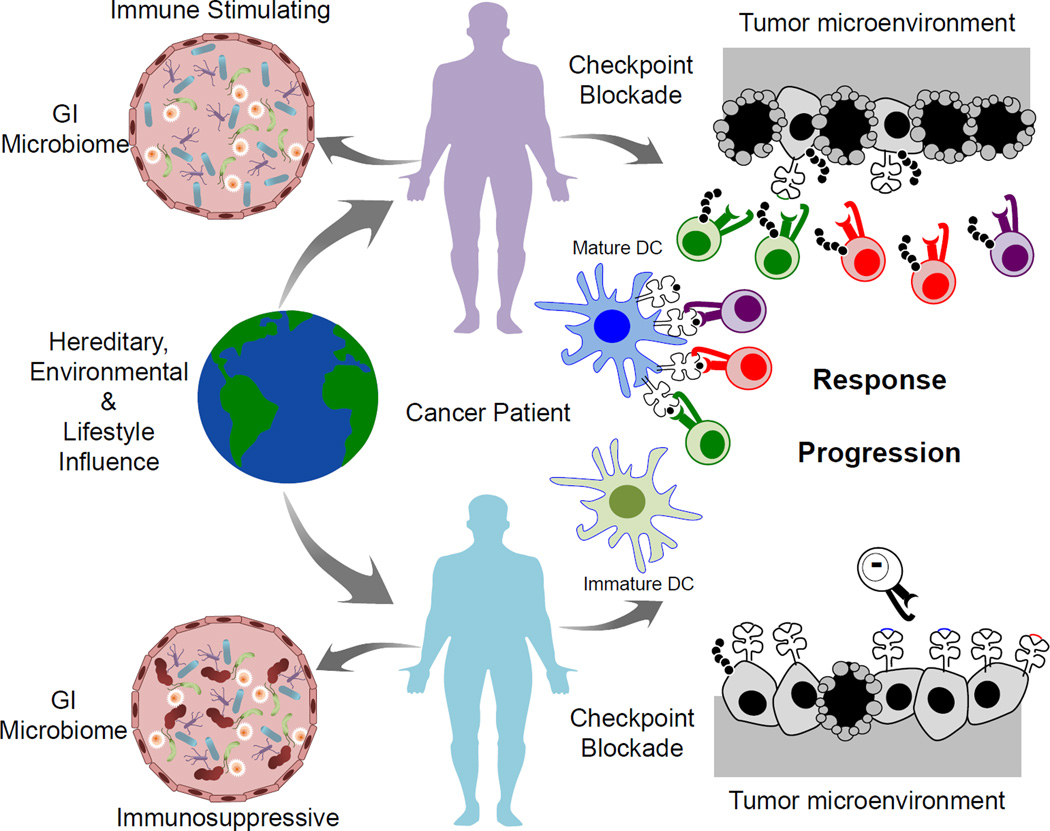
Figure 3. The host microbiome may contribute to responses to immunotherapy.
Many factors can shape immune responses, including hereditary, environmental and lifestyle factors, as well as the host microbiome. Recent studies have suggested a role for the gastrointestinal microbiome in contributing to response and resistance to checkpoint blockade immunotherapy in melanoma. The proposed mechanism behind this is via modulation of dendritic cell function affecting downstream antigen presentation and priming of anti-tumor CD4 and CD8 T cells. Microbiome constitution may either be immune stimulating or immunosuppressive, and thereby either promote or inhibit activity of checkpoint blockade and other immunotherapies.
Conclusion
As immune monitoring techniques evolve, it is becoming increasingly possible to identify determinants of treatment response and to gain mechanistic insight into immune mechanisms of response and resistance. A key feature as we move forward is the need to perform longitudinal assessment throughout the course of therapy, as static assessments are limited and do not take into account the dynamics of anti-tumor immune responses. Furthermore, we as a field must better understand tumor heterogeneity as it relates to anti-tumor immune responses, and must better understand and optimize concordance between tissue-based, blood-based and novel imaging techniques to assess immune responses. Emerging techniques hold promise for less invasive and more robust assessment of anti-tumor immune responses, especially with advances in single cell based technologies and tissue imaging. Novel frameworks and bioinformatics strategies will allow the integration of the extensive data that will be generated from these technologies and preliminary work is already revealing the power of this comprehensive and systematic assessment. Through this approach, we will realize the potential to obtain a dynamic and comprehensive understanding of tumor-microenvironment interactions, as well as their relationship to therapeutic response and resistance.
Highlights.
Fresh tissue is more reliable for immune monitoring than archival tissue.
Pre-surgical trials are important for adequate tissue collection.
Immune system is dynamic, and longitudinal monitoring on therapy is needed.
“Hot” and “cold” tumors show different pathophysiology and treatment responses.
High dimensional single cell profiling highlights extensive immune heterogeneity.
Acknowledgments
Jennifer Wargo acknowledges funding from the Kenedy foundation grant 0727030. Sangeetha Reddy was supported by the National Institute of Health T32 training grant 2T32CA009666-21.
Padmanee Sharma acknowledges funding from the National Institute of Health grant R01 CA 163793 grant, Institutional Core Grant P30 CA016672, CPRIT grant RP120108, SU2C-CRI-AACR Cancer Immunotherapy Dream Team grant SU2C-AACR-DT1012, and the PCF Immunology Challenge Grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jennifer A. Wargo, Department of Surgical Oncology, Genomic Medicine, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, Texas, 77030.
Sangeetha M. Reddy, Division of Cancer Medicine, The University of Texas MD Anderson Cancer Center, 1400 Holcombe Blvd, Unit 463, Houston, TX 77030.
Alexandre Reuben, Department of Surgical Oncology, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, Texas, 77030.
Padmanee Sharma, Department of Genitourinary Medical Oncology, 1155 Pressler Street, Unit 1374, Houston, TX 77030.
References
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Posevitz-Fejfar A, Posevitz V, Gross CC, et al. Effects of blood transportation on human peripheral mononuclear cell yield, phenotype and function: implications for immune cell biobanking. PLoS One. 2014;9:e115920. doi: 10.1371/journal.pone.0115920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang J, Diaz N, Adelsberger J, et al. The effects of storage temperature on PBMC gene expression. BMC Immunol. 2016;17:6. doi: 10.1186/s12865-016-0144-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Paoli-Iseppi R, Johansson PA, Menzies AM, et al. Comparison of whole-exome sequencing of matched fresh and formalin fixed paraffin embedded melanoma tumours: implications for clinical decision making. Pathology. 2016;48:261–266. doi: 10.1016/j.pathol.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558–562. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 6.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carbognin L, Pilotto S, Milella M, et al. Differential Activity of Nivolumab, Pembrolizumab and MPDL3280A according to the Tumor Expression of Programmed Death-Ligand-1 (PD-L1): Sensitivity Analysis of Trials in Melanoma, Lung and Genitourinary Cancers. PLoS One. 2015;10:e0130142. doi: 10.1371/journal.pone.0130142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hugo W, Shi H, Sun L, et al. Non-genomic and Immune Evolution of Melanoma Acquiring MAPKi Resistance. Cell. 2015;162:1271–1285. doi: 10.1016/j.cell.2015.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chin LWJ, Spring DJ, Kantarjian H, Futreal PA. Cancer Genomics in Clinical Context. Trends in Cancer. 2015;1:36–43. doi: 10.1016/j.trecan.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 10.Chen PL, Roh W, Reuben A, et al. Immune infiltrate in early on-treatment biopsies is highly predictive of response to immune checkpoint blockade. In Society for Melanoma Research Congress. 2015 [Google Scholar]
- 11.Ng Tang D, Shen Y, Sun J, et al. Increased frequency of ICOS+ CD4 T cells as a pharmacodynamic biomarker for anti-CTLA-4 therapy. Cancer Immunol Res. 2013;1:229–234. doi: 10.1158/2326-6066.CIR-13-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 13.Reuben A, Spencer C, Roszik J, et al. Molecular and immune heterogeneity in synchronous melanoma metastases. Journal for ImmunoTherapy of Cancer. 2015;3(Suppl 2):P262. [Google Scholar]
- 14.Zhang J, Fujimoto J, Zhang J, et al. Intratumor heterogeneity in localized lung adenocarcinomas delineated by multiregion sequencing. Science. 2014;346:256–259. doi: 10.1126/science.1256930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reuben A, Spencer C, Prieto P, et al. Genomic and immune heterogeneity in synchronous melanoma metastases contribute to differential tumor growth and response to therapy. Manuscript submitted for publication. 2016 [Google Scholar]
- 16.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 17.Liakou CI, Kamat A, Tang DN, et al. CTLA-4 blockade increases IFNgamma-producing CD4+ICOShi cells to shift the ratio of effector to regulatory T cells in cancer patients. Proc Natl Acad Sci U S A. 2008;105:14987–14992. doi: 10.1073/pnas.0806075105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spranger S. Mechanisms of tumor escape in the context of the T cell-inflamed and the non-T cell-inflamed tumor microenvironment. Int Immunol. 2016 doi: 10.1093/intimm/dxw014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature. 2015;523:231–235. doi: 10.1038/nature14404. [DOI] [PubMed] [Google Scholar]
- 20. Peng W, Chen JQ, Liu C, et al. Loss of PTEN Promotes Resistance to T Cell-Mediated Immunotherapy. Cancer Discov. 2016;6:202–216. doi: 10.1158/2159-8290.CD-15-0283. • This paper provides an example of how oncogenic signaling pathways affect the tumor microenvironment and mediate efficacy of immunotherapies, thereby providing rationale for combination therapies.
- 21.Gadiot J, Hooijkaas AI, Kaiser AD, et al. Overall survival and PD-L1 expression in metastasized malignant melanoma. Cancer. 2011;117:2192–2201. doi: 10.1002/cncr.25747. [DOI] [PubMed] [Google Scholar]
- 22.Galon J, Mlecnik B, Bindea G, et al. Towards the introduction of the 'Immunoscore' in the classification of malignant tumours. J Pathol. 2014;232:199–209. doi: 10.1002/path.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stack EC, Wang C, Roman KA, Hoyt CC. Multiplexed immunohistochemistry, imaging, and quantitation: a review, with an assessment of Tyramide signal amplification, multispectral imaging and multiplex analysis. Methods. 2014;70:46–58. doi: 10.1016/j.ymeth.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 24. Giesen C, Wang HA, Schapiro D, et al. Highly multiplexed imaging of tumor tissues with subcellular resolution by mass cytometry. Nat Methods. 2014;11:417–422. doi: 10.1038/nmeth.2869. •• Novel multiplex technology detailed in this article is used to simultaneous image 32 markers of human breast tissue to provide high resolution comprehensive profiling. This technology can be expanded to image up to 100 markers.
- 25.Tsujikawa TBR, Azimi V, Rassi EE, et al. Multiplex immunohistochemistry for immune profiling of HPV-associated head and neck cancer. Journal for ImmunoTherapy of Cancer. 2015;3(Suppl 2):P419. [Google Scholar]
- 26.Angelo M, Bendall SC, Finck R, et al. Multiplexed ion beam imaging of human breast tumors. Nat Med. 2014;20:436–442. doi: 10.1038/nm.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martens A, Wistuba-Hamprecht K, Geukes Foppen MH, et al. Baseline peripheral blood biomarkers associated with clinical outcome of advanced melanoma patients treated with ipilimumab. Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-15-2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dobrovolskiene NT, Cicenas S, Kazlauskaite N, et al. CD8(high)CD57(+) T-cell population as an independent predictor of response to chemoradiation therapy in extensive-stage small cell lung cancer. Lung Cancer. 2015;90:326–333. doi: 10.1016/j.lungcan.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Ornatsky O, Bandura D, Baranov V, et al. Highly multiparametric analysis by mass cytometry. J Immunol Methods. 2010;361:1–20. doi: 10.1016/j.jim.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 30.Bendall SC, Nolan GP. From single cells to deep phenotypes in cancer. Nat Biotechnol. 2012;30:639–647. doi: 10.1038/nbt.2283. [DOI] [PubMed] [Google Scholar]
- 31.Levine JH, Simonds EF, Bendall SC, et al. Data-Driven Phenotypic Dissection of AML Reveals Progenitor-like Cells that Correlate with Prognosis. Cell. 2015;162:184–197. doi: 10.1016/j.cell.2015.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Behbehani GK, Samusik N, Bjornson ZB, et al. Mass Cytometric Functional Profiling of Acute Myeloid Leukemia Defines Cell-Cycle and Immunophenotypic Properties That Correlate with Known Responses to Therapy. Cancer Discov. 2015;5:988–1003. doi: 10.1158/2159-8290.CD-15-0298. • Application of high dimensional mass cytometry is used to identify mechanisms underlying differences in relapse rates among acute myelogenous leukemia subtypes.
- 33. Spitzer MH, Gherardini PF, Fragiadakis GK, et al. IMMUNOLOGY. An interactive reference framework for modeling a dynamic immune system. Science. 2015;349:1259425. doi: 10.1126/science.1259425. •• This article higlights the complexity of the immune cell network and how a systematic approach is needed to integrate the big data obtained from the high throughput data obtained from mass cytometry and other single cell technologies.
- 34.Tran E, Ahmadzadeh M, Lu YC, et al. Immunogenicity of somatic mutations in human gastrointestinal cancers. Science. 2015;350:1387–1390. doi: 10.1126/science.aad1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roszik JJA, Siroy A, Haydu LE, et al. A novel algorithm applicable to cancer next-generation sequencing panels to predict total tumor mutation load and correlation with clinical outcomes in melanoma. J Clin Oncol. 2015;33(suppl) abstr 9071. [Google Scholar]
- 36.Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Allen EM, Miao D, Schilling B, et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science. 2015;350:207–211. doi: 10.1126/science.aad0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kelderman S, Schumacher TN, Kvistborg P. Mismatch Repair-Deficient Cancers Are Targets for Anti-PD-1 Therapy. Cancer Cell. 2015;28:11–13. doi: 10.1016/j.ccell.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 41.Polak P, Karlic R, Koren A, et al. Cell-of-origin chromatin organization shapes the mutational landscape of cancer. Nature. 2015;518:360–364. doi: 10.1038/nature14221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cooper ZA, Frederick DT, Juneja VR, et al. BRAF inhibition is associated with increased clonality in tumor-infiltrating lymphocytes. Oncoimmunology. 2013;2:e26615. doi: 10.4161/onci.26615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Postow MA, Manuel M, Wong P, et al. Peripheral T cell receptor diversity is associated with clinical outcomes following ipilimumab treatment in metastatic melanoma. J Immunother Cancer. 2015;3:23. doi: 10.1186/s40425-015-0070-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Snyder A, Chan TA. Immunogenic peptide discovery in cancer genomes. Curr Opin Genet Dev. 2015;30:7–16. doi: 10.1016/j.gde.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yuan J, Hegde PS, Clynes R, et al. Novel technologies and emerging biomarkers for personalized cancer immunotherapy. J Immunother Cancer. 2016;4:3. doi: 10.1186/s40425-016-0107-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348:69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 48.Tran E, Turcotte S, Gros A, et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 2014;344:641–645. doi: 10.1126/science.1251102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gros A, Parkhurst MR, Tran E, et al. Prospective identification of neoantigen-specific lymphocytes in the peripheral blood of melanoma patients. Nat Med. 2016 doi: 10.1038/nm.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kelderman S, Heemskerk B, Fanchi L, et al. Antigen-specific TIL therapy for melanoma: A flexible platform for personalized cancer immunotherapy. Eur J Immunol. 2016 doi: 10.1002/eji.201545849. [DOI] [PubMed] [Google Scholar]
- 51.Hacohen N, Fritsch EF, Carter TA, et al. Getting personal with neoantigen-based therapeutic cancer vaccines. Cancer Immunol Res. 2013;1:11–15. doi: 10.1158/2326-6066.CIR-13-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carreno BM, Magrini V, Becker-Hapak M, et al. Cancer immunotherapy. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science. 2015;348:803–808. doi: 10.1126/science.aaa3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Geiss GK, Bumgarner RE, Birditt B, et al. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat Biotechnol. 2008;26:317–325. doi: 10.1038/nbt1385. [DOI] [PubMed] [Google Scholar]
- 54.Das R, Verma R, Sznol M, et al. Combination therapy with anti-CTLA-4 and anti-PD-1 leads to distinct immunologic changes in vivo. J Immunol. 2015;194:950–959. doi: 10.4049/jimmunol.1401686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shalek AK, Satija R, Adiconis X, et al. Single-cell transcriptomics reveals bimodality in expression and splicing in immune cells. Nature. 2013;498:236–240. doi: 10.1038/nature12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Han A, Glanville J, Hansmann L, Davis MM. Linking T-cell receptor sequence to functional phenotype at the single-cell level. Nat Biotechnol. 2014;32:684–692. doi: 10.1038/nbt.2938. •• The importance of coupling single cell technologies is demonstrated by performing both T cell receptor sequencing and phenotyping to identify T cell clonal ancestry and differentiation.
- 57.Ryan JF, Hovde R, Glanville J, et al. Successful immunotherapy induces previously unidentified allergen-specific CD4+ T-cell subsets. Proc Natl Acad Sci U S A. 2016;113:E1286–E1295. doi: 10.1073/pnas.1520180113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Avraham R, Haseley N, Brown D, et al. Pathogen Cell-to-Cell Variability Drives Heterogeneity in Host Immune Responses. Cell. 2015;162:1309–1321. doi: 10.1016/j.cell.2015.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 60.Liu Y, Gu Y, Cao X. The exosomes in tumor immunity. Oncoimmunology. 2015;4:e1027472. doi: 10.1080/2162402X.2015.1027472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hurley J, Hu L, Brock G, et al. Profiling exosomal mRNAs in patients undergoing immunotherapy for malignant melanoma. J Clin Oncol. 2015;33(suppl) abstr e22159. [Google Scholar]
- 62.Orru V, Steri M, Sole G, et al. Genetic variants regulating immune cell levels in health and disease. Cell. 2013;155:242–256. doi: 10.1016/j.cell.2013.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brodin P, Jojic V, Gao T, et al. Variation in the human immune system is largely driven by non-heritable influences. Cell. 2015;160:37–47. doi: 10.1016/j.cell.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hamid O, Schmidt H, Nissan A, et al. A prospective phase II trial exploring the association between tumor microenvironment biomarkers and clinical activity of ipilimumab in advanced melanoma. J Transl Med. 2011;9:204. doi: 10.1186/1479-5876-9-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Breunis WB, Tarazona-Santos E, Chen R, et al. Influence of cytotoxic T lymphocyte-associated antigen 4 (CTLA4) common polymorphisms on outcome in treatment of melanoma patients with CTLA-4 blockade. J Immunother. 2008;31:586–590. doi: 10.1097/CJI.0b013e31817fd8f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Queirolo P, Morabito A, Laurent S, et al. Association of CTLA-4 polymorphisms with improved overall survival in melanoma patients treated with CTLA-4 blockade: a pilot study. Cancer Invest. 2013;31:336–345. doi: 10.3109/07357907.2013.793699. [DOI] [PubMed] [Google Scholar]
- 67.Tarhini A, LaFramboise W, Petrosko P, et al. Clustered genomic variants specific to patients who develop immune-related colitis after ipilimumab for prediction of toxicity. J Clin Oncol. 2014;32:5s. 2014 (suppl; abstr 9024) [Google Scholar]
- 68.Sivan A, Corrales L, Hubert N, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350:1084–1089. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Vetizou M, Pitt JM, Daillere R, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350:1079–1084. doi: 10.1126/science.aad1329. • The microbiome is demonstrated to modulate responses to immunotherapy, which can be manipulated by removing or re-introducing back Bacteroides species.
- 70.Goldszmid RS, Dzutsev A, Viaud S, et al. Microbiota modulation of myeloid cells in cancer therapy. Cancer Immunol Res. 2015;3:103–109. doi: 10.1158/2326-6066.CIR-14-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]