Abstract
Objectives
Delirium disproportionately affects patients with dementia and is associated with adverse outcomes. The diagnosis of delirium superimposed on dementia (DSD), however, can be challenging due to several factors including the absence of caregivers or the severity of pre-existing cognitive impairment. Altered level of consciousness has been advocated as a possible useful indicator of delirium in this population. Here we evaluated the performance of the Richmond Agitation and Sedation Scale (RASS) and the modified-RASS (m-RASS) – an ultra-brief measure of the level of consciousness – in the diagnosis of DSD.
Design
Multicenter prospective observational study. RASS and m-RASS results were analysed together, labelled RASS/m-RASS).
Setting
Acute geriatric wards, inhospital rehabilitation, emergency department.
Participants
Patients 65 years and older with dementia.
Measurements
Delirium was diagnosed with the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) or with the DRS-R-98 or with the 4AT. Dementia was detected with the Clinical Dementia Rating (CDR) Scale, the Short Form Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) or via the clinical records.
Results
Of the 645 patients included, 376 (58%) had delirium. According to the instrument used to evaluate delirium the prevalence was 66% with the 4AT, 23% with the DSM and 100% with the DRS-R-98. Overall a RASS/m-RASS score other than 0 was 70.5% sensitive (95% CI: 65.9% – 75.1%) and 84.8% (CI: 80.5% – 89.1%) specific for DSD. Using a RASS/m-RASS value >+1 or <−1 as a cut-off, the sensitivity was 30.6% (CI: 25.9% – 35.2%) and the specificity was 95.5% (CI: 93.1% – 98.0%). The sensitivity and the specificity did not greatly vary according to the method of delirium diagnosis and the dementia ascertainment, though the specificity was slightly higher when the DSM and the IQCODE were used.
Conclusion
In older patients admitted to different clinical settings the RASS and m-RASS analyzed as a single group had moderate sensitivity and very high specificity for the detection of DSD. Level of consciousness is therefore a valuable clinical indicator that should form part of delirium screening strategies, though for higher sensitivity other methods of assessment should be used.
Keywords: delirium, dementia, diagnosis, RASS, m-RASS
INTRODUCTION
Delirium is a common neuropsychiatric disorder and when it occurs in patients with dementia, this condition is referred to as delirium superimposed on dementia, or DSD.1 The prevalence of DSD ranges between 22% to 89% in community and hospital populations.1 It is associated with higher health care costs, worse functional outcomes, and higher mortality rates when compared to patients with dementia alone.1–5
It is recognized in clinical practice and research that it can be challenging to distinguish delirium from dementia, particularly when the dementia is severe.6 Tests of attention can be used to detect inattention in delirium, but performance on these tests is also commonly impaired in the presence of severe dementia.7 There is little research specifically addressing the issue of the best methods of detecting DSD. In a recent systematic review, we found that very few studies had specifically determined how existing assessment methods perform in the diagnosis of DSD.8 Of 1,569 participants analyzed in the systematic review, 401 had dementia, and 50 had delirium superimposed on dementia. Six delirium tools were evaluated. The survey found a small evidence to support the CAM and the CAM-ICU to detect DSD in the general ward and ICU settings, respectively. With respect to specialists’ opinions, Richardson and colleagues performed a survey and found 41% of responders (N = 85) felt that it was always possible to distinguish delirium from Behavioral and Psychological Symptoms in Dementia (BPSD), while 48% (N = 118) felt that this was only possible in some circumstances.6 However, the majority of responders believed that motor fluctuations and/or altered level of consciousness were important for the diagnosis of DSD. In those who did use a specific test for level of consciousness, the Richmond Agitation and Sedation Scale (RASS) was the most popular in both clinical practice and research studies.6
The RASS was developed by Sessler and validated by Ely and colleagues as a measure of arousal, sedation, and level of consciousness in critically ill patients (Table 1).9, 10 The RASS has been recently modified and adapted for use in general wards (i.e. the modified RASS or m-RASS).11 Both the RASS and the m-RASS are ultra-brief assessments of the level of consciousness and recent studies have suggested that they have high specificity for delirium in geriatrics wards and emergency department.11–13 Leonard and colleagues14 recently reported how delirium can be distinguished from dementia by virtue of the disproportionate impairment of vigilance and attention.
Table 1.
The Richmond Agitation and Sedation Scale (RASS)9, 10 and the modified Richmond Agitation and Sedation Scale (m-RASS).11
| Score | RASS | m-RASS |
|---|---|---|
| +4 |
Combative: Combative, violent, immediate danger to staff |
Combative: No attention; overtly combative, violent, immediate danger to staff |
| +3 |
Very agitated: Pulls to remove tubes or catheters, aggressive |
Very agitated: Very distractible; repeated calling or touch required to get or keep eye contact or attention; cannot focus; pulls or removes tube(s) or catheter(s); aggressive; fights environment not people |
| +2 |
Agitated: Frequent non-purposefeul movement, fight ventilator |
Slightly agitated: Easily distractible; rapidly loses attention; resists care or uncooperative; frequent non-purposeful movement |
| +1 |
Restless: Anxious, apprehensive, movements non aggressive |
Restless: Slighlty distactrible; pays attention most of the time; anxious, but cooperative; movements non aggressive or vigorous |
| 0 |
Alert and Calm: Spontaneously pays attention to caregiver |
Alert and Calm: Pays attention; makes eye contact, aware of surroundings; responds immediately and appropriately to calling name and touch |
| −1 |
Drowsy: Not fully alert, but has sustained awakening to voice (eye opening & contact >10 sec) |
Wakes easily: Slightly drowsy; eye contact >10 sec; not fully alert, but has sustained awakening; eye opening/eye contact to voice >10 seconds |
| −2 |
Light sedation: Briefly awakens to voice (eyes open & contact |
Wakes slowly: Very drowsy; pays attention some of the time; briefly awakens with eye contact to voice < 10 seconds |
| −3 |
Moderate sedation: Movement or eye opening to voice (no eye contact) |
Difficult to wake: Repeated calling or touch required to get or keep eye contact or attention; needs repeated stimuli (touch or voice) for attention, movement, or eye opening to voice (but no eye contact) |
| −4 |
Deep sedation: No response to voice, but movement or eye opening to physical stimulation |
Can’t stay awake: Arousable but no attention; no response to voice, but movement or eye opening to physical stimulation |
| −5 |
Unarousable: No response to voice or physical stimulation |
Unarousable: No response to voice or physical stimulation |
Because the RASS and the m-RASS simply require the rater to observe the patient and no collateral history is needed, they may be particularly useful in detecting delirium in patients with dementia, especially when the patient’s cognitive baseline (pre-existing attention or other cognitive deficits, determining acute onset and fluctuations etc.) is difficult to establish. However, patients in the advanced stages of dementia may be more likely to have baseline impairments in level of consciousness,15, 16 and so the specificity of the RASS may be decreased. Therefore we sought to determine, in a multicenter prospective cohort study, the diagnostic performances of the RASS and the m-RASS for the detection of delirium in older patients with dementia admitted to different clinical settings (i.e., acute geriatric wards, inhospital rehabilitation, emergency department).
METHODS
Sample and study design
This was a multicenter secondary analysis of previous prospective cohort studies that have evaluated delirium in patients with dementia. The local institutional review board or appropriate ethics committee in each institution reviewed and approved this study. The pooled dataset is derived from five different groups in different clinical settings (Table 1). The dataset consists of 645 patients with dementia.
Group 1 (N=114): this group was drawn from a prospective multicenter study designed to evaluate the performance of various attention tests in different subgroup of older patients (i.e. patients without delirium and dementia; patients with delirium only; patients with dementia only; patients with delirium and dementia). Patients were enrolled in different clinical settings in four European Countries: old-age psychiatry consultation-liaison (University of Limerick, Ireland); inpatient Rehabilitation (Department of Rehabilitation, Fondazione Camplani, Cremona, Italy); acute geriatric ward (San Gerardo Hospital, Monza, Italy); general surgery and orthopedic surgery (University of Basel, Switzerland); psychiatric ward and acute geriatric ward (Centro Hospitalar e Universitário de Coimbra, Portugal). In this group delirium was diagnosed using the Diagnostic and Statistical Manual of Mental Disorder, 5th Edition (DSM-5), and dementia was ascertained using the Short Form-Informant Questionnaire on Cognitive Decline in the Elderly (SF-IQCODE).
Group 2 (N=404): this group of patients was drawn from patients admitted to the acute geriatric ward of the San Gerardo Hospital, Monza, Italy May 2015 and May 2015. In this group of patients delirium was diagnosed using the 4 “A”s Test (4AT)17 and dementia was ascertained using the clinical records.
Group 3 (N=35): this group included only patients with delirium and dementia admitted to an inhospital rehabilitation unit. This population has been previously described.18, 19 The presence of dementia was determined by reviewing patient medical records. Additionally, two expert neuropsychologists confirmed and rated the severity of dementia by interviewing the caregivers at the time of enrollment using the Clinical Dementia Rating (CDR) Scale20 and the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE), with a cut-off of 3.3 used to indicate cognitive impairment.21 Delirium was diagnosed with the Diagnostic and Statistical Manual of Mental Disorders (4th edition, text revision) (DSM-IV-TR) criteria, using a standardized approach.17
Group 4 (N=40): this group was previously described in Jabbar et al.22 In this group, delirium was diagnosed with the DRS-R-98, while dementia was defined as the presence of persistent cognitive impairment for at least six months prior to the assessment and/or DSM-IV criteria based on all available information at the time of assessment including clinical case notes and collateral history from family and/or carers.
Group 5 (N=39): The fifth group of patients has also been previously described.12 In this group were included older patients admitted to an academic, tertiary care emergency department July 2009 to February 2012. Delirium was diagnosed using a comprehensive consultation-liaison psychiatrist assessment with the DSM-IV-TR criteria, while dementia was ascertained using the clinical records and IQCODE.
In each of these studies the modified-Richmond Agitation and Sedation Scale (m-RASS)11 (Groups 1 and 3) or the Richmond Agitation and Sedation Scale (RASS) (Groups 2, 4 and 5) was performed.9, 10 The RASS9, 10 is a commonly used in the ICU to assess arousal, level of sedation and consciousness (Table 1). It ranges from –5 (unarousable) to + 4 (combative), and 0 indicates a normal level of alertness. The m-RASS11 also ranges from −5 (unarousable) to + 4 (combative). The scale was modified to be used in non-ICU wards and it includes an element of judgment regarding attention, which is different from the RASS.
Statistical analysis
Continuous variables are reported as medians and interquartile ranges (IQRs) and categorical variables as proportions. RASS and m-RASS results were analyzed together, labeled RASS/m-RASS. Sensitivities, specificities, positive likelihood ratios, and negative likelihood ratios with their 95% confidence intervals (95% CIs) were calculated, stratifying according to the delirium (i.e., DSM-5, 4AT, DRS-R-98) and dementia evaluation (IQCODE-SF, CDR, clinical records). Diagnostic performances were calculated for three cut points: 1) a RASS/m-RASS other than 0 (RASS/m-RASS > 0 or < 0); 2) a RASS/m-RASS > +1 or < –1. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Carey, NC).
RESULTS
The study included a total of 645 patients with dementia. The median age was 84 (Interquartile Range, IQR: 78, 89) (Table 3). 376 (58%) patients had delirium according to the reference standard assessments. The prevalence of delirium was 29.8% in the Group 1, 3, and 5 where the DSM was used to assess for delirium, 63.9% in Group 4 where the 4AT was used to assess for delirium. Finally in the Group 5 the prevalence of delirium was 100% since it included only patients with delirium assessed with the DRS-R-98). Dementia prevalence was 70.9% in Group 2 and 4 where the clinical records were used to assess for dementia prevalence, 23.7% in Group 1 and 5 where the IQCODE was used to assess for dementia. In Group 3 the prevalence of dementia was 100% since it included patients with dementia evaluated with the CDR.
Table 3.
Patient characteristics for all patients and stratified by delirium.
| Variable | All Patients N=632 |
Delirium Negative n=265 |
Delirium Positive n=367 |
|---|---|---|---|
| Median Age (IQR) | 84 (78, 89) | 82 (77, 88) | 85 (80, 89) |
| Gender | |||
| Female | 405 (64.1%) | 169 (63.8%) | 235 (64.1%) |
| Male | 227 (35.9%) | 96 (36.2%) | 134 (36.5%) |
| Enrollment Site | |||
| Basel (Switzerland) | 5 (0.8%) | 1 (0.4%) | 4 (1.1%) |
| Coimbra (Portugal) | 14 (2.2%) | 7 (2.6%) | 7 (1.9%) |
| Cremona (Italy) | 49 (7.8%) | 7 (2.6%) | 42 (11.4%) |
| Limerick (Ireland) | 54 (8.5%) | 7 (2.6%) | 47 (12.8%) |
| Monza (Italy) | 411 (59.9%) | 165(39.9%) | 246 (67.0%) |
| Nashville (USA) | 99 (15.7%) | 78 (29.0%) | 21 (5.7%) |
| Dementia Method | |||
| CDR | 35 (5.5%) | 0 (0.0%) | 35 (9.5%) |
| Clinical Records | 444 (70.3%) | 165 (62.3%) | 279 (76.0%) |
| SF-IQCODE | 153 (23.2%) | 100 (37.7%) | 53 (14.4%) |
| Delirium Reference Standard | |||
| 4AT | 404 (63.9%) | 165 (62.3%) | 239 (65.1%) |
| DRS-R-98 | 40 (6.3%) | 0 (0.0%) | 40 (10.9%) |
| DSM | 188 (29.8%) | 100 (37.7%) | 88 (24.0%) |
| RASS/m-RASS score | |||
| RASS/m-RASS =0 | 339 (53.6%) | 228 (86.0%) | 111 (30.3%) |
| RASS/m-RASS = −1 | 129 (20.4%) | 26 (9.8%) | 103 (28.1%) |
| RASS/m-RASS = −2 | 40 (6.3%) | 4 (1.5%) | 36 (9.8%) |
| RASS/m-RASS = −3 | 14 (2.2%) | 3 (1.1%) | 11 (3.0%) |
| RASS/m-RASS = +1 | 50 (7.9%) | 3 (1.1%) | 47 (12.8%) |
| RASS/m-RASS = +2 | 42 (6.7%) | 1 (0.4%) | 41 (11.2%) |
| RASS/m-RASS= +3 | 12 (1.9%) | 0 (0.0%) | 12 (3.3%) |
| RASS/m-RASS= +4 | 6 (1.0%) | 0 (0.0%) | 6 (1.6%) |
Abbreviations: Clinical Dementia Rating (CDR) Scale; Short Form Informant Questionnaire on Cognitive Decline in the Elderly (SF-IQCODE); Diagnostic and Statistical Manual of Mental Disorders (DSM); Delirium Rating Scale-Revised (DRS-R-98)
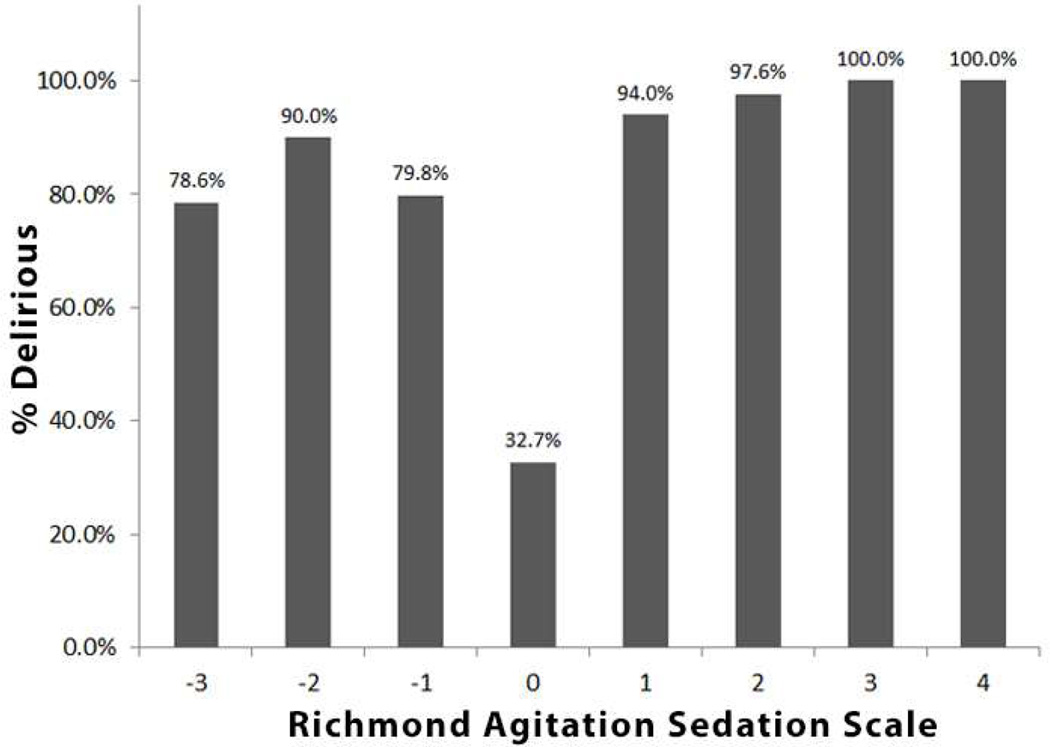
Figure 1 shows the proportion of delirious patients according to the RASS/m-RASS score. Patients with a RASS/m-RASS score ≤ 4 are not shown since are not considered assessable for delirium.
Figure 1.
Proportion of delirious patients according to the RASS/m-RASS score. Patients with a RASS/m-RASS score ≤ 4 are not shown since are not considered assessable for delirium.
The diagnostic performances of the RASS/m-RASS for the overall population can be seen in Table 4. A RASS/m-RASS other than 0 was 70.5% (95%CI: 65.9% – 75.1%) sensitive and 84.8% (95%CI: 80.5% – 89.1%) specific. The specificity of the RASS/m-RASS incrementally increased with higher degrees of impairment increasing to 95.5% with a RASS/m-RASS value >+1 or <−1 but at the expense of sensitivity.
Table 4.
Diagnostic performance of the Richmond Agitation Sedation Scale/modified Richmond Agitation Sedation Scale for delirium for all patients and stratified by delirium standard and dementia ascertainment method.
| Stratified | Cut-off | Sensitivity (95%CI) | Specificity (%95CI) * | +LR (95%CI) * | −LR (95%CI) * | |
|---|---|---|---|---|---|---|
| Total n = 632 |
RASS other than 0 |
69.8% (65.1% – 74.5%) | 86.0% (81.9% – 90.2%) | 5.00 (3.68 – 6.79) | 0.35 (0.30 – 0.41) | |
| RASS >+1 or <−1 |
28.9% (24.2% – 33.5%) | 97.0% (94.9% – 99.0%) | 9.57 (4.75 – 19.29) | 0.73 (0.68 – 0.78) | ||
| Stratified by delirium reference standard |
DSM n= 188 |
RASS other than 0 |
83.0% (75.1% – 90.8%) | 83.0% (75.6% – 90.4%) | 4.88 (3.13 – 7.60) | 0.21 (0.13 – 0.33) |
| RASS >+1 or <−1 |
21.6% (13.0% – 30.2%) | 98.0% (95.3% – 100.0%) | 10.80 (2.59 – 45.05) | 0.80 (0.71 – 0.90) | ||
| DRS-R-98 n = 40 |
RASS other than 0 |
92.5% (84.3% – 100.0%) | - | - | - | |
| RASS >+1 or <−1 |
62.5% (47.5% – 77.5%) | - | - | - | ||
| 4AT n = 404 |
RASS other than 0 |
61.1% (54.9% – 67.3%) | 87.9% (82.9% – 92.9%) | 5.04 (3.30 – 7.69) | 0.44 (0.37 – 0.52) | |
| RASS >+1 or <−1 |
25.9% (20.4% – 31.5%) | 96.4% (93.5% – 99.2%) | 7.13 (3.17 – 16.10) | 0.77 (0.71 – 0.83) | ||
| Stratified by dementia ascertainment method |
CDR n = 35 |
RASS other than 0 |
68.6% (53.2% – 84.0%) | - | - | - |
| RASS >+1 or <−1 |
14.3% 2.7% – 25.9%) | - | - | - | ||
| Clinical records n = 444 |
RASS other than 0 |
65.6% (60.0% – 71.2%) | 87.9% (82.9% – 92.9%) | 5.41 (3.56 – 8.23) | 0.39 (0.33 – 0.46) | |
| RASS >+1 or <−1 |
31.2% (25.7% – 36.6%) | 96.4% (93.5% – 99.2%) | 8.58 (3.84– 19.7) | 0.71 (0.66 – 0.78) | ||
| SF_IQCODE n = 153 |
RASS other than 0 |
92.5% (85.3% – 99.6%) | 83.0% (75.6% – 90.4%) | 5.44 (3.50 – 8.44) | 0.09 (0.04 – 0.23) | |
| RASS >+1 or <−1 |
26.4% (14.5% – 38.3%) | 98.0% (95.3% – 100.0%) | 13.21 (3.12 – 55.95) | 0.75 (0.64 – 0.88) |
The empty cell indicates that the diagnostic characteristic could not be calculated because there were no patients without delirium in that subgroup.
+LR = positive likelihood ratio; −LR = negative likelihood ratio
When stratified by delirium reference standard, the specificities of each RASS/m-RASS cut-off were remarkably consistent. However, the RASS/m-RASS’ sensitivity was more variable based upon the delirium reference standard used. For example, a RASS/m-RASS other than 0 was 62.5% (95% CI: 56.5% – 68.5%) when the 4AT was used as the delirium reference standard, whereas the sensitivity was 92.5% with the DRS-R-98. The sensitivity and specificity of the RASS/m-RASS remained consistent also when stratified by care location (Appendix 1).
The RASS/m-RASS’ specificities for all cut-offs were also similar between dementia ascertainment methods. However, the RASS/m-RASS’ sensitivity was more variable based upon the dementia ascertainment method used. For a RASS/m-RASS other than 0, the sensitivity was 66.7% (95% CI 53.2% – 84.0%) when the CDR was used to define patients with dementia, whereas the sensitivity was 92.5% (95% CI: 85.3% to 99.6%) when the SF-IQCODE was used.
DISCUSSION
In this multicenter prospective cohort study we found the RASS/m-RASS, analyzed in a combined dataset, to be specific for the diagnosis of DSD in older patients admitted to acute geriatric wards, inhospital rehabilitation, emergency department, especially in patients with higher degrees of impaired level of consciousness (RASS/m-RASS value >+1 or <−1). Overall a RASS/m-RASS score other than 0 had a good specificity which progressively increased with a RASS/m-RASS value >+1 or <−1. The RASS/m-RASS’ specificity did not greatly vary according to the method of delirium diagnosis and the dementia ascertainment, though the sensitivity was slightly higher when the DSM and the SF-IQCODE were used to diagnose delirium and to detect the presence of dementia, respectively.
Our findings are in line with previous studies investigating the accuracy of the RASS and the m-RASS for the detection of delirium.11–13 However, none of the previous studies has focused specifically on patients with delirium and dementia. Han and colleagues12 found a RASS score other than 0 to have a very good sensitivity (84%, 95% CI: 73.8% to 94.2%) and specificity (87.6%, 95 % CI: 84.2% to 91.1%) for the detection of delirium in 406 older patients admitted to an emergency department (ED). Interestingly, a RASS score > +1 or < –1 was nearly diagnostic for delirium in this population,12 providing the busy ED setting with a fast and valuable tool for the screening-diagnosis of delirium. Two other studies have investigated the performance of the RASS for delirium assessment in other clinical settings. Chester and colleagues reported that any abnormal m-RASS score in a single-day evaluation had high specificity for delirium detection in 95 older medical patients.11 The specificity increased to 99.6% and the sensitivity fell to 34% when considering an abnormal m-RASS ≥2 or ≤2.11 In 30 acute hip fracture older patients the RASS had a high sensitivity and specificity for delirium.13 The high specificity and lower sensitivity of the RASS/m-RASS in our study is similar to the study of Chester and colleagues,11 with higher RASS/m-RASS scores almost diagnostic for delirium. These findings are also supported by findings from the initial 4AT validation study, in which the binary level of alertness item (normal/abnormal) showed 96.1% specificity and 53.2% sensitivity for delirium in an inpatient geriatric population.17
The findings are of interest considering the difficulties in the detection of delirium in patients with dementia and they also reflect previous investigation evaluating the fluctuation of motor performances for the detection of delirium. Indeed the RASS/m-RASS are measures of arousal, level of sedation and consciousness. However, the negative and positive scores have also been used to characterize motor performances in patients with delirium.18, 19, 23 Motor performances have indeed been shown in previous investigations to be useful for DSD detection. Bellelli reported a clear change in the functional status of older patients with DSD and with a recovery after delirium resolution.24 Similarly, Meagher and colleagues found a higher prevalence of motor retardation and agitation in patients with DSD compared to patients with dementia alone.25 Interestingly, Voyer26 recently validated the RADAR, a new delirium screening tool for hospital and nursing home including patients with dementia. The tool requires the nurse evaluating the patients to answer three questions while administering the medications 1) Was the patient drowsy?; 2) Did the patient have trouble following your instructions?; 3) Were the patient’s movements slowed down? In the validation study item 3 (i.e. were the patient’s movements slowed down) was the item most commonly listed as positive (88%).26 The importance of motor fluctuations in the evaluation of patients with dementia has also been acknowledged in a recent survey of delirium experts.8 Most of the responders have indicated the RASS as a possible measure of motor fluctuation but to date there is not a clear indication. Indeed others have indicated the Glasgow Coma Scale or the Observational Scale of Level of Arousal (OSLA)13 as alternative measures of motor fluctuation. Additionally the easy of use of the RASS could indeed promote its application for a rapid first evaluation of delirium or for chart review purposes. Indeed, the m-RASS has been advocated for its simplicity and brevity to be used in delirium prediction models.27 The RASS has also been shown to be a good marker of negative outcomes (i.e., hospital stay, discharge to skilled nursing facility, and mortality) in critically ill patients and acute medical older patients.28–30
Flaherty and colleagues have previously suggested the importance of including the assessment of the mental status as the sixth vital sign.31 It is well accepted to measure daily blood pressure, heart rate, respiratory rate, temperature and pain but it is not a standard of care to include a measure of mental status. Alertness was suggested as a simple way to evaluate mental status at the bedside, increasing the attention of health care providers to the detection of delirium and its management. The RASS/m-RASS might be well suitable for this scope, given the easy of its use and its ability to signaling delirium, especially in dementia patients. Indeed there has been a proposal to use a pictorial representation of the RASS using older male patients’ faces to facilitate the use of this scale by a wide range of health care providers.32 Therefore future studies should further evaluate the possibilities to integrate this proposed method similarly to the visual analogic scale for pain evaluation.
Other authors have also tried to increase the detection of delirium providing medical personnel with screening methods. Measures of inattention – a cardinal feature of delirium-have been proposed for this purpose. Indeed the “months of the year backwards" was found to have a sensitivity of 83% (95% confidence interval [CI]: 69%–93%) and specificity of 69% (95% CI: 61%–76%) for delirium detection.33 The best 2-item screen was the combination of "months of the year backwards" and "what is the day of the week?" with a sensitivity of 93% (95% CI: 81%–99%) and specificity of 64% (95% CI: 56%–70%).33 In another study the simultaneous evaluation of the “months of the year backwards" and the assessment for subjective/objective confusion provided a sensitivity of 93.8% (95% CI: 82.8%–98.6%) and a specificity of 84.7% (95% CI: 79.2%–89.2%).34 Nonetheless, patients with severe inattention might not be formally evaluated with the use of tests such as the “months of the year backwards" leading clinicians to consider these patients as untestable with the risk of missing delirium in the most severe cases. The use of the RASS/m-RASS as a screening method might overcome these limitations.
Our study has important strengths. This is the first multicenter observational study evaluating the performances of the RASS and m-RASS for the detection of delirium in a large group of patients with dementia in different clinical settings. The diagnosis of delirium was carried out with rigorous evaluations. We were able to compare the performances of the RASS and m-RASS using different tools to diagnose delirium and with different methods to evaluate the presence of dementia. Limitations of the study include a greater number of patients evaluated with the 4AT. However, this tool has been validated against the DSM-IV criteria and indeed the findings of the performance of the RASS/m-RASS to detect delirium were similar when the 4AT and DSM criteria were used.
CONCLUSION
In older patients admitted to different clinical settings the RASS and/or m-RASS are useful tools for delirium screening in patients with dementia. A RASS/m-RASS score other than 0 has moderate sensitivity and high specificity for the detection of delirium in patients with dementia. The accuracy of the tests increases with a higher degree of impairment of level of consciousness. The sensitivity and the specificity did not greatly vary according to the method of delirium diagnosis and the dementia ascertainment, though the specificity was slightly higher when the DSM and the SF-IQCODE were used to diagnose delirium and to detect the presence of dementia. The RASS or m-RASS are very quick and should be part of delirium screening strategies in routine practice. However, because the sensitivity is only moderate (because not all patients with DSD have altered level of consciousness), additional methods such as informant history and cognitive testing would increase the rates of detection.
Supplementary Material
Table 2.
Description of setting, delirium assessment and dementia ascertainment according to the enrollment site
| Enrollment | Setting | Delirium diagnosis |
Diagnosis of dementia |
|---|---|---|---|
| Group 1 | |||
| -Cremona (Italy) | Inhospital rehabilitation | DSM-IV | SF-IQCODE |
| -Limerick (Ireland) | Old-age psychiatry consultation-liaison |
DSM-IV | SF-IQCODE |
| -Coimbra (Portugal) | Psychiatry, acute geriatric ward |
DSM-IV | SF-IQCODE |
| -Basel (Switzerland) | General surgery and orthopedic surgery |
DSM-IV | SF-IQCODE |
| -Monza (Italy) | Acute geriatric ward | DSM-IV | SF-IQCODE |
| Group 2 | |||
| Monza (Italy) | Acute geriatric ward | 4AT | Clinical records |
| Group 3 | |||
| Cremona (Italy) | Inhospital rehabilitation | DSM-IV | Clinical Dementia Rating Scale (CDR) |
| Group 4 | |||
| Limerick (Ireland) | Palliative care | DRS-R-98 | Clinical records |
| Group 5 | |||
| Nashville (TN, USA) | Emergency Department | DSM-IV | SF-IQCODE |
Short Form Informant Questionnaire on Cognitive Decline in the Elderly (SF-IQCODE).
Acknowledgments
None
Footnotes
Conflict of interests: None
Bibliography
- 1.Fick DM, Agostini JV, Inouye SK. Delirium superimposed on dementia: a systematic review. Journal of the American Geriatrics Society. 2002;50:1723–1732. doi: 10.1046/j.1532-5415.2002.50468.x. [DOI] [PubMed] [Google Scholar]
- 2.Sampson EL, Blanchard MR, Jones L, Tookman A, King M. Dementia in the acute hospital: prospective cohort study of prevalence and mortality. The British journal of psychiatry : the journal of mental science. 2009;195:61–66. doi: 10.1192/bjp.bp.108.055335. [DOI] [PubMed] [Google Scholar]
- 3.Fick DM, Steis MR, Waller JL, Inouye SK. Delirium superimposed on dementia is associated with prolonged length of stay and poor outcomes in hospitalized older adults. Journal of hospital medicine : an official publication of the Society of Hospital Medicine. 2013;8:500–505. doi: 10.1002/jhm.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellelli G, Frisoni GB, Turco R, Lucchi E, Magnifico F, Trabucchi M. Delirium superimposed on dementia predicts 12-month survival in elderly patients discharged from a postacute rehabilitation facility. The journals of gerontology Series A, Biological sciences and medical sciences. 2007;62:1306–1309. doi: 10.1093/gerona/62.11.1306. [DOI] [PubMed] [Google Scholar]
- 5.Morandi A, Davis D, Fick DM, Turco R, Boustani M, Lucchi E, Guerini F, Morghen S, Torpilliesi T, Gentile S, Maclullich AM, Trabucchi M, Bellelli G. Delirium superimposed on dementia strongly predicts worse outcomes in older rehabilitation inpatients. Journal of the American Medical Directors Association. 2014;15:349–354. doi: 10.1016/j.jamda.2013.12.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richardson S, Teodorczuk A, Bellelli G, Davis DH, Neufeld KJ, Kamholz BA, Trabucchi M, MacLullich AM, Morandi A. Delirium superimposed on dementia: a survey of delirium specialists shows a lack of consensus in clinical practice and research studies. International psychogeriatrics / IPA. 2015:1–9. doi: 10.1017/S1041610215002288. [DOI] [PubMed] [Google Scholar]
- 7.Tieges Z, Brown LJ, MacLullich AM. Objective assessment of attention in delirium: a narrative review. International journal of geriatric psychiatry. 2014;29:1185–1197. doi: 10.1002/gps.4131. [DOI] [PubMed] [Google Scholar]
- 8.Morandi A, McCurley J, Vasilevskis EE, Fick DM, Bellelli G, Lee P, Jackson JC, Shenkin SD, Marcotrabucchi Schnelle J, Inouye SK, Ely EW, MacLullich A. Tools to detect delirium superimposed on dementia: a systematic review. J Am Geriatr Soc. 2012;60:2005–2013. doi: 10.1111/j.1532-5415.2012.04199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O'Neal PV, Keane KA, Tesoro EP, Elswick RK. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338–1344. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 10.Ely EW, Truman B, Shintani A, Thomason JW, Wheeler AP, Gordon S, Francis J, Speroff T, Gautam S, Margolin R, Sessler CN, Dittus RS, Bernard GR. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS) JAMA : the journal of the American Medical Association. 2003;289:2983–2991. doi: 10.1001/jama.289.22.2983. [DOI] [PubMed] [Google Scholar]
- 11.Chester JG, Beth Harrington M, Rudolph JL. Serial administration of a modified Richmond Agitation and Sedation Scale for delirium screening. Journal of hospital medicine : an official publication of the Society of Hospital Medicine. 2012;7:450–453. doi: 10.1002/jhm.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han JH, Vasilevskis EE, Schnelle JF, Shintani A, Dittus RS, Wilson A, Ely EW. The Diagnostic Performance of the Richmond Agitation Sedation Scale for Detecting Delirium in Older Emergency Department Patients. Acad Emerg Med. 2015;22:878–882. doi: 10.1111/acem.12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tieges Z, McGrath A, Hall RJ, Maclullich AM. Abnormal level of arousal as a predictor of delirium and inattention: an exploratory study. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2013;21:1244–1253. doi: 10.1016/j.jagp.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Leonard M, McInerney S, McFarland J, Condon C, Awan F, O'Connor M, Reynolds P, Meaney AM, Adamis D, Dunne C, Cullen W, Trzepacz PT, Meagher DJ. Comparison of cognitive and neuropsychiatric profiles in hospitalised elderly medical patients with delirium, dementia and comorbid delirium-dementia. BMJ open. 2016;6:e009212. doi: 10.1136/bmjopen-2015-009212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leonard MM, Agar M, Spiller JA, Davis B, Mohamad MM, Meagher DJ, Lawlor PG. Delirium diagnostic and classification challenges in palliative care: subsyndromal delirium, comorbid delirium-dementia, and psychomotor subtypes. Journal of pain and symptom management. 2014;48:199–214. doi: 10.1016/j.jpainsymman.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 16.Voyer P, Cole MG, McCusker J, Belzile E. Prevalence and symptoms of delirium superimposed on dementia. Clinical nursing research. 2006;15:46–66. doi: 10.1177/1054773805282299. [DOI] [PubMed] [Google Scholar]
- 17.Bellelli G, Morandi A, Davis DH, Mazzola P, Turco R, Gentile S, Ryan T, Cash H, Guerini F, Torpilliesi T, Del Santo F, Trabucchi M, Annoni G, Maclullich AM. Validation of the 4AT, a new instrument for rapid delirium screening: a study in 234 hospitalised older people. Age and ageing. 2014;43:496–502. doi: 10.1093/ageing/afu021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morandi A, Lucchi E, Turco R, Morghen S, Guerini F, Santi R, Gentile S, Meagher D, Voyer P, Fick DM, Schmitt EM, Inouye SK, Trabucchi M, Bellelli G. Delirium superimposed on dementia: A quantitative and qualitative evaluation of informal caregivers and health care staff experience. Journal of psychosomatic research. 2015;79:272–280. doi: 10.1016/j.jpsychores.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morandi A, Lucchi E, Turco R, Morghen S, Guerini F, Santi R, Gentile S, Meagher D, Voyer P, Fick D, Schmitt EM, Inouye SK, Trabucchi M, Bellelli G. Delirium superimposed on dementia: A quantitative and qualitative evaluation of patient experience. Journal of psychosomatic research. 2015;79:281–287. doi: 10.1016/j.jpsychores.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berg L. Clinical Dementia Rating (CDR) Psychopharmacology bulletin. 1988;24:637–639. [PubMed] [Google Scholar]
- 21.Jorm AF, Scott R, Cullen JS, MacKinnon AJ. Performance of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) as a screening test for dementia. Psychological medicine. 1991;21:785–790. doi: 10.1017/s0033291700022418. [DOI] [PubMed] [Google Scholar]
- 22.Jabbar F, Leonard M, Meehan K, O'Connor M, Cronin C, Reynolds P, Meaney AM, Meagher D. Neuropsychiatric and cognitive profile of patients with DSM-IV delirium referred to an old age psychiatry consultation-liaison service. International psychogeriatrics / IPA. 2011;23:1167–1174. doi: 10.1017/S1041610210002383. [DOI] [PubMed] [Google Scholar]
- 23.Peterson JF, Pun BT, Dittus RS, Thomason JW, Jackson JC, Shintani AK, Ely EW. Delirium and its motoric subtypes: a study of 614 critically ill patients. Journal of the American Geriatrics Society. 2006;54:479–484. doi: 10.1111/j.1532-5415.2005.00621.x. [DOI] [PubMed] [Google Scholar]
- 24.Bellelli G, Speciale S, Morghen S, Torpilliesi T, Turco R, Trabucchi M. Are fluctuations in motor performance a diagnostic sign of delirium? Journal of the American Medical Directors Association. 2011;12:578–583. doi: 10.1016/j.jamda.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 25.Meagher DJ, Leonard M, Donnelly S, Conroy M, Saunders J, Trzepacz PT. A comparison of neuropsychiatric and cognitive profiles in delirium, dementia, comorbid delirium-dementia and cognitively intact controls. Journal of neurology, neurosurgery, and psychiatry. 2010;81:876–881. doi: 10.1136/jnnp.2009.200956. [DOI] [PubMed] [Google Scholar]
- 26.Voyer P, Champoux N, Desrosiers J, Landreville P, McCusker J, Monette J, Savoie M, Carmichael PH, Richard H, Richard S. RADAR: A Measure of the Sixth Vital Sign? Clinical nursing research. 2016;25:9–29. doi: 10.1177/1054773815603346. [DOI] [PubMed] [Google Scholar]
- 27.Rudolph JL, Doherty K, Kelly B, Driver JA, Archambault E. Validation of a Delirium Risk Assessment Using Electronic Medical Record Information. Journal of the American Medical Directors Association. 2015 doi: 10.1016/j.jamda.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 28.Yevchak AM, Han JH, Doherty K, Archambault EG, Kelly B, Chandrasekhar R, Ely EW, Rudolph JL. Impaired Arousal in Older Adults Is Associated With Prolonged Hospital Stay and Discharge to Skilled Nursing Facility. Journal of the American Medical Directors Association. 2015;16:586–589. doi: 10.1016/j.jamda.2015.01.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bellelli G, Mazzone A, Morandi A, Latronico N, Perego S, Zazzetta S, Mazzola P, Annoni G. The Effect of an Impaired Arousal on Short- and Long-Term Mortality of Elderly Patients Admitted to an Acute Geriatric Unit. Journal of the American Medical Directors Association. 2015 doi: 10.1016/j.jamda.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Vasilevskis EE, Pandharipande PP, Graves AJ, Shintani A, Tsuruta R, Ely EW, Girard TD. Validity of a Modified Sequential Organ Failure Assessment Score Using the Richmond Agitation-Sedation Scale. Critical care medicine. 2016;44:138–146. doi: 10.1097/CCM.0000000000001375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flaherty JH, Rudolph J, Shay K, Kamholz B, Boockvar KS, Shaughnessy M, Shapiro R, Stein J, Weir C, Edes T. Delirium is a serious and under-recognized problem: why assessment of mental status should be the sixth vital sign. Journal of the American Medical Directors Association. 2007;8:273–275. doi: 10.1016/j.jamda.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 32.Flaherty JH, Shay K, Weir C, Kamholz B, Boockvar KS, Shaughnessy M, Shapiro R, Gordon S, Stein J, Rudolph JL. The development of a mental status vital sign for use across the spectrum of care. Journal of the American Medical Directors Association. 2009;10:379–380. doi: 10.1016/j.jamda.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 33.Fick DM, Inouye SK, Guess J, Ngo LH, Jones RN, Saczynski JS, Marcantonio ER. Preliminary development of an ultrabrief two-item bedside test for delirium. Journal of hospital medicine : an official publication of the Society of Hospital Medicine. 2015;10:645–650. doi: 10.1002/jhm.2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Regan NA, Ryan DJ, Boland E, Connolly W, McGlade C, Leonard M, Clare J, Eustace JA, Meagher D, Timmons S. Attention! A good bedside test for delirium? Journal of neurology, neurosurgery, and psychiatry. 2014;85:1122–1131. doi: 10.1136/jnnp-2013-307053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.