Abstract
Evidence gleaned from recent studies on the role of tumor-infiltrating lymphocytes (TILs) suggests that cancer is not only a genetic disease but also an immunologic disease. Head and Neck Squamous Cell Carcinoma (HNSCC) has been a significant model to study cancer cell-immune cell interactions. First, immune cell infiltration is an important feature of these tumors. Second, HNSCC frequently develops resistance to immunogenic cytotoxicity, which provides a window to decipher how tumors engage the immune system to establish immune tolerance. Finally, chemoradiation therapy, as a central modality for HNSCC treatment, has been shown to elicit immune activation. The presence of effector immune cells in the tumor microenvironment is often associated with superior clinical response to adjuvant therapy. On the other hand, an activated immune system, in addition to limiting tumor initiation and progression, could also exert selective pressure to promote the growth of less immunogenic tumors, as a pivotal immunoediting process. But it remains unclear how cancer cell signaling regulates tumor immunogenicity and how to mitigate HNSCC-potentiated TIL suppression. In this review, we will revisit the prognostic role of TILs in HNSCC, and collectively discuss how cancer cell machinery impacts upon the plasticity of TILs.
Keywords: Tumor-Infiltrating Lymphocytes (TIL), Innate Immunity, Oral Cancer, Head and Neck Cancer, Type 1 Interferon, Autophagy, Immunogenicity, Prognosis, Anti-Tumor Immunity, Immunotherapy
Head and Neck Squamous Cell Carcinomas (HNSCC) are comprised of a number of epithelial malignancies arising in the mucosa of the upper aerodigestive tract. Immune cell infiltration is a prominent pathologic feature of HNSCC. First, due to the close proximity of these tumors to lymphoid tissue in the Waldeyer’s ring, dense infiltrates of immune cells are frequently an integral component of the lesion. In addition, some types of HNSCC are driven by viral infection, such as human papillomavirus (HPV)-associated oropharyngeal squamous cell carcinoma, and Epstein-Barr virus (EBV)-associated nasopharyngeal carcinoma. Both types of HNSCC contain dense lymphocytic infiltrates and are believed to evolve with immune cells specific for viral antigens. In fact, some of the earliest literature on TILs were focused on the B- T- cell subpopulation characterization in nasopharyngeal carcinoma [1]. Last but not least, standard care of HNSCC patients, such as chemoradiation therapy, could elicit anti-tumor immune response. Classification of tumors based on the dynamics and phenotypes of TILs is rapidly emerging as a powerful prognostic tool to guide treatment. Initial descriptions of TILs appeared in the literature about 40 years ago [1], this review will discuss some of the most commonly studied historical topics on the clinical value of TILs, and the most urgent questions that challenge tumor immunology research related to HNSCC.
Does the number of T-cells in the tumor microenvironment matter?
Although TILs had been appreciated as a component of solid tumors in 1976, it was not until 10 years later that the subpopulations of TILs in HNSCC were characterized [2]. In the initial cohort, 40 patients with HNSCC were included, and short-term disease-free interval and actuarial survival were correlated with the number of CD4+ infiltrating T-cells [2]. In addition, the earlier stages of HNSCC were associated with greater number of TILs [2, 3]. These findings promoted enthusiasm for designing novel neoadjuvant clinical trials for immunotherapy. Early attempts of immunotherapeutic approaches focused on the elicitation and expansion of effector cells. These trials frequently employed an IL-2-based regimen, aiming to promote the expansion of effector immune cells. Peritumoral injection of IL-2 indeed increased the percentage of CD3−CD56+ NK cells [4]. Although one study showed that perilymphatic IL-2 injection did not change the number of T-cells in peripheral blood and restore suppressed immune function [5], another group showed that regional perilymphatic injections of a mixture of cytokines significantly increased the total lymphocyte counts, CD3+ T-cells, CD4+ T-cells, and CD8+ T-cells. The overall lymphocytic infiltration increased 4.7 fold [6]. Guided by a similar philosophy of promoting the proliferation of effector immune cells, tumor vaccine clinical trials were later developed to increase the specificity and intensity of anti-tumor immune response. Unfortunately, these clinical trials did not appear to significantly improve the survival of HNSCC patients, which has remained almost unchanged in the past several decades [7, 8]. These observations may be in part due to the fact that cytokines and the components of early vaccines have a relatively short half-life, and cannot continuously elicit an “inflamed” tumor microenvironment to promote effector immune cell homing to the tumor.
The recent revitalized interest in immunotherapy prompts many studies on the prognostic value of TILs. The results are remarkably consistent with what was found 40 years ago. A recent study examined formalin-fixed paraffin-embedded (FFPE) HNSCC tissues from 161 patients with a median follow-up of 48 months. High CD3 or CD8 expression on TILs was considered an independent factor for favorable overall survival, local progression-free survival, and distant metastases-free survival [9]. Although in a small cohort of oral cavity carcinoma patients, the number of CD4 or CD8 T-cells was not associated with overall survival [10], a recent larger retrospective study with 278 patients showed that increased CD4 and CD8 TIL levels were significant independent prognostic variables associated with favorable overall survival and recurrence-free survival [11]. A comprehensive meta-analysis of the prognostic value of the number of TILs for HNSCC patients has not been published; but meta-analyses for patients with lung cancer and breast cancer showed that high TIL numbers were independently associated with a favorable outcome in both cancer types [12, 13]. Notably, HPV-driven HNSCC has a distinct clinical response profile to chemoradiation therapy. Although HPV+ HNSCC is often accompanied by nodal metastasis, the overall survival and progression-free survival are usually longer than HPV− tumors. One hypothesis is that the immune response against viral antigens could drive the elimination of tumor cells [14, 15]. In a recent study, the number of TILs was used to classify HPV+ tumors into different risk groups, with the most dense immune infiltrates being associated with a significantly improved survival [16]. Specifically, CD8+ and FOXP3+ T-cells were positively associated with disease-specific survival in patients with HPV+ oropharyngeal cancer [17].
Radiotherapy and chemotherapy are two major treatment modalities for HNSCC patients. In addition to the direct induction of DNA damage in tumor cells with irradiation or a platinum-based regimen, anti-tumor immune activation has been repetitively identified as an important mechanism underlying the treatment efficacy [18]. In agreement with this notion, high levels of CD3+ CD8+ T-cell infiltration are indicative of a favorable response to chemoradiotherapy [19]. A study shows that the prognostic value of CD3+ and CD8+ T-cells depends on the infiltration pattern. It is the TILs that are within the tumor cells or in close proximity to tumor parenchyma that would bear prognostic value. In contrast, CD3+ T-cells in the tumor stroma or the periphery of the tumor islands do not exhibit a prognostic value [20]. In our recent study, we noticed that above median levels of CD4+, CD8+, and FOXP3+ T-cell infiltration was correlated with extended survival in patients who received chemoradiation therapy. Interestingly, in patients receiving surgery, only a modest improvement in overall survival was noticed in those with high CD8 counts [11]. We have also previously noted that T-cells in peripheral blood also bear prognostic value. In an induction chemotherapy Phase II trial to identify HNSCC patients that were sensitive to chemoradiation therapy, CD4+ T-cells in the peripheral blood could predict response to neoadjuvant chemotherapy in patients with laryngeal cancer, but not with oropharyngeal tumors [21]. In an analysis of 124 articles that focus on the prognostic values of T-cells, the presence of CD8 cytotoxic T-cells appears to be generally associated with an improved survival [22].
Regulatory T (Treg) cell infiltration is another frequently studied biomarker for prognosis. Most studies use CD4+CD25+FOXP3+ population as markers for Tregs. Treg shares many common activation markers with effector T-cells, such as OX40, CTLA4, and PD-1. But due to its intrinsic immunosuppressive function, checkpoint blockade in this population of T-cells does not reverse its suppressive property, in contrast to functional restoration of “exhausted” effector T-cells. Despite its inhibitory impact on the anti-tumor Tc1/TH1 response, FOXP3+ Tregs were associated with favorable outcome in several types of solid tumors [23]. In a small cohort of 35 HNSCC patients, FOXP3+ Tregs were found to be related to prolonged overall survival [24]. A more recent study using FFPE specimens from 278 patients also suggested that high level of FOXP3+ cells were associated with a favorable prognosis [11]. In addition, CD4+ FOXP3+ T-cells were positively correlated with superior locoregional control [25]. Consistently, in a pan-solid tumor analysis, Treg infiltrates appeared to be associated with a favorable survival outcome [23]. Although it is not entirely unclear why higher Treg infiltrates were found to be beneficial in clinical outcomes, Tregs are usually enriched in a pro-Tc1/TH1 environment so that immune activation is kept in check. Thus the presence of high level of Tregs may suggest a background of high Tc1/TH1 response that promotes a favorable outcome. Indeed, multiple immunosuppressive markers, such as indoleamine-2,3-dioxygenase (IDO), programmed death ligand 1 (PD-L1), and FOXP3, were associated with T-cell-inflamed tumors and a better prognosis [26, 27]. FOXP3+CD4+ T-cells were found to be in the same area as CD8+ T-cells [26]. As these immunosuppressive markers are downstream targets of both type I and type II interferon [28], the evidence collectively suggests that these inhibitory markers were induced because of the presence of a functional effector T-cell infiltrate and a favorable “inflamed” tumor microenvironment. A meta-analysis of the prognostic value of Treg numbers in HNSCC would be confirmative of these reported associations.
T helper 17 (TH17) cells inform a T-cell subpopulation that promotes anti-tumor immunity. A recent phenotypic analysis of TH17 cells revealed that they resembled terminally differentiated memory T-cells, and strongly promoted long-term anti-tumor immunity [29]. Increased TH17 population was associated with a better prognosis in patients with cervical adenocarcinoma [30]. A cytokine macrophage inhibitory factor (MIF) that strongly promoted the generation of TH17 was significantly associated with a better survival in patients with nasopharyngeal carcinomas [31].
T-cell-based immunoscore represents an emerging dimension for cancer classification. Although a T-cell-centered immunoscore for oral cancer is still under development, it has shown promising prognostic value in colorectal cancer. The presence of high density of CD45RO+ memory T-cells was significantly correlated with an increased expression of Tc1/TH1 genes [32]. A combinatorial assessment of CD8+ and CD45RO+ T-cells in the center of the tumor and invasive margins showed that high densities of these tumor-associated T-cell populations were indicative of a significantly improved overall survival and disease-free survival [32]. Intriguingly, the T-cell-based stratification system was found superior to conventional histopathologic methods [33]. Microsatellite instability is a genetic feature in a subset of colorectal tumors, and it is often linked to a favorable prognosis. Notably, microsatellite-instable tumors exhibited increased densities of TH1, effector, and memory T-cell populations. A T-cell-based immunoscore could even better predict patients’ prognosis than microsatellite instability [34]. A cytotoxic immune signature was inversely correlated with distant metastasis in colorectal cancer patients [35]. One potential challenge of using a T-cell-based immunoscore as a prognostic marker is that the quantitation methods of T-cells usually vary among different research groups and institutions. Recent advances in digital pathology encourage the utilization of quantitative immunohistochemistry and quantitative multiplex immunofluorescence as part of the scoring practice. This effort also confirmed the correlation between a high CD8+ T-cell count and a favorable clinical response of melanoma patients to treatment [36].
Are immune cells in the tumor microenvironment different from those in the peripheral blood?
As early as in late 1980s, effector immune cells in tumors were found to be more suppressed than those in peripheral blood [37]. For example, NK cells-mediated tumor lysis activity was severely damaged in TILs and those from the tumor-containing draining lymph nodes [37]. About the same time, significant differences in effector function were noted between TILs and lymphocytes from the peripheral blood in oral cancer patients [38]. The past several decades have witnessed a significant increase in our knowledge of the sub-classification of T-cell populations, markers of different T-cell subsets, and checkpoint receptor signaling pathways. In agreement with previous findings, a recent study showed that TILs of HNSCC demonstrated significantly suppressed Tc1/TH1 phenotype in comparison with CD8+ T-cells from matching patients’ peripheral blood [39]. Importantly, a phosphatase SHP-2 was found to be overexpressed in TILs and correlated with the checkpoint receptor PD-1 expression. In addition, SHP-2 activation suppressed phospho-STAT1/T-bet-mediated Tc1/TH1 response [39]. Consistent with these findings, the subpopulations positive for the checkpoint receptors, such as CTLA-4, TIM-3, and PD-1, were significantly more frequent in the Tregs within the tumor microenvironment, compared to the Tregs in circulating blood [40]. Tregs from the TILs also exhibited more potent inhibitory effect than those from the peripheral blood [40]. However, the pro-TH1 population TH17 cells in the TILs demonstrated enhanced capability of producing IFN-γ compared to their counterparts in the peripheral blood [31].
Are non-T-cell TILs bystanders?
Despite the predominance of T-cells in the TIL population, almost all types of immune cells including natural killer (NK) cells, neutrophils, B-cells, macrophages, and dendritic cells are present in the tumor microenvironment. The presence and functional significance of these subsets remain to be fully elucidated, but there is increasing evidence that B cells, neutrophils and complex crosstalk via cytokines may influence whether the environment is tumorigenic or cytotoxic. The relationship between cancer and inflammation is complex and usually context-dependent. Although pro-inflammatory responses and chronic inflammation mediated by the aforementioned immune cells have been reported to promote cancer initiation [41], proper inflammatory response is critical in establishing an effective anti-tumor Tc1/TH1 response in established tumor cells.
In a recent study of neutrophils in the TIL, an analysis of 90 patients with squamous cell carcinoma of the esophagus showed that an increased neutrophil infiltrate was a poor prognostic factor, and that the peritumoral neutrophil-to-lymphocyte ratio was significantly associated with disease progression in patients who received curative surgery [42]. Interestingly, a recent comprehensive bioinformatics study involving ~18,000 human tumors of 39 types of malignancy revealed consistent results that neutrophils were markers for poor clinical responses. In the same study, increased level of T-cells was correlated with improved survival; and intra-tumoral γδT-cells emerged as the most significant biomarker for a favorable prognosis across all cancer types [23]. Importantly, this study employed a new machine learning tool to interrogate the clinical association of 22 leukocyte subsets. Aside from T-cell subsets, intra-tumoral plasma cells were identified as novel immune biomarkers indicative of superior survival [23]. In agreement with these findings, tumor-infiltrating immune cell profile exhibited distinct patterns between early stage and advanced stage colorectal cancers; and the number of B-cells was positively correlated with survival [43].
How do tumor cells modulate the intensity of immune response?
Pilot clinical results suggested that the response rates to checkpoint inhibitor pembrolizumab in HNSCC patients were about 20%, with the majority of patients non-responsive [44]. There are two major challenges that curb the efficacy of current immunotherapy. (1) How do cancer cells inhibit a “T-cell-inflamed” tumor microenvironment? (2) How do cancer cells establish immune tolerance by interfering with immunogenic cytotoxicity?
One of the most important conceptual advances in tumor immunology is the emerging classification of cancer into immunogenic “hot” tumors and hypoimmunogenic “cold” tumors. Compelling evidence suggests that IFN-I signaling in cancer cells and tumor microenvironment critically modulates the recruitment and activation of effector T-cells. Indeed, IFN-I have been shown to alleviate tumor burden in a wide spectrum of tumor models [45–47]. Cancer cell autonomous IFN-I is indispensable for chemotherapy-induced anti-tumor immunity, and IFN-I signatures in cancer cells were associated with superior prognosis [48]. Similarly, the efficacy of radiotherapy also depends on the activation of IFN-I signaling [49–51]. Mechanistically, radiotherapy triggers DNA damage, which may cause a release of damaged DNA into the cytoplasm [52, 53]. Cytoplasmic DNA is detected by a class of DNA sensors such as cyclic GMP-AMP synthase (cGAS), which produces a second messenger cyclic GMP-AMP (cGAMP) [54, 55]. This second messenger activates the adaptor protein Stimulator of Interferon Genes (STING) to trigger IFN-I signaling (Figure 1) [56, 57]. A number of IFN-I downstream target genes are chemokines, such as CXCL9 and CXCL10, which promote the recruitment of T-cells to tumor bed. Indeed, significantly lower expression levels of CCL1, CCL26, CCR6, CXCL2, CXCL12, CXCL13, and CXCL16 were noticed in cancer; and patients with CXCL13 deletion showed a lower density of B-cells and follicular helper T-cells [43]. Importantly, patients with chemokine defects are more likely to present with disease relapse [43]. Epigenetic silencing of TH1 cytokines CXCL9 and CXCL10 in cancer cells severely compromised the recruitment of effector T-cells, and blockade of epigenetic repression improved checkpoint-targeted immunotherapy [58].
Figure 1. IFN-I signaling in cancer regulates its immunogenicity.
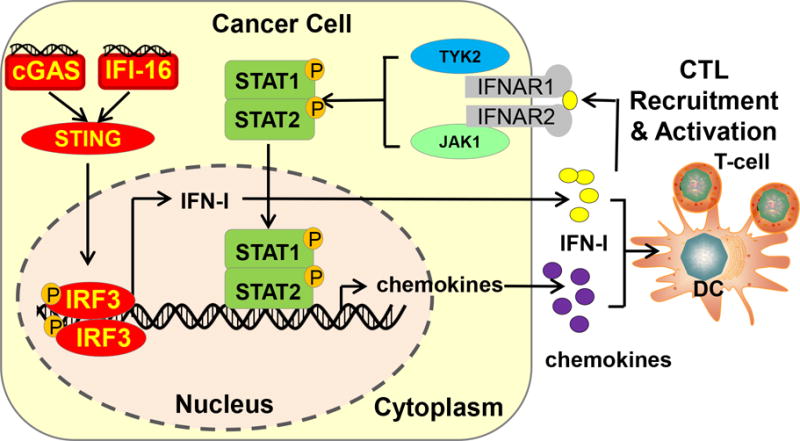
“Hot” immunogenic tumors could be better recognized by the immune system than “cold” tumors. IFN-I-derived from cancer cells could launch a large interferon-stimulated genes (ISG) transcriptional program and promote effector immune cell trafficking to the tumor bed. In addition, IFN-I could also promote APC cross-priming of T-cells.
In addition to IFN-I signaling in tumor cells, IFN-I activation in the tumor microenvironment separates “T-cell-inflamed” tumors from “non-T-cell-inflamed” tumors [59]. IFN-I potently activates antigen-presenting cells and promotes dendritic cell cross-priming. In fact, implantation of tumors in IFN-I receptor-deficient Ifnar1−/− mice lead to significantly increased tumor burden [45]. This IFN-I-primed inflamed status facilitates the T-cell trafficking to tumors. Notably, IFN-I-inducing STING agonists have demonstrated promising adjuvant potential in improving melanomas response to checkpoint block therapy [60]. In addition, because IFN-I and IFN-γ both induce immunosuppressive markers including IDO, PD-L1 and FOXP3+ Tregs, checkpoint blockade may be most effective in tumors with an inflamed microenvironment [46].
Due to the significance of IFN-I signaling in promoting tumor immunogenicity, cancer cells could employ a set of mechanisms to suppress STING-mediated IFN-I activation. However it remains elusive how cancer inhibits IFN-I induction. The discovery of cancer cell factors that modulate IFN-I will likely reveal key molecular machinery underlying tumor immunogenicity. We and others have identified a group of IFN-I “checkpoint” NLRs (NOD-like receptors, also known as nucleotide-binding domain, lots of leucine rich repeats-containing proteins). For example NLRX1, NLRC3, and NLRP4 could all dampen IFN-I signaling [61–67]. This NLR subset usually exhibits broad tissue expression pattern, including cancer cells. Better understanding how these molecules regulate pro-inflammatory signaling in tumor will reveal key mechanistic candidates that dampen T-cell trafficking to tumor microenvironment.
It has been suggested that the genomic mutations in melanoma drive the presentation of tumor-associated mutant neoantigens on the cell surface, which promotes the clonal diversity of anti-tumor immunity and underlies the successful clinical outcome of immunotherapy [68]. Recent studies of HNSCC cancer genomics showed that every HNSCC cell harbors more than 200 mutations [69, 70]; yet patient responses to immunotherapeutic agents are not optimal [71, 72]. Besides possible inhibition of IFN-I signaling, HNSCC may employ other mechanisms to establish immune tolerance. Autophagy, an evolutionarily conserved process that recycles damaged organelles and protein aggregates, has been closely associated with tumor initiation and response to treatment [73]. Most previous studies on autophagy heavily focus on its role in protecting tumor cells from treatment-induced metabolic crisis. Indeed, independent groups have found that autophagy promotes resistance in tumor cells to chemoradiation therapy [73]. Recently, it is increasingly appreciated that selective autophagy could potently promote cancer resistance to activated effector immune cells. Both NK and CD8+ CTL deliver cytotoxic proteins, including perforin and GMZB, to tumor cells and activate the extrinsic apoptotic caspase cascade. GMZB is a target of autophagosomes, and can be rapidly degraded by autophagy [74–76]. Deficiency in autophagy-promoting proteins, such as BECN1 or TUFM, increased cancer cell sensitivity to NK-mediated cytotoxicity [63, 77]. Conceptually in agreement, hypoxia-induced autophagy also promoted cancer cell resistance to both NK cells and CTL. Knocking down autophagy-promoting proteins restored the level of GMZB in tumor cells and sensitized tumor to effector immune cells [74, 76].
Autophagy has a context-dependent role in cancer. Genetic evidence shows that autophagy prevents tumor initiation, as disruption of an autophagy-promoting gene BECN1 resulted in increased tumorigenesis [78]. But in established tumors, autophagy promotes resistance to a variety to cytotoxic mechanisms, including immunogenic cytotoxicity [79, 80]. Interestingly, a group found that autophagy may regulate tumor cell immunogenicity through the regulation of the release of danger-associated molecular patterns (DAMP). DAMPs may be secreted by dying tumor cells such as adenosine triphosphate (ATP). ATP could activate the NLRP3-dependent inflammasome, which controls the secretion of mature IL-1β in a caspase-1-dependent fashion [81, 82]. IL-1β and other IL-1β-dependent pro-inflammatory cytokines promote the maturation of dendritic cells. Thus autophagy may regulate cell immunogenicity in an ATP-inflammasome-IL1-β-dependent fashion [83]. In particular, since an autophagy-defect could drive spontaneous tumor development [78], it is possible that evasion from autophagy-dependent immunosurveillance contributes to tumor initiation. Evidence gleaned from TIL studies suggest that cancer is not only a genetic disease, but also an immunologic disease. From an immuno-oncology point of view, the dual roles of autophagy in cancer initiation and response to treatments can be, at least partially, attributed to its impact on cancer cell-immune cell interaction. During the tumor initiation stage, autophagy-regulated ATP release from transforming cells could alert the innate immune system, which protects the host from cancer development. Should this immunosurveillance mechanism fails and tumors become established, autophagy is promoting resistance to immunogenic cytotoxicity by targeting the effector molecule GZMB.
Last but not least, immunogenic cell death is initiated by the effector immune cells, and its outcome depends on the successful activation of the pro-apoptotic caspases cascade [84]. Importantly, recent genomic characterization of HNSCC revealed novel mutations involving cell death pathways, especially mutations affecting CASP8 [85]. The encoded protein Caspase-8 is responsible for activating the extrinsic apoptotic pathway. Mutations of CASP8 in HNSCC were found to be diffuse and suggestive of loss-of-function in nature [85]. Interestingly, these mutations were also associated with cytolytic activity of immune cells through a bioinformatics analysis [86]. This evidence suggests that changes in CASP8, either through genetic mutations or transcriptional regulations could have a major impact on the outcome of cell death, in response to immunogenic cytotoxicity.
How do cancer initiating cells (CICs) respond to TILs?
The recent evolving theme in CICs suggests this subset of cancer cells is highly tumorigenic and more resistant to treatments [87]. There have been excellent reviews regarding the response profiles of CICs to chemoradiation therapy [88]. A general consensus is such that CICs represent a small resistant population in tumor cells, with high ALDH activity and CD44 expression. The initial characterization of HNSCC CICs is evidenced by the fact that HNSCC cells with high ALDH activity showed superior tumorigenic potential when implanted as a xenograft [89]. Interestingly, several human HNSCC cell lines harbor a CIC-like population, which could be harnessed as a tool to investigate whether this small population of tumor cells represent as a major hurdle against a successful neoadjuvant therapy [87, 90]. The studies on the interaction between CICs and immune system are beginning to emerge. Interestingly, CD44+ HNSCC cells exhibited lower levels HLA-A2 and TAP2 expression, the latter of which is essential for assembling the MHC Class I-tumor antigen peptide complex [91]. These data indicate that CIC-like cells in HNSCC may be less immunogenic than the bulk of the tumor cells. Notably, CD44+ HNSCC cells exhibited selective PD-L1 expression compared to CD44− cells; and the decreased immunogenicity of CD44+ HNSCC cells can be partially restored by PD-1 blockade [92]. As CICs are functionally defined by the increased tumorigenic potential of a subset of tumor cells, it is very likely that the maintenance and renewal of these CICs could vary depending on the nutrient condition and treatments. In addition, it is noteworthy that CICs can be effectively targeted by immune cells, as this population has shown susceptibility to co-culture with NK cells [93, 94]. CICs also present specific tumor-associated antigens on the cell surface, which can be potentially targeted by effector T-cells [95]. But one factor that limits the efficacy of immunotherapy is the CICs-potentiated immuno-suppressive environment [96]. The general mechanisms that tumor cells employ to suppress TIL infiltration could be applied to CICs as well. Future studies on how unique signaling pathways in CICs suppress immune cell trafficking and interfere with immunogenic cytotoxicity would be essential in the design of immunotherapeutic approaches targeting this population.
What are the challenges of using TIL as prognostic markers for HNSCC?
As we have discussed above, TILs number and functional subsets are indicative of host anti-tumor immune response, and associated with patients’ clinical outcomes. A worldwide task force has been committed to classify colorectal tumors based on the TIL profile [97]. The Society for Immunotherapy of Cancer (SITC) has launched an Immunoscore Validation Project to verify the prognostic significance of the T-cell-based immunoscore in more than 5,000 colon cancer patients. These effort will complement the clinical benefits of the American Joint Committee on Cancer (AJCC) staging system, and provide a reference value that not only predicts patients’ survival but also response to treatments.
Importantly, TILs are not only telltale markers for tumor response to treatment, but also represent a new dimension for therapeutic intervention. However there are several urgent scientific challenges that need to be addressed to realize the potential of precision medicine-informed immunotherapy: (1) Although high CD3+ CD8+ T-cell and Treg counts appear to be prognostically beneficial, the current immunoscore scheme has not yet fully taken into account cancer cell factors that modulate tumor immunogenicity. The significance of cancer cell autonomous IFN-I signaling in establishing an “inflamed” microenvironment has been established from studies in several types of solid tumors, but its role in HNSCC remains elusive. In addition, IFN-I signatures involve a complex set of genes, detailed analysis of high throughput sequencing data would be essential to determine which IFN-I pathways are associated with a favorable cytotoxic T-cell response. (2) HNSCC is often used as an umbrella terminology to describe a number of histologic subtypes, which may include conventional squamous cell carcinoma, verrucous carcinoma, basaloid squamous cell carcinoma, papillary squamous cell carcinoma, lymphoepithelial carcinoma, spindle cell carcinoma, adenosquamous carcinoma adenocarcinoma, and sinonasal undifferentiated carcinoma. Although some of these subtypes are not as common as conventional squamous cell carcinoma, different histologic subtypes are associated with varied prognosis; for example, basaloid squamous cell carcinoma is usually associated with a worse prognosis, while papillary squamous cell carcinoma and verrucous carcinoma may behave better over time [69]. The interpretation of TILs associated with different histologic subtypes needs to be further refined. (3) The number and functions of TILs are dynamic in nature. After TIL is initially evaluated in a biopsy specimen, subsequent treatments, such as chemoradiotherapy, may significantly change the infiltrating profile. Thus the prognostic value of initial interpretation on a biopsy specimen may be also confounded by the dynamics. Thus development of an expanded panel including TIL and cancer cell factors that underlie its immunogenic potential would hold key to realize the potential of precision immunotherapy. (4) Although autophagy promotes resistance to immunogenic cytotoxicity, selective inhibition of autophagy in tumor cells is technically challenging. The current inhibitor of autophagy, such as hydrochloroquine, has an unfavorable pharmacologic dynamics [98]. In addition, it potently interferes with dendritic cell activation, which dampens adaptive immunity [99, 100]. Thus in order to bypass this limitation in tumors with high level of autophagy, alternative approaches to promote a T-cell-inflamed tumor microenvironment and increase effector immune cell density would be critically beneficial and more practical.
In conclusion, an immunoscore based on TIL phenotype has demonstrated great promise in complementing the current AJCC staging system to better classify patients. A coordinated effort in developing an immunoscoring system to integrate both TIL phenotypes and cancer biomarkers for immunogenicity would have a significant impact on the staging system of HNSCC. Discovery of major cancer cell machinery that impedes a favorable “inflamed” tumor microenvironment holds key to expand the patient pool who respond to novel adjuvant immunotherapy. Approaches that center on the improvement of TIL trafficking and activation will synergize with a checkpoint-blockade-based regimen.
Acknowledgments
Financial Support: DE024173 (YL), DE021139 (JN), DE023220 (JN), and CA097248 (GTW, JN)
References
- 1.Klein E, Becker S, Svedmyr E, Jondal M, Vanky F. Tumor infiltrating lymphocytes. Annals of the New York Academy of Sciences. 1976;276:207–16. doi: 10.1111/j.1749-6632.1976.tb41647.x. [DOI] [PubMed] [Google Scholar]
- 2.Wolf GT, Hudson JL, Peterson KA, Miller HL, McClatchey KD. Lymphocyte subpopulations infiltrating squamous carcinomas of the head and neck: correlations with extent of tumor and prognosis. Otolaryngology–head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 1986;95:142–52. doi: 10.1177/019459988609500203. [DOI] [PubMed] [Google Scholar]
- 3.Boheim K, Denz H, Boheim C, Glassl H, Huber H. An immunohistologic study of the distribution and status of activation of head and neck tumor infiltrating leukocytes. Arch Otorhinolaryngol. 1987;244:127–32. doi: 10.1007/BF00458563. [DOI] [PubMed] [Google Scholar]
- 4.Whiteside TL, Letessier E, Hirabayashi H, Vitolo D, Bryant J, Barnes L, et al. Evidence for local and systemic activation of immune cells by peritumoral injections of interleukin 2 in patients with advanced squamous cell carcinoma of the head and neck. Cancer research. 1993;53:5654–62. [PubMed] [Google Scholar]
- 5.Melioli G, Margarino G, Scala M, Mereu P, Bertoglio S, Schenone G, et al. Perilymphatic injections of recombinant interleukin-2 (rIL-2) partially correct the immunologic defects in patients with advanced head and neck squamous cell carcinoma. Laryngoscope. 1992;102:572–8. doi: 10.1288/00005537-199205000-00018. [DOI] [PubMed] [Google Scholar]
- 6.Verastegui E, Barrera JL, Zinser J, Del Rio R, Meneses A, De La Garza J, et al. A natural cytokine mixture (IRX-2) and interference with immune suppression induce immune mobilization and regression of head and neck cancer. Int J Immunopharmacol. 1997;19:619–27. doi: 10.1016/s0192-0561(97)00059-3. [DOI] [PubMed] [Google Scholar]
- 7.Jemal A, Clegg LX, Ward E, Ries LA, Wu X, Jamison PM, et al. Annual report to the nation on the status of cancer, 1975–2001, with a special feature regarding survival. Cancer. 2004;101:3–27. doi: 10.1002/cncr.20288. [DOI] [PubMed] [Google Scholar]
- 8.Edwards BK, Noone AM, Mariotto AB, Simard EP, Boscoe FP, Henley SJ, et al. Annual Report to the Nation on the status of cancer, 1975–2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer. 2013 doi: 10.1002/cncr.28509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balermpas P, Rodel F, Rodel C, Krause M, Linge A, Lohaus F, et al. CD8+ tumour-infiltrating lymphocytes in relation to HPV status and clinical outcome in patients with head and neck cancer after postoperative chemoradiotherapy: A multicentre study of the German cancer consortium radiation oncology group (DKTK-ROG) International journal of cancer Journal international du cancer. 2016;138:171–81. doi: 10.1002/ijc.29683. [DOI] [PubMed] [Google Scholar]
- 10.Wolf GT, Chepeha DB, Bellile E, Nguyen A, Thomas D, McHugh J, et al. Tumor infiltrating lymphocytes (TIL) and prognosis in oral cavity squamous carcinoma: a preliminary study. Oral oncology. 2015;51:90–5. doi: 10.1016/j.oraloncology.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen N, Bellile E, Thomas D, McHugh J, Rozek L, Virani S, et al. Tumor infiltrating lymphocytes and survival in patients with head and neck squamous cell carcinoma. Head & neck. 2016 doi: 10.1002/hed.24406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kotoula V, Chatzopoulos K, Lakis S, Alexopoulou Z, Timotheadou E, Zagouri F, et al. Tumors with high-density tumor infiltrating lymphocytes constitute a favorable entity in breast cancer: a pooled analysis of four prospective adjuvant trials. Oncotarget. 2015 doi: 10.18632/oncotarget.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geng Y, Shao Y, He W, Hu W, Xu Y, Chen J, et al. Prognostic Role of Tumor-Infiltrating Lymphocytes in Lung Cancer: a Meta-Analysis. Cell Physiol Biochem. 2015;37:1560–71. doi: 10.1159/000438523. [DOI] [PubMed] [Google Scholar]
- 14.Gunn GR, Zubair A, Peters C, Pan ZK, Wu TC, Paterson Y. Two Listeria monocytogenes vaccine vectors that express different molecular forms of human papilloma virus-16 (HPV-16) E7 induce qualitatively different T cell immunity that correlates with their ability to induce regression of established tumors immortalized by HPV-16. J Immunol. 2001;167:6471–9. doi: 10.4049/jimmunol.167.11.6471. [DOI] [PubMed] [Google Scholar]
- 15.Psyrri A, Sasaki C, Vassilakopoulou M, Dimitriadis G, Rampias T. Future directions in research, treatment and prevention of HPV-related squamous cell carcinoma of the head and neck. Head and neck pathology. 2012;6(Suppl 1):S121–8. doi: 10.1007/s12105-012-0361-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.King EV, Ottensmeier CH, Thomas GJ. The immune response in HPV oropharyngeal cancer. Oncoimmunology. 2014;3:e27254. doi: 10.4161/onci.27254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wansom D, Light E, Thomas D, Worden F, Prince M, Urba S, et al. Infiltrating lymphocytes and human papillomavirus-16–associated oropharyngeal cancer. Laryngoscope. 2012;122:121–7. doi: 10.1002/lary.22133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Medler TR, Cotechini T, Coussens LM. Immune response to cancer therapy: mounting an effective antitumor response and mechanisms of resistance. Trends Cancer. 2015;1:66–75. doi: 10.1016/j.trecan.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balermpas P, Michel Y, Wagenblast J, Seitz O, Weiss C, Rodel F, et al. Tumour-infiltrating lymphocytes predict response to definitive chemoradiotherapy in head and neck cancer. British journal of cancer. 2014;110:501–9. doi: 10.1038/bjc.2013.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balermpas P, Rodel F, Weiss C, Rodel C, Fokas E. Tumor-infiltrating lymphocytes favor the response to chemoradiotherapy of head and neck cancer. Oncoimmunology. 2014;3:e27403. doi: 10.4161/onci.27403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dewyer NA, Wolf GT, Light E, Worden F, Urba S, Eisbruch A, et al. Circulating CD4-positive lymphocyte levels as predictor of response to induction chemotherapy in patients with advanced laryngeal cancer. Head & neck. 2014;36:9–14. doi: 10.1002/hed.23263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nature reviews Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 23.Gentles AJ, Newman AM, Liu CL, Bratman SV, Feng W, Kim D, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nature medicine. 2015;21:938–45. doi: 10.1038/nm.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bron L, Jandus C, Andrejevic-Blant S, Speiser DE, Monnier P, Romero P, et al. Prognostic value of arginase-II expression and regulatory T-cell infiltration in head and neck squamous cell carcinoma. International journal of cancer Journal international du cancer. 2013;132:E85–93. doi: 10.1002/ijc.27728. [DOI] [PubMed] [Google Scholar]
- 25.Badoual C, Hans S, Rodriguez J, Peyrard S, Klein C, Agueznay Nel H, et al. Prognostic value of tumor-infiltrating CD4+ T-cell subpopulations in head and neck cancers. Clin Cancer Res. 2006;12:465–72. doi: 10.1158/1078-0432.CCR-05-1886. [DOI] [PubMed] [Google Scholar]
- 26.Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, et al. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med. 2013;5:200ra116. doi: 10.1126/scitranslmed.3006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spranger S, Gajewski TF. Tumor-intrinsic oncogene pathways mediating immune avoidance. Oncoimmunology. 2016;5:e1086862. doi: 10.1080/2162402X.2015.1086862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shah S, Caruso A, Cash H, Waes CV, Allen CT. Pools of programmed death-ligand within the oral cavity tumor microenvironment: Variable alteration by targeted therapies. Head & neck. 2016 doi: 10.1002/hed.24269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kryczek I, Zhao E, Liu Y, Wang Y, Vatan L, Szeliga W, et al. Human TH17 cells are long-lived effector memory cells. Sci Transl Med. 2011;3:104ra0. doi: 10.1126/scitranslmed.3002949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Punt S, van Vliet ME, Spaans VM, de Kroon CD, Fleuren GJ, Gorter A, et al. FoxP3(+) and IL-17(+) cells are correlated with improved prognosis in cervical adenocarcinoma. Cancer immunology, immunotherapy: CII. 2015;64:745–53. doi: 10.1007/s00262-015-1678-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li J, Mo HY, Xiong G, Zhang L, He J, Huang ZF, et al. Tumor microenvironment macrophage inhibitory factor directs the accumulation of interleukin-17-producing tumor-infiltrating lymphocytes and predicts favorable survival in nasopharyngeal carcinoma patients. The Journal of biological chemistry. 2012;287:35484–95. doi: 10.1074/jbc.M112.367532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pages F, Kirilovsky A, Mlecnik B, Asslaber M, Tosolini M, Bindea G, et al. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:5944–51. doi: 10.1200/JCO.2008.19.6147. [DOI] [PubMed] [Google Scholar]
- 33.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science (New York, NY) 2006;313:1960–4. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 34.Mlecnik B, Bindea G, Angell HK, Maby P, Angelova M, Tougeron D, et al. Integrative Analyses of Colorectal Cancer Show Immunoscore Is a Stronger Predictor of Patient Survival Than Microsatellite Instability. Immunity. 2016;44:698–711. doi: 10.1016/j.immuni.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 35.Mlecnik B, Bindea G, Kirilovsky A, Angell HK, Obenauf AC, Tosolini M, et al. The tumor microenvironment and Immunoscore are critical determinants of dissemination to distant metastasis. Sci Transl Med. 2016;8:327ra26. doi: 10.1126/scitranslmed.aad6352. [DOI] [PubMed] [Google Scholar]
- 36.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–71. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mickel RA, Kessler DJ, Taylor JM, Lichtenstein A. Natural killer cell cytotoxicity in the peripheral blood, cervical lymph nodes, and tumor of head and neck cancer patients. Cancer research. 1988;48:5017–22. [PubMed] [Google Scholar]
- 38.Mukhopadhyaya R, Tatake RJ, Krishnan N, Rao RS, Fakih AR, Naik SL, et al. Immunoreactivity of lymphocytes from draining lymph nodes, peripheral blood and tumor infiltrates from oral cancer patients. J Clin Lab Immunol. 1989;30:21–5. [PubMed] [Google Scholar]
- 39.Li J, Jie HB, Lei Y, Gildener-Leapman N, Trivedi S, Green T, et al. PD-1/SHP-2 inhibits Tc1/Th1 phenotypic responses and the activation of T cells in the tumor microenvironment. Cancer research. 2015;75:508–18. doi: 10.1158/0008-5472.CAN-14-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jie HB, Gildener-Leapman N, Li J, Srivastava RM, Gibson SP, Whiteside TL, et al. Intratumoral regulatory T cells upregulate immunosuppressive molecules in head and neck cancer patients. British journal of cancer. 2013;109:2629–35. doi: 10.1038/bjc.2013.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DiDonato JA, Mercurio F, Karin M. NF-kappaB and the link between inflammation and cancer. Immunological reviews. 2012;246:379–400. doi: 10.1111/j.1600-065X.2012.01099.x. [DOI] [PubMed] [Google Scholar]
- 42.Wang J, Jia Y, Wang N, Zhang X, Tan B, Zhang G, et al. The clinical significance of tumor-infiltrating neutrophils and neutrophil-to-CD8+ lymphocyte ratio in patients with resectable esophageal squamous cell carcinoma. J Transl Med. 2014;12:7. doi: 10.1186/1479-5876-12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bindea G, Mlecnik B, Tosolini M, Kirilovsky A, Waldner M, Obenauf AC, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013;39:782–95. doi: 10.1016/j.immuni.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 44.Starr P. Encouraging Results for Pembrolizumab in Head and Neck Cancer. Am Health Drug Benefits. 2015;8:16. [PMC free article] [PubMed] [Google Scholar]
- 45.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nature reviews. 2006;6:836–48. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 46.Gajewski TF, Woo SR, Zha Y, Spaapen R, Zheng Y, Corrales L, et al. Cancer immunotherapy strategies based on overcoming barriers within the tumor microenvironment. Current opinion in immunology. 2013;25:268–76. doi: 10.1016/j.coi.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 47.Woo SR, Corrales L, Gajewski TF. The STING pathway and the T cell-inflamed tumor microenvironment. Trends in immunology. 2015;36:250–6. doi: 10.1016/j.it.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sistigu A, Yamazaki T, Vacchelli E, Chaba K, Enot DP, Adam J, et al. Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nature medicine. 2014;20:1301–9. doi: 10.1038/nm.3708. [DOI] [PubMed] [Google Scholar]
- 49.Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. The Journal of clinical investigation. 2014;124:687–95. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burnette BC, Liang H, Lee Y, Chlewicki L, Khodarev NN, Weichselbaum RR, et al. The efficacy of radiotherapy relies upon induction of type i interferon-dependent innate and adaptive immunity. Cancer research. 2011;71:2488–96. doi: 10.1158/0008-5472.CAN-10-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, et al. STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity. 2014;41:843–52. doi: 10.1016/j.immuni.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barber GN. STING: infection, inflammation and cancer. Nature reviews. 2015;15:760–70. doi: 10.1038/nri3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ahn J, Barber GN. Self-DNA, STING-dependent signaling and the origins of autoinflammatory disease. Current opinion in immunology. 2014;31:121–6. doi: 10.1016/j.coi.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 54.Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science (New York, NY. 2013;339:786–91. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu J, Sun L, Chen X, Du F, Shi H, Chen C, et al. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science (New York, NY) 2013;339:826–30. doi: 10.1126/science.1229963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ishikawa H, Ma Z, Barber GN. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461:788–92. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455:674–8. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peng D, Kryczek I, Nagarsheth N, Zhao L, Wei S, Wang W, et al. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature. 2015;527:249–53. doi: 10.1038/nature15520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Woo SR, Fuertes MB, Corrales L, Spranger S, Furdyna MJ, Leung MY, et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity. 2014;41:830–42. doi: 10.1016/j.immuni.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fu J, Kanne DB, Leong M, Glickman LH, McWhirter SM, Lemmens E, et al. STING agonist formulated cancer vaccines can cure established tumors resistant to PD-1 blockade. Sci Transl Med. 2015;7:283ra52. doi: 10.1126/scitranslmed.aaa4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Allen IC, Moore CB, Schneider M, Lei Y, Davis BK, Scull MA, et al. NLRX1 protein attenuates inflammatory responses to infection by interfering with the RIG-I-MAVS and TRAF6-NF-kappaB signaling pathways. Immunity. 2011;34:854–65. doi: 10.1016/j.immuni.2011.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lei Y, Wen H, Ting JP. The NLR protein, NLRX1, and its partner, TUFM, reduce type I interferon, and enhance autophagy. Autophagy. 2013;9:432–3. doi: 10.4161/auto.23026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lei Y, Wen H, Yu Y, Taxman DJ, Zhang L, Widman DG, et al. The mitochondrial proteins NLRX1 and TUFM form a complex that regulates type I interferon and autophagy. Immunity. 2012;36:933–46. doi: 10.1016/j.immuni.2012.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moore CB, Bergstralh DT, Duncan JA, Lei Y, Morrison TE, Zimmermann AG, et al. NLRX1 is a regulator of mitochondrial antiviral immunity. Nature. 2008;451:573–7. doi: 10.1038/nature06501. [DOI] [PubMed] [Google Scholar]
- 65.Xia X, Cui J, Wang HY, Zhu L, Matsueda S, Wang Q, et al. NLRX1 negatively regulates TLR-induced NF-kappaB signaling by targeting TRAF6 and IKK. Immunity. 2011;34:843–53. doi: 10.1016/j.immuni.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang L, Mo J, Swanson KV, Wen H, Petrucelli A, Gregory SM, et al. NLRC3, a member of the NLR family of proteins, is a negative regulator of innate immune signaling induced by the DNA sensor STING. Immunity. 2014;40:329–41. doi: 10.1016/j.immuni.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cui J, Li Y, Zhu L, Liu D, Songyang Z, Wang HY, et al. NLRP4 negatively regulates type I interferon signaling by targeting the kinase TBK1 for degradation via the ubiquitin ligase DTX4. Nature immunology. 2012;13:387–95. doi: 10.1038/ni.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carreno BM, Magrini V, Becker-Hapak M, Kaabinejadian S, Hundal J, Petti AA, et al. Cancer immunotherapy. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science (New York, NY) 2015;348:803–8. doi: 10.1126/science.aaa3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lei Y, Lui VW, Grandis JR, Egloff AM. Identification of mutations in the PYRIN-containing NLR genes (NLRP) in Head and Neck Squamous Cell Carcinoma. PloS one. 2014;9:e85619. doi: 10.1371/journal.pone.0085619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stransky N, Egloff AM, Tward AD, Kostic AD, Cibulskis K, Sivachenko A, et al. The mutational landscape of head and neck squamous cell carcinoma. Science (New York, NY) 2011;333:1157–60. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reeves TD, Hill EG, Armeson KE, Gillespie MB. Cetuximab therapy for head and neck squamous cell carcinoma: a systematic review of the data. Otolaryngology–head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2011;144:676–84. doi: 10.1177/0194599811399559. [DOI] [PubMed] [Google Scholar]
- 72.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–7. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.White E. Deconvoluting the context-dependent role for autophagy in cancer. Nature reviews Cancer. 2012;12:401–10. doi: 10.1038/nrc3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Baginska J, Viry E, Berchem G, Poli A, Noman MZ, van Moer K, et al. Granzyme B degradation by autophagy decreases tumor cell susceptibility to natural killer-mediated lysis under hypoxia. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:17450–5. doi: 10.1073/pnas.1304790110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Messai Y, Noman MZ, Janji B, Hasmim M, Escudier B, Chouaib S. The autophagy sensor ITPR1 protects renal carcinoma cells from NK-mediated killing. Autophagy. 2015:0. doi: 10.1080/15548627.2015.1017194. [DOI] [PubMed] [Google Scholar]
- 76.Noman MZ, Janji B, Kaminska B, Van Moer K, Pierson S, Przanowski P, et al. Blocking hypoxia-induced autophagy in tumors restores cytotoxic T-cell activity and promotes regression. Cancer research. 2011;71:5976–86. doi: 10.1158/0008-5472.CAN-11-1094. [DOI] [PubMed] [Google Scholar]
- 77.Lei Y, Kansy BA, Li J, Cong L, Liu Y, Trivedi S, et al. EGFR-targeted mAb therapy modulates autophagy in head and neck squamous cell carcinoma through NLRX1-TUFM protein complex. Oncogene. 2016 doi: 10.1038/onc.2016.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liang XH, Yu J, Brown K, Levine B. Beclin 1 contains a leucine-rich nuclear export signal that is required for its autophagy and tumor suppressor function. Cancer research. 2001;61:3443–9. [PubMed] [Google Scholar]
- 79.White E. The role for autophagy in cancer. The Journal of clinical investigation. 2015;125:42–6. doi: 10.1172/JCI73941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Viry E, Baginska J, Berchem G, Noman MZ, Medves S, Chouaib S, et al. Autophagic degradation of GZMB/granzyme B: a new mechanism of hypoxic tumor cell escape from natural killer cell-mediated lysis. Autophagy. 2014;10:173–5. doi: 10.4161/auto.26924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nature immunology. 2011;12:408–15. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gross O, Thomas CJ, Guarda G, Tschopp J. The inflammasome: an integrated view. Immunological reviews. 2011;243:136–51. doi: 10.1111/j.1600-065X.2011.01046.x. [DOI] [PubMed] [Google Scholar]
- 83.Michaud M, Martins I, Sukkurwala AQ, Adjemian S, Ma Y, Pellegatti P, et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science (New York, NY) 2011;334:1573–7. doi: 10.1126/science.1208347. [DOI] [PubMed] [Google Scholar]
- 84.Kepp O, Senovilla L, Vitale I, Vacchelli E, Adjemian S, Agostinis P, et al. Consensus guidelines for the detection of immunogenic cell death. Oncoimmunology. 2014;3:e955691. doi: 10.4161/21624011.2014.955691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Network CGA. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517:576–82. doi: 10.1038/nature14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rooney MS, Shukla SA, Wu CJ, Getz G, Hacohen N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell. 2015;160:48–61. doi: 10.1016/j.cell.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chinn SB, Darr OA, Peters RD, Prince ME. The role of head and neck squamous cell carcinoma cancer stem cells in tumorigenesis, metastasis, and treatment failure. Front Endocrinol (Lausanne) 2012;3:90. doi: 10.3389/fendo.2012.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dionne LK, Driver ER, Wang XJ. Head and Neck Cancer Stem Cells: From Identification to Tumor Immune Network. Journal of dental research. 2015;94:1524–31. doi: 10.1177/0022034515599766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:973–8. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Owen JH, Hauff SJ, Tang AL, Graham MP, Czerwinski MJ, Kaddoura M, et al. UM-SCC-103: a unique tongue cancer cell line that recapitulates the tumorigenic stem cell population of the primary tumor. Ann Otol Rhinol Laryngol. 2014;123:662–72. doi: 10.1177/0003489414531910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chikamatsu K, Takahashi G, Sakakura K, Ferrone S, Masuyama K. Immunoregulatory properties of CD44+ cancer stem-like cells in squamous cell carcinoma of the head and neck. Head & neck. 2011;33:208–15. doi: 10.1002/hed.21420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee Y, Shin JH, Longmire M, Wang H, Kohrt HE, Chang HY, et al. CD44+ Cells in Head and Neck Squamous Cell Carcinoma Suppress T-Cell-Mediated Immunity by Selective Constitutive and Inducible Expression of PD-L1. Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-15-2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tallerico R, Todaro M, Di Franco S, Maccalli C, Garofalo C, Sottile R, et al. Human NK cells selective targeting of colon cancer-initiating cells: a role for natural cytotoxicity receptors and MHC class I molecules. J Immunol. 2013;190:2381–90. doi: 10.4049/jimmunol.1201542. [DOI] [PubMed] [Google Scholar]
- 94.Tseng HC, Arasteh A, Paranjpe A, Teruel A, Yang W, Behel A, et al. Increased lysis of stem cells but not their differentiated cells by natural killer cells; de-differentiation or reprogramming activates NK cells. PloS one. 2010;5:e11590. doi: 10.1371/journal.pone.0011590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yamada R, Takahashi A, Torigoe T, Morita R, Tamura Y, Tsukahara T, et al. Preferential expression of cancer/testis genes in cancer stem-like cells: proposal of a novel sub-category, cancer/testis/stem gene. Tissue antigens. 2013;81:428–34. doi: 10.1111/tan.12113. [DOI] [PubMed] [Google Scholar]
- 96.Hirohashi Y, Torigoe T, Tsukahara T, Kanaseki T, Kochin V, Sato N. Immune Responses to Human Cancer Stem-like Cells/Cancer-initiating Cells. Cancer science. 2015 doi: 10.1111/cas.12830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Galon J, Pages F, Marincola FM, Angell HK, Thurin M, Lugli A, et al. Cancer classification using the Immunoscore: a worldwide task force. J Transl Med. 2012;10:205. doi: 10.1186/1479-5876-10-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Amaravadi RK, Lippincott-Schwartz J, Yin XM, Weiss WA, Takebe N, Timmer W, et al. Principles and current strategies for targeting autophagy for cancer treatment. Clin Cancer Res. 2011;17:654–66. doi: 10.1158/1078-0432.CCR-10-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ertel W, Morrison MH, Ayala A, Chaudry IH. Chloroquine attenuates hemorrhagic shock-induced suppression of Kupffer cell antigen presentation and major histocompatibility complex class II antigen expression through blockade of tumor necrosis factor and prostaglandin release. Blood. 1991;78:1781–8. [PubMed] [Google Scholar]
- 100.Thome R, Issayama LK, DiGangi R, Bombeiro AL, da Costa TA, Ferreira IT, et al. Dendritic cells treated with chloroquine modulate experimental autoimmune encephalomyelitis. Immunol Cell Biol. 2014;92:124–32. doi: 10.1038/icb.2013.73. [DOI] [PubMed] [Google Scholar]


