Abstract
Background
The popularity of electronic cigarettes (ECs) has increased dramatically despite their unknown health consequences. Because the abuse liability of ECs is one of the leading concerns of the Food and Drug Administration (FDA), models to assess it are urgently needed to inform FDA regulatory decisions regarding these products. The purpose of this study was to assess the relative abuse liability of an EC liquid compared to nicotine alone in rats. Because this EC liquid contains non-nicotine constituents that may enhance its abuse liability, we hypothesized that it would have greater abuse liability than nicotine alone.
Methods
Nicotine alone and nicotine dose-equivalent concentrations of EC liquid were compared in terms of their acute effects on intracranial self-stimulation (ICSS) thresholds, acquisition of self-administration, reinforcing efficacy (i.e., elasticity of demand), blockade of these behavioral effects by mecamylamine, nicotine pharmacokinetics and nicotinic acetylcholine receptor binding and activation.
Results
There were no significant differences between formulations on any measure, except that EC liquid produced less of an elevation in ICSS thresholds at high nicotine doses.
Conclusions
Collectively, these findings suggest that the relative abuse liability of this EC liquid is similar to that of nicotine alone in terms of its reinforcing and reinforcement-enhancing effects, but that it may have less aversive/anhedonic effects at high doses. The present methods may be useful for assessing the abuse liability of other ECs to inform potential FDA regulation of those products.
Keywords: Intracranial self-stimulation, Self-administration, Rat, Nicotine, E-cigarettes, Behavioral economics, Tobacco Control Policy
1. INTRODUCTION
Electronic cigarettes (ECs) are devices that deliver an inhalable aerosol containing nicotine and other constituents (e.g., propylene glycol, minor alkaloids, flavorants; Brandon et al., 2015; Harrell et al., 2014; Orellana-Barrios et al., 2015; Walton et al., 2015). ECs are being marketed as a safer or less addictive alternative to conventional tobacco cigarettes despite the lack of scientific evidence to support these claims (Brandon et al., 2015; Harrell et al., 2014; Orellana-Barrios et al., 2015; Walton et al., 2015). In fact, there is concern that ECs could increase the health burden of tobacco dependence by undermining prevention or cessation efforts (Brandon et al., 2015; Lauterstein et al., 2014; Orellana-Barrios et al., 2015; Walton et al., 2015). Despite the unknown health consequences of ECs, their use is rapidly increasing, particularly among adolescents and current smokers (Lauterstein et al., 2014; Porter et al., 2015). For example, EC use tripled in high school and middle school students between 2013 and 2014, and ECs are now more popular than tobacco cigarettes in youth (Arrazola et al., 2015). In light of these issues, the FDA Center for Tobacco Products (CTP) now has the authority to regulate ECs under the Family Smoking Prevention and Tobacco Control Act (FSPTCA), which also provides the FDA CTP regulatory authority over cigarettes, cigarette tobacco, roll-your-own tobacco, and smokeless tobacco. Establishing methodology for evaluating the relative abuse liability and adverse effects of ECs is therefore essential for informing potential FDA CTP regulatory policy regarding these products and for anticipating the impact of ECs on public health (Brandon et al., 2015; Breland et al., 2014; Cobb et al., 2015).
Preclinical models are crucial for tobacco product evaluation because they can address issues that cannot be studied experimentally in humans (Donny et al., 2012). Most preclinical models of tobacco addiction involve administration of nicotine and/or other constituents (e.g., minor alkaloids, acetaldehyde) in isolation from the thousands of other chemicals in tobacco. This approach may not be sufficient to evaluate the abuse liability of tobacco products because other compounds may contribute to tobacco abuse, either positively or negatively. Ultimately, it is the collective action of these compounds in tobacco, smoke, or EC aerosol that determines the abuse liability of a product (Brennan et al., 2013a, 2014; Harris et al., 2012, 2015b).
To address these limitations, our laboratory and others have evaluated the addiction-related effects of extracts that are derived directly from tobacco or tobacco smoke and contain a extensive mixture of tobacco constituents (Ambrose et al., 2007; Brennan et al., 2013a, 2014, 2013c; Costello et al., 2014; Harris et al., 2012, 2015b; Touiki et al., 2007). Several of these studies have reported greater abuse liability for extracts compared to nicotine alone (e.g., Brennan et al., 2013a, 2014; Costello et al., 2014). One interpretation is that certain non-nicotine constituents present in extracts (e.g., minor alkaloids, MAO inhibitors) contribute to the greater abuse liability because they can mimic or enhance nicotine’s addiction-related effects when studied in isolation (Bardo et al., 1999; Belluzzi et al., 2005; Dwoskin et al., 1999; Foddai et al., 2004; Guillem et al., 2005; Villegier et al., 2007). Many EC liquids also contain behaviorally active non-nicotine constituents (Etter et al., 2013; Goniewicz et al., 2014; Kosmider et al., 2014). In addition to the same minor alkaloids present in tobacco smoke, some EC liquids contain acetaldehyde, which is self-administered by rats (Myers et al., 1982, 1984; Takayama and Uyeno, 1985) and can enhance the reinforcing and other behavioral effects of nicotine (Belluzzi et al., 2005; Cao et al., 2007). Also, a common vehicle in EC liquids is propylene glycol, which is self-administered in alcohol-preferring rodents (Hillman and Schneider, 1975) and can also have sedative or anxiolytic effects (Da Silva and Elisabetsky, 2001; Lin et al., 1998; Singh et al., 1982; Zaroslinski et al., 1971). To our knowledge, preclinical studies of the abuse liability of EC liquids have not yet been conducted.
The primary goal of the present study was to compare the effects of nicotine alone and nicotine dose-equivalent concentrations of EC liquid in animal models of tobacco addiction. We used a product (Aroma E-Juice Dark Honey Whole Tobacco Alkaloid (WTA)) that is designed to more closely simulate traditional tobacco cigarettes than typical ECs by including higher levels of minor alkaloids than other ECs (www.aromaejuice.com). As such, we hypothesized that this EC liquid would exhibit greater abuse liability than nicotine alone.
We assessed abuse liability using two common behavioral models. The first involved examining the acute effects of nicotine alone and EC liquid in an intracranial self-stimulation (ICSS) assay. Low to moderate doses of nicotine and other addictive drugs lower the minimal (i.e., threshold) electrical stimulation intensity that supports ICSS (e.g., Harrison et al., 2002; Huston-Lyons and Kornetsky, 1992; Kornetsky et al., 1979; Negus and Miller, 2014; Paterson et al., 2008). This may reflect the ability of drugs to enhance the reinforcing effects of non-drug stimuli (e.g., sensory stimuli, food), a phenomenon that may contribute to addiction (Caggiula et al., 2009; Chaudhri et al., 2006; Wise, 2002). This assay provides excellent predictive validity for identifying whether or not a drug will be abused in humans (nominal scaling of drugs), as well as for identifying the relative degree of abuse potential between drugs (ordinal or ratio scaling of drugs; Kornetsky and Esposito, 1979; Kornetsky et al., 1979; Negus and Miller, 2014). Further supporting the sensitivity of this measure, some addictive drugs that do not produce addiction-related effects in other assays (e.g., hallucinogens) nonetheless reduce ICSS thresholds (Wise, 1996, 2002; Wise et al., 1992). At high doses, nicotine and other drugs disrupt brain reinforcement systems and elevate ICSS thresholds (Fowler et al., 2011; Kenny et al., 2003; Spiller et al., 2009). This represents a putative measure of a drug’s aversive or anhedonic effects that can limit its intake (Fowler and Kenny, 2012, 2013; Fowler et al., 2011). The relative abuse liability of nicotine alone and EC liquid was also examined in an i.v. self-administration (SA) assay. Differences in rate of acquisition of SA and resistance of consumption to increases in response requirements (i.e., elasticity of demand) were assessed. Combined, these behavioral models provide convergent evidence for the abuse liability of nicotine (see Fowler et al., 2011) and SA is specifically recommended by the FDA for comparing the relative abuse liability of novel compounds to established drugs (Food and Drug Administration, 2010). We also compared formulations in terms of their binding and activation of nicotinic acetylcholine receptors (nAChRs) and nicotine pharmacokinetics to determine whether these factors might mediate the observed behavioral effects.
2. MATERIAL AND METHODS
2.1. General methods
2.1.1. Animals
Male adult Holtzman rats (Harlan, Indianapolis, IN) weighing 300–350 grams at arrival were used. Upon arrival, all rats were individually housed in a temperature- and humidity controlled colony room with unlimited access to food and water under a reversed 12-h light/dark cycle (lights off at 11:00 hr) for one week. Rats were then food restricted to 18 g/day for the remainder of the experiment. Protocols were approved by the Institutional Animal Care and Use Committee of the Minneapolis Medical Research Foundation in accordance with the NIH Guide for the Care and Use of Laboratory Animals and the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council 2011).
2.1.2. Apparatus
For ICSS (Experiment 1), testing occurred in operant conditioning chambers (Med Associates, St. Albans, VT) placed inside sound-attenuated cubicles with exhaust fans. A 5-cm wide metal wheel manipulandum was fixed to the front wall. Brain stimulation was administered with constant-current stimulators (Model #PHM-152, Med-Associates). Rats were connected to the stimulation circuit through bipolar leads (Plastics One, Roanoke, VA) attached to gold-contact swivel commutators (Plastics One).
For drug self-administration (Experiment 4), each operant conditioning chamber (Med-Associates) was made of aluminum and clear polycarbonate walls, an aluminum ceiling, and a stainless steel grid floor. Two response levers were located on the front wall on either side of an aperture for delivery of food pellets (not used in this study). A third lever was located on the right side of the back wall of the chamber. White stimulus lights were located above each response lever. A house light mounted centrally at the top of the back panel provided ambient illumination. Each chamber was placed inside a sound-attenuating cubicle equipped with an exhaust fan that provided masking noise. Infusion pumps (PHM-100, Med-Associates, St. Albans, VT) placed next to the operant chamber inside the cubicle delivered infusions through Tygon tubing connected to a fluid swivel (Instech Inc., Plymouth Meeting, PA) mounted on a counter-balanced arm above the center of the chamber. Tubing ran from the swivel through a spring leash connected to a vascular access harness (VAH95AB, Instech Inc., Plymouth Meeting, PA) on the back of the rat. For both ICSS and drug self-administration studies, MED-PC IV (Med Associates, St Albans, VT) software was used for operating the apparatus and recording data.
2.1.3. Initial EC liquid constituent analysis
Concentrations of nicotine and minor alkaloids (nornicotine, anabasine, and anatabine) in the EC liquid were analyzed by liquid chromatography-tandem mass spectrometry (LC- MS/MS) by modification of a previously described method (Rangiah et al., 2011). Briefly, the EC liquid was mixed with stable isotope-labeled nicotine and nornicotine, anatabine, and anabasine (internal standards), diluted with 10 mM ammonium acetate containing 5% methanol, and analyzed by LC-MS/MS on a Hypercarb column (Thermo Scientific), using 10 mM ammonium acetate (with 0.001% formic acid) and methanol as mobile phase.
2.1.4. Drugs
Nicotine bitartrate and mecamylamine (MEC) were obtained from Sigma Chemical Co. (St. Louis, MO) and dissolved in sterile saline. Whole Tobacco Alkaloid (WTA) EC refill liquid (Dark Honey Tobacco flavor in 10 ml vials) was obtained from Aroma E-Juice (http://www.aromaejuice.com, Scottsdale, AZ). According to the label, the refill liquid contained 80% vegetable glycerine (VG) and 20% propylene glycol (PG), and had a nicotine concentration of 24 mg/ml. The nicotine concentration was determined in each 10 ml vial of EC liquid used (see below), allowing dilution in saline to the nicotine concentrations required for the current studies. The pH of the solutions was adjusted to 7.4 with dilute NaOH or HCL, and heparin (30 units/ml) was added to help maintain catheter patency for the self-administration study. Nicotine and mecamylamine doses are expressed as the base and salt, respectively.
2.1.5. Routine nicotine assay
Nicotine concentrations in nicotine alone and EC liquid solutions used for behavioral studies were measured by gas chromatography with nitrogen phosphorus detection, according to standard protocol in our laboratory (Harris et al., 2008; Hieda et al., 1999; LeSage et al., 2003). The average measured nicotine concentration across vials used in the behavioral studies was 24.78 ± 0.73 SEM mg/ml (range 21.66 to 27.70 mg/ml).
2.2. Experiment 1: Effects of nicotine alone and EC liquid on ICSS
2.2.1. Surgery and training
Surgery, apparatus, and training procedures used here are described in detail elsewhere (Harris et al., 2010, 2011; Roiko et al., 2009). Briefly, animals were anesthetized with i.m. ketamine (75 mg/kg)/dexmedetomidine (0.025 mg) and implanted with a bipolar stainless steel electrode in the medial forebrain bundle at the level of the lateral hypothalamus. Rats were later trained to respond for electrical brain stimulation using a modified version of the Kornetsky and Esposito (1979) discrete-trial current-threshold procedure (Markou and Koob, 1992) as routinely used in our laboratory (Harris et al., 2010, 2011; Roiko et al., 2009). Each session was approximately 45 minutes, and the main dependent measures were ICSS thresholds (a measure of brain reinforcement-pathway function) and response latencies (a measure of non-specific (e.g., motor) effects).
2.2.2. Phase 1: Acute dose-response determinations
Animals (N = 12) were tested in daily ICSS sessions conducted Mon-Fri until thresholds were stable (i.e., less than 10% coefficient of variation over a 5-day period and no apparent trend). To habituate animals to the injection procedure, saline was administered s.c. 10 minutes prior to ICSS at least once and until thresholds were unaltered by the injection. Effects of 10-minute pretreatment with nicotine alone (half of the animals) or EC liquid (the other half) were subsequently determined at nicotine doses of 0, 0.06, 0.125, 0.25, 0.50, 0.75, 1.0, or 1.25 mg/kg. These doses bracket the range of nicotine doses that reduce or increase ICSS thresholds when administered acutely (Bauco and Wise, 1994; Harris et al., 2012; Harrison et al., 2002; Huston-Lyons and Kornetsky, 1992; Spiller et al., 2009). Nicotine and EC liquid injections typically occurred on Tuesdays and Fridays, provided that thresholds were within baseline range on intervening days. Doses were administered in a counterbalanced order. Following completion of dose-response testing, animals were tested for ICSS under drug-free conditions for at least 2 weeks and until ICSS thresholds were stable. All rats then underwent the same procedure as described above with the exception that formulation (i.e., nicotine alone versus EC liquid) was crossed-over within each subject.
2.2.3. Phase 2: Effects of mecamylamine pretreatment
Rats from Phase 1 were tested for ICSS under drug-free conditions for at least 2 weeks and until ICSS thresholds were stable. To habituate animals to the injection procedure, saline was administered 15 minutes and 10 minutes prior to ICSS testing twice per week (Tuesdays and Fridays) until no effect was apparent. On each subsequent test day, rats were injected with mecamylamine (MEC) (0, 0.03, 0.1, 0.3, or 1.0 mg/kg) 15 minutes prior to ICSS testing. Five minutes later, rats were injected with nicotine alone (half of the rats) or EC liquid (the other half) at a nicotine (NIC) dose of 0.25 mg/kg. These or similar doses of MEC block the acute effects of nicotine in other behavioral assays (e.g., Biala et al., 2010; Clarke and Kumar, 1983; LeSage et al., 2012), while this dose of nicotine reliably reduces ICSS thresholds (Harris et al., 2015a, 2015b; Harrison et al., 2002). Rats were also tested under control conditions in which they received saline followed by saline (i.e., MEC 0 + NIC 0) or the highest dose of mecamylamine followed by saline (i.e., MEC 1.0 + NIC 0) prior to ICSS testing. Test days typically occurred on Tuesdays and Fridays, provided that thresholds were within baseline range on intervening days, and the order of treatment conditions was counterbalanced across animals. Following completion of all treatment conditions, animals were tested for ICSS under drug-free conditions for at least 2 weeks and until ICSS thresholds were stable. All rats then underwent the same procedure as described above except that formulation (i.e., nicotine alone or EC liquid) was crossed-over within each subject.
2.2.4. Statistical analyses
For Phase 1, baseline ICSS threshold and latency values (in μA and sec, respectively) were compared between formulations (i.e., nicotine alone versus EC liquid) using mixed effects linear regression with fixed effects for formulation and formulation testing order and a random effect for rat. ICSS threshold and latency values during test sessions, expressed as a percent of baseline (i.e., mean during last 5 sessions prior to each dose-response determination), were initially analyzed to account for the crossover repeated measures study design using a mixed effects linear regression model with fixed effects for dose, formulation, formulation testing order, and a dose by formulation interaction, and a random effect for rat. There was no evidence of a cross-over order effect and therefore paired t-tests were conducted for all comparisons. Data for Phase 2 were analyzed in the same manner, except that NIC + MEC condition rather than dose was used as a factor. Means ± standard error of the mean (SEM) are reported unless otherwise noted. In all experiments, p-values ≤0.05 were considered statistically significant. The p-values reported for multiple comparisons were adjusted within each experiment to reduce the false discovery rate using the Benjamini and Yekutieli method (Benjamini and Yekutieli, 2001), a variant of the Benjamini and Hochberg procedure (Benjamini and Hochberg, 1995) that deals with potential dependency of the test statistics.
2.3. Experiment 2: Binding affinities and receptor activation of nicotine alone and EC liquid at nAChRs
Radioligand binding and 86RB+ efflux assays were conducted using solutions of nicotine alone and EC liquid (nicotine content for both solutions = 0.6 mg/ml) by the National Institute of Mental Health-Psychoactive Drug Screening Program. Complete descriptions of these assays, which are based on procedures reported in (Xiao et al., 2006, 1998), are available at http://pdsp.med.unc.edu.
2.3.1. Experiment 2a: Radioligand binding assay
Radioligand binding assays of nAChRs to [3H]-epibatidine were conducted using human embryonic kidney (HEK) 293 cell lines stably expressing human α4β2, α3β4, α2β2, α2β4, α3β2, or α4β4 nAChR subtypes, or the rat α7 nAChR subtype. Assays were also conducted using α4β2 or α7 nAChRs expressed in rat forebrain or cortex, respectively. Cells containing the above nAChR subtypes were harvested, washed, homogenized, and centrifuged. The resulting washed membranes were then incubated with [3H]-epibatidine for 4 hours at room temperature. Nonspecific binding was assessed in parallel incubations in the presence of 300 uM nicotine. Bound and free ligands were separated by vacuum filtration, and the filter-retained radioactivity was measured by liquid scintillation counting. Specific binding was defined as the difference between total binding and nonspecific binding.
Primary binding assays were performed with 100 pM [3H]-epibatidine and 10 uM of each test formulation in quadruplicate. Test formulations with a minimum of 25% inhibition of radioligand-specific binding were subjected to secondary binding assays to determine binding affinity. In these assays, 0.5 nM [3H]-epibatidine and 10 concentrations of each test formulation were tested in singlets to generate a competition binding curve.
2.3.2. Experiment 4b: 86RB+ efflux assay
Agonist and antagonist activities of test formulations at nAChRs were assessed by measuring 86RB+ efflux in HEK293 cells grown in well-plates and stably expressing human α4β2 or α3β4 nAChR subtypes. Cells were incubated in growth medium containing 86RBCl (2 uCi/ml) for 4 hr. The loading mixture was aspirated, and the cells were washed with buffer. One ml of buffer, with or without each test formulation, was then added to each well. After a 2 min incubation period, the assay buffer was collected and the amount of 86RB+ in the buffer was determined. NaOH was then added to each well to lyse the cells, and the lysate was collected for determination of the amount of 86RB+ in the cells. Radioactivity of assay samples and lysates were measured by liquid scintillation counting. The total amount of 86RB+ loaded (cpm) was calculated as the sum of the assay sample and the lysate of each well. The amount of 86RB+ efflux was expressed as a percentage of the 86RB + loaded. “Stimulated 86RB+ efflux” was defined as the difference between efflux in the presence versus absence of each test formulation.
For assessing agonist activity, a primary functional assay was performed in which 4 concentrations of each test formulation (0.1, 1, 10 and 100 uM) were applied. Agonist activity was scaled as a percentage of the stimulation by 100 uM nicotine (100%). Test formulations producing a concentration-dependent activation, and 25% stimulation at any concentration, were subjected to secondary functional assays in which 8 concentrations of each test formulation were tested. Antagonist activity was assessed in a similar manner, except that concentrations were tested in the presence of 100 uM nicotine. Antagonist activity was scaled as a percentage of the inhibition of the 86RB+ efflux stimulated by 100 uM nicotine. All efflux assays were performed in quadruplicate.
2.3.3. Statistical analyses
Data for the nAChR binding and functional studies were analyzed by nonlinear least-squares regression to obtain Ki or EC50 values, respectively (see http://pdsp.med.unc.edu. for further details). Statistically significant differences between formulations were defined as non-overlapping 95% confidence intervals for Ki or EC50 derived from each nonlinear regression.
2.4. Experiment 3: Nicotine pharmacokinetics
2.4.1. Procedures
Rats were anesthetized with i.m. fentanyl (0.1 mg/kg)/dexmedetomidine (0.05 mg) and i.p. propofol (100ml/kg) and implanted with an i.v. jugular and femoral catheter using general procedures described in (Grebenstein et al., 2015; Harris et al., 2008; Harris et al., 2009). Rats were then infused with nicotine alone or e-liquid (0.1 mg/kg; n = 10 /formulation) over 10 seconds via the jugular catheter, and blood was obtained via the femoral catheter at 1, 5, 15, 30, and 60 min for measurement of serum nicotine concentrations. Immediately following the last sample, rats were decapitated and trunk blood and brain were collected. Timing of sample collection coincided with the timing of behavioral testing in Experiments 1 and 4.
2.4.2 Nicotine assay
Serum and brain nicotine levels were measured using gas chromatography with nitrogen-phosphorous detection (Jacob et al., 1981). Brain nicotine levels were corrected for brain blood content (Hieda et al., 1999).
2.4.3 Statistical analyses
Serum nicotine concentrations at each time point were analyzed using two-factor ANOVA with formulation as a between-subject factor and time point as a within-subject factor. Brain nicotine concentrations and brain:serum nicotine concentration ratios at the 60 minute time point were compared using separate two-sided two-sample t-tests.
2.5. Experiment 4: Self-administration of nicotine alone and EC liquid
2.5.1. Surgery
Each rat was implanted with a chronic indwelling catheter into the right jugular vein under i.m. ketamine (75–90 mg/kg)/dexmedetomidine (0.25 mg/kg) anesthesia, described in detail elsewhere (Harris et al., 2008; Lesage et al., 2002). The catheter was externalized between the scapulae and attached to a vascular-access harness (VAH95AB, Instech Laboratories, Plymouth Meeting, PA) that allowed connection to a fluid swivel via a tether for drug administration. Animals were allowed to recover for at least four days after surgery, during which time they received daily i.v. infusions of heparinized saline and ceftriaxone (5.25 mg) and s.c. injections of buprenorphine (0.05 mg/kg; first two days only) for analgesia. Infusions of methohexital (0.1 ml, 10 mg/ml, i.v.) were administered weekly on Fridays after behavioral testing to check catheter patency. If a catheter became occluded, another catheter was implanted into the ipsilateral femoral vein. Failure of this second catheter resulted in the removal of the animal from the study.
2.5.4. SA Acquisition
Acquisition of SA was examined in a total of 36 rats (18 for each formulation). Procedures were similar to those routinely used in our laboratory (e.g., Lesage, 2009; LeSage et al., 2004). Before access to nicotine, each rat was allowed to habituate to the operant chamber for at least five sessions and active lever pressing showed no trend over five consecutive sessions (mean 7.3, range 5–12). During each 2-h habituation session, the house light and stimulus light above the drug lever were illuminated, but pressing either lever had no programmed consequence. Following habituation, nicotine alone or EC liquid became available during 2 hr sessions under an FR 1 schedule, under which each press of the drug response lever produced an infusion of nicotine alone (0.06 mg/kg/inf) or EC liquid (equivalent nicotine dose). This nicotine dose was used because it lies on the descending limb of the dose response curve for nicotine self-administration (NSA), allowing any weaker aversive effects of EC liquid seen in the ICSS experiment to manifest as a higher infusion rate compared to nicotine alone. Using a higher unit dose also allowed a wider range of consumption to be assessed during demand curve assessment (see below). Infusions were delivered in a volume of 0.1 ml/kg in approximately 1 sec. Responses on the other two levers were recorded but had no programmed consequences. Infusions were signaled by offset of the house light and stimulus light over the drug lever. Both lights remained off for the duration of the infusion. After the infusion, the house light was illuminated but the stimulus light remained off during a subsequent 15-sec timeout (TO), during which responses on all levers were recorded but had no programmed consequences. Once robust responding developed under the FR 1 schedule, the FR value was gradually increased to FR 3 across several sessions. Rats were considered to have acquired self-administration when they earned at least five infusions per session and the mean ratio of active to inactive lever presses was at least 2:1 for three consecutive sessions. Self-administration was judged stable when at least five infusions were earned per session and there was no trend in the number of infusions per session across three consecutive sessions. Sessions were run five days per week (Monday through Friday).
2.5.5. Elasticity of Demand
Fourteen of the rats that acquired stable self-administration at FR 3 (7 for each formulation) were used to assess elasticity of demand. During this phase, the FR value was increased weekly, typically on Mondays. On some occasions, the FR value was increased on a Tuesday due to a holiday on Monday. Thus, each FR value was in effect for four or five sessions, until consumption decreased to zero on the last two or three consecutive days of the week, respectively. The FR value increased to yield a progression of unit prices similar to that used in previous studies using a unit dose-reduction protocol (Grebenstein et al., 2013; Grebenstein et al., 2015), with values of 6, 9, 12, 15, 20, 25, 32, 40, 50, and 62. The catheter of one rat failed before reaching zero consumption. Data for this rat were included up to the last FR value at which catheter patency was confirmed, where consumption had decreased by 86%.
2.5.6. Mecamylamine Pretreatment
Fifteen of the rats that acquired stable self-administration at FR3 (7 nicotine, 8 e-liquid) were used to examine the effects of acute mecamylamine (MEC) pretreatment. Initially, rats received two s.c. injections of saline 15 min prior to the self-administration session (Tuesday and Friday). If this disrupted responding, additional injections (up to three) were given twice per week (Tuesday and Friday) until rats acclimated to the injection procedure and no disruption of behavior was evident. Effects of acute MEC (0.1, 0.3, 0.6, 1.0, and 2.0 mg/kg) were then assessed, with injections given 15 min prior to Tuesday and Friday sessions. The order of doses was randomized for each rat. After the acute dose response assessment, responding was allowed to stabilize for at least two weeks in 11 rats that still had patent catheters (five nicotine and six e-liquid) before assessing the effects of repeated mecamylamine treatment. During this two-week phase, rats received a saline injection on Monday, followed by daily mecamylamine pretreatment on Tue-Fri, with the 1.0 mg/kg MEC dose tested in the first week and the 2.0 mg/kg dose tested in the following week.
2.5.7. Data Analysis
Mean lever presses on the active and inactive levers (totaled across inactive levers), number of infusions, and nicotine intake across the last three sessions at each FR value were the primary dependent measures. For analysis of the acquisition (initial 10 days) and MEC pretreatment phases, these measures were analyzed via a mixed effects linear regression model accounting for the repeated measures within rats using a random effect and fixed effects for formulation, session, and a formulation by session interaction. The p-values reported for multiple comparisons were adjusted within each experiment to reduce the false discovery rate using the Benjamini and Yekutieli (2001) method.
To examine elasticity of demand during the FR escalation phase, exponential demand curve analysis was conducted according to Hursh and Silberberg (2008), utilizing the equation:
| (1) |
The dependent variable, Q, is the quantity consumed. The independent variable, C , is the cost of nicotine based on the unit price (FR/unit dose). Thus, in the present study, unit price was manipulated by varying the FR size as the unit dose remained constant. The free parameters, Q0 and α are estimated from the best-fit function and refer to the maximum level of consumption at zero price (i.e., level or “intensity” of demand) and the rate of change in consumption with increases in unit price, respectively. The range of the exponential function, k, is a constant specifying the range of consumption in log units. The k value is held constant across all data sets being compared (set to 2.0 in the present study), because changes in k impact the value of α. The exponential term, Q0 C, represents the standardized price of a commodity, which corrects for variations in price due to potential differences in potency of the commodities being compared. It also serves to correct for differences in drug potency between subjects. The α parameter is considered a measure of reinforcing strength or “essential value” (i.e., the degree to which a given commodity (e.g., drug) is capable of maintaining behavior under constraints of increasing price). The value of α is inversely related to reinforcing strength so that drugs that produce rapidly declining (elastic) demand curves have higher α values and lower reinforcing strength than demand curves with slower declining (inelastic) demand curves. Therefore, α served as the index of elasticity of demand for, or reinforcing efficacy of, nicotine or e-liquid in the present study. Other demand measures of interest included: Q0, the level or intensity of demand as described above; Omax, the maximal response output; and Pmax, the unit price at which maximal response output occurred and demand changes from relatively inelastic to relatively elastic (i.e. the point of unit elasticity).
As mentioned above, the unit price at which zero consumption occurred differed between rats, resulting in missing data for some rats at higher unit prices. By using only those rats exposed to higher prices, an average of consumption at those prices would be larger than the true average if all rats were included. Therefore, to fit group demand curves, it was assumed that rats would continue to exhibit zero consumption at unit prices above the unit price at which zero consumption was measured. Therefore, missing data were treated as zero consumption. This approach provides a more accurate portrayal of the true group consumption at high unit prices. However, statistical comparison of demand parameters between groups was done via two-sided two-sample t-tests comparing the means of parameter estimates derived from curves fit to individual-subject data.
3. RESULTS
3.1. EC liquid constituent analysis
Levels of minor alkaloids (expressed as % of nicotine) in EC liquid were either lower (nornicotine, anabasine) or within the range (anatabine) of those reported for Kodiak and Camel Snus smokeless tobacco extracts in our previous study (Harris et al., 2015b) (Table 1).
Table 1.
Nicotine and minor alkaloid levels (μg/mL) in Aroma E-Juice Dark Honey WTA EC liquid. Alkaloid levels from Kodiak and Camel Snus smokeless tobacco extracts used in our previous study (Harris et al. 2015a) are also shown for comparison. Data in parentheses indicate relative levels of each minor alkaloid (expressed as % of nicotine) in that solution. Total Minor = Nornicotine + Anabasine + Anatabine.
| Nicotine | Nornicotine | Anabasine | Anatabine | Total Minor | |
|---|---|---|---|---|---|
| EC liquid | 20665 | 7.1 (0.03%) | 37.1 (0.2%) | 192.8 (0.9%) | 237.0 (1.15%) |
| Kodiak | 3430 | 21.7 (0.6%) | 14.2 (0.4%) | 41.8 (1.2%) | 77.7 (2.3%) |
| Camel Snus | 2110 | 25.9 (1.2%) | 12.8 (0.6%) | 18.9 (0.9%) | 57.6 (2.7%) |
3.2 Experiment 1: Effects of nicotine alone and EC liquid on ICSS
3.2.1 Phase 1: Acute dose-response determinations
Baseline ICSS thresholds (78.5 ± 5.8 μA versus 76.4 ± 5.6 μA) and response latencies (2.20 ± 0.06 seconds versus 2.17 ± 0.08 seconds) did not differ between the nicotine alone and e-liquid dose-response determinations.
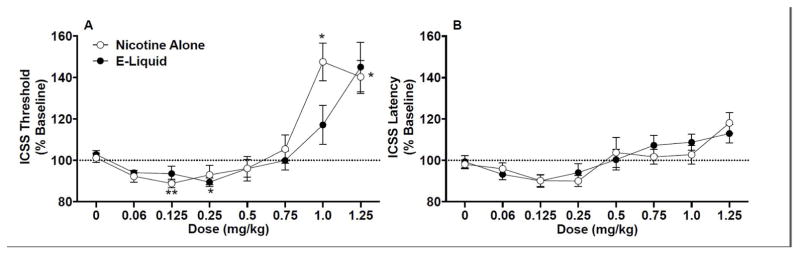
Analysis of ICSS threshold data indicated no effect of formulation, but there was a significant main effect of dose (F(7,165)=23.2, p <0.0001) and a marginally significant dose x formulation interaction (F(7,165)=1.95, p = 0.06). Although thresholds were lower for e-liquid compared to nicotine alone at the 1.0 mg/kg dose, this difference was not statistically significant (Fig. 1a). Thresholds also did not differ between formulations at any other dose. For nicotine alone, ICSS thresholds were significantly reduced compared to saline at 0.125 mg/kg (t(11) = 7.24, p < 0.01) and elevated compared to saline at 1.0 mg/kg (t(11) = 4.47, p < 0.05) and 1.25 mg/kg (t(11) = 4.48, p < 0.05) (Fig 1A). For EC liquid, ICSS thresholds were significantly reduced compared to saline at 0.25 mg/kg (t(11) = 4.66, p < 0.05). However, in contrast to nicotine alone, no dose of EC liquid produced a statistically significant increase in ICSS thresholds.
Figure 1.
ICSS thresholds (A) and response latencies (B) (expressed as percent of baseline, mean ± SEM) following injection of nicotine alone or EC liquid (e-liquid) (0 – 1.25 mg/kg) in Phase 1 of Experiment 1. *,** Significantly different from saline (0 mg/kg) for that formulation, p < 0.05 or 0.01.
There was a significant main effect of dose on ICSS response latencies (F(7,165)=11.4, p <0.0001), but no significant effect of formulation or dose x formulation interaction (Fig 1B). Latencies did not significantly differ from saline at any dose for either formulation (Fig 1B).
3.2.2 Phase 2: Effects of mecamylamine pretreatment
Three animals were lost to attrition prior to completion of this experiment due to removal of ICSS headcap or loss of stability of ICSS thresholds. Analysis of baseline data for the remaining 9 animals indicated that the nicotine alone and EC liquid dose-response determinations did not differ in terms of baseline ICSS thresholds (97.6 ± 7.4 μA versus 91.5 ± 10.4 μA) or response latencies (2.22 ± 0.11 seconds versus 2.13 ± 0.06 sec).
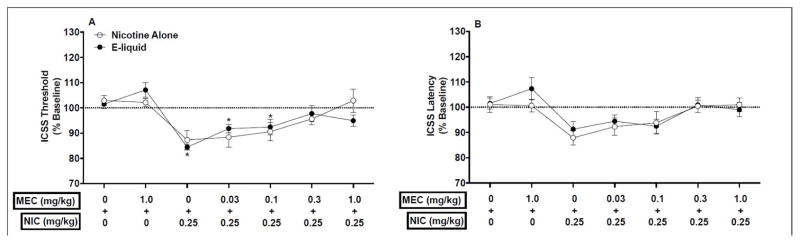
Mecamylamine similarly attenuated the ICSS threshold-lowering effects of nicotine alone and EC liquid. Analysis of ICSS threshold data indicated a significant main effect of condition (F(6,104)=15.2, p <0.0001), but no effect of formulation or condition x formulation interaction. Thresholds did not differ between formulations under any condition (Fig 2A). For nicotine alone, ICSS thresholds were lower compared to saline alone (i.e., MEC 0 + NIC 0 condition) under the MEC 0 + NIC 0.25, MEC 0.03 + NIC 0.25, and MEC 0.1 + NIC 0.25, conditions, but these effects only approached significance (p < 0.1, Fig 2A). Thresholds were similar to saline alone under the MEC 1.0 + NIC 0, MEC 0.3 + NIC 0.25, and MEC 1.0 + NIC 0.25 conditions (Fig 2A). For EC liquid, ICSS thresholds were reduced compared to the MEC 0 + NIC 0 condition under the MEC 0 + NIC 0.25 (t(8) = 6.51, p < 0.05) and MEC 0.03 + NIC 0.25 (t(8) = 4.61, p < 0.05) conditions, but not under the other conditions.
Figure 2.
ICSS thresholds (A) and response latencies (B) (expressed as percent of baseline, mean ± SEM) following injection of MEC (0 – 1.0 mg/kg) + nicotine alone or EC liquid (e-liquid) (0 or 0.25 mg/kg) in Phase 2 of Experiment 1. * Significantly different from MEC 0 + NIC 0 for that formulation, p < 0.05.
There was a significant main effect of condition on ICSS latencies (F(6,104)=6.25, p <0.0001), but no effect of formulation or interaction. Latencies did not differ from MEC 0 + NIC 0 under any condition for either formulation (Fig 2B).
3.2. Experiment 2: nAChR binding affinity and activation profiles of nicotine alone and EC liquid
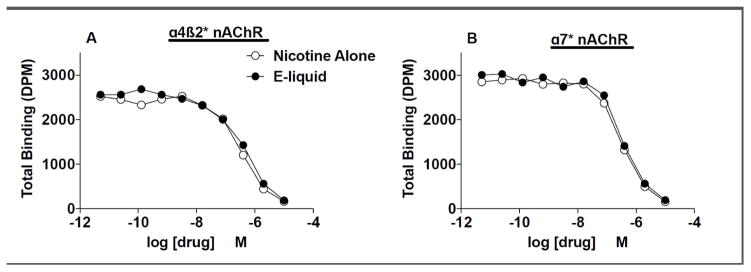
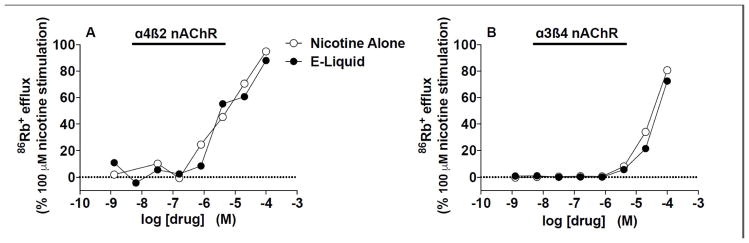
Nicotine alone and EC liquid had similar nAChR binding affinities at all nAChR subtypes studied (Table 2, see Fig. 3A and 3B for competition binding curves for α4β2 and α7 nAChRs expressed in rat brain). Nicotine alone and EC liquid also produced similar α4β2 or α3β4 nAChR activation in HEK 393 cells (see Table 3 and Figure 4). There was no evidence of antagonist activity of the formulations at either α4β2 or α3β4 nAChRs (data not shown).
Table 2.
Binding affinities for nicotine alone and EC liquid at nAChRs expressed in HEK 393 cells or rat brain (denoted by *) and labeled by [3H]-epibatidine. Values represent Ki (nM) and 95% CI (in parentheses) derived from a single competition binding curve at each nAChR subtype.
| Nicotine Alone | EC liquid | |
|---|---|---|
| α4β2 | 15.2 (11.5–20.1) | 17.2 (13.0–22.7) |
| α4β2* | 28.7 (21.4–38.4) | 38.5 (28.7–51.6) |
| α7 | 838.7 (254.6–2762.0) | 799.2 (232.1–2751.0) |
| α7* | 23.0 (18.4–28.7) | 28.1 (22.5–35.1) |
| α3β4 | 426.2 (141.9–1280.0) | 698.5 (248.5–1963.0) |
| α2β2 | 11.2 (7.0–18.0) | 15.6 (9.7–24.9) |
| α2β4 | 131.3 (65.9–261.4) | 173.4 (88.1–341.0) |
| α3β2 | 21.2 (13.3–33.9) | 37.0 (23.4–58.7) |
| α4β4 | 52.2 (36.8–74.1) | 54.3 (38.2–77.0) |
Figure 3.
Competition by nicotine alone and EC liquid (e-liquid) for α4β2 (A) or α7 (B) nAChR binding sites expressed in rat brain and labeled by [3H]-epibatidine. Ki values for formulations at these and other nAChRs are shown in Table 2.
Table 3.
86Rb+ efflux stimulated by nicotine alone and EC liquid (0.6 mg/ml) in α4β2 or α3β4 nAChRs expressed in HEK 393 cells. Agonist activity is scaled as % of the stimulation by 100 μM nicotine (100%). Values represent EC50 values (in M) and 95% confidence intervals (in parentheses) at each nAChR subtype. Data are derived from a single experiment run in quadruplicate.
| Nicotine Alone | EC liquid | |
|---|---|---|
| α4β2 | 8.2 (0.9–77.35) | 3.1 (0.8–11.2) |
| α3β4 | 42.8 (32.8–55.9) | 190.2 (19.0–1906.0) |
Figure 4.
86Rb+ efflux stimulated by nicotine alone and EC liquid (e-liquid) in α4β2 or α3β4 nAChRs expressed in HEK 393 cells. EC50 values for formulations at these nAChRs are shown in Table 3.
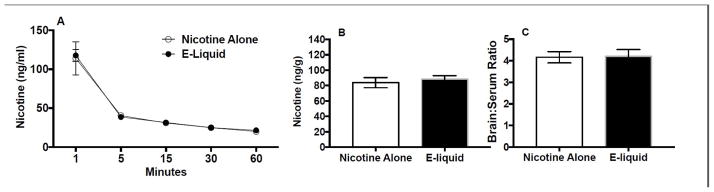
3.3. Experiment 3: Nicotine pharmacokinetics
There was a significant main effect of time on nicotine serum levels (F(4,56) = 44.25, p < 0.0001), but no effect of formulation or interaction. Serum nicotine levels did not differ between formulations at any time point (Fig. 5A). Brain nicotine levels (Fig. 5B) and brain/serum nicotine concentration ratios (Fig. 5C) did not differ at the 60 min time point.
Figure 5.
(A) Time course of serum nicotine levels following i.v. infusion of nicotine alone or EC liquid (e-liquid) (0.1 mg/kg). Brain nicotine levels (B) and brain/serum nicotine concentration ratios (C) at the 60 minute time point are also shown.
3.4. Experiment 4: Self-administration of nicotine alone and EC liquid
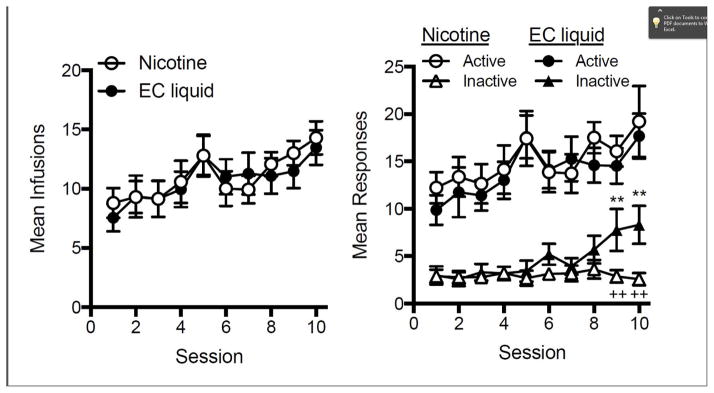
Fig. 6 shows infusion and response rates during the initial 10 days of acquisition for each group. There was a significant main effect of session on mean infusion rate (left panel, F(9,243) = 4.86, p < 0.0001) and mean active lever presses (right panel, F(9,242) = 3.50, p < 0.001), but no significant main effect of formulation or session x formulation interaction for either measure. Within each group, mean active lever presses was significantly higher than inactive lever presses (right panel, main effect: F(1,247) = 319.96, p < 0.0001 and F(1,265) = 162.32, p < 0.0001, for nicotine and EC liquid, respectively). Active lever pressing was higher than inactive lever pressing on all sessions in the nicotine group, as well as on all sessions except 1 and 9 in the EC liquid group (right panel). There was no significant effect of formulation on mean inactive lever presses, but there was a significant main effect of session (F(9,243) = 2.47, p < 0.05) and a session x formulation interaction (F(9,243) = 2.59, p < 0.01). Inactive lever pressing was higher on sessions 9 and 10 in the EC liquid group compared to the nicotine group (t(243) = 3.24 and 3.78, p < 0.01 and 0.001, respectively) and compared to session one in the EC liquid group (t(243) = 3.76 and 4.17, p < 0.001 and 0.001, respectively). However, by the time behavior stabilized on the FR 3 schedule, there was no difference in inactive lever pressing between formulations (5.39 ± 1.08 and 7.95 ± 1.59 responses for nicotine and EC liquid groups, respectively, data not shown).
Figure 6.
Mean (±SEM) infusions (left panel) or responses (right panel) during the first 10 session under the FR 1 schedule for each group. Each point represents the mean of 14–15 rats. ** Different from session 1, p < 0.01. ++ Different from EC liquid, p < 0.01.
There was a significant main effect of FR value on mean infusions (F(10,75) = 56.2, p < 0.0001), but no effect of formulation or formulation x FR interaction (data not shown). Consequently, no significant differences in mean demand curve parameters were observed between groups (Fig. 7 and Table 4). Demand curve fits were generally good at describing the relationship between consumption and unit price in individual subjects (Table 4).
Figure 7.
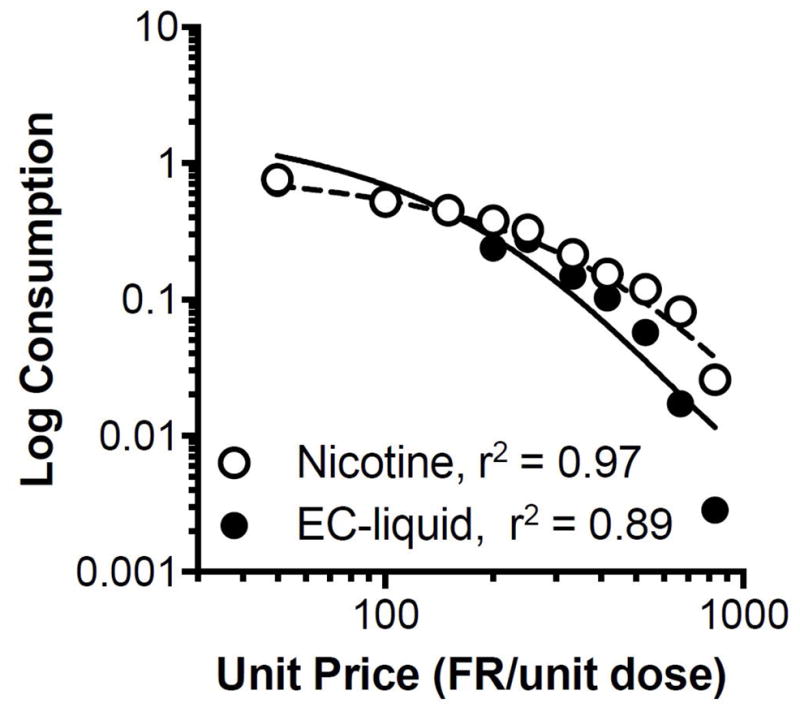
Group (N=7 each) demand curves for each nicotine formulation. Zero consumption was entered for missing data at higher unit prices in rats that exhibited zero consumption at lower prices. This approach provides a more accurate portrayal of the true group consumption at high unit prices.
Table 4.
Exponential demand curve parameter estimates for individual rats in each group (k set to 2.0 globally).
| Subject ID | α | Q0 | r2 | Pmax | Omax |
|---|---|---|---|---|---|
| Nicotine Alone | |||||
| 8543 | 0.00370 | 1.30 | 0.93 | 60.35 | 24.60 |
| 8544 | 0.00200 | 1.40 | 0.81 | 103.67 | 45.50 |
| 8547 | 0.00053 | 1.30 | 0.94 | 421.31 | 171.70 |
| 8548 | 0.00180 | 1.40 | 0.79 | 115.19 | 50.50 |
| 1125 | 0.00092 | 0.70 | 0.85 | 450.75 | 98.90 |
| 1128 | 0.00223 | 0.97 | 0.69 | 136.03 | 41.40 |
| 1129 | 0.00050 | 1.00 | 0.84 | 580.57 | 182.00 |
| Mean | 0.00166 | 1.15 | 0.84 | 266.80 | 87.80 |
| SEM | 0.00043 | 0.10 | 0.03 | 79.50 | 24.57 |
| EC Liquid | |||||
| 8545 | 0.00150 | 1.10 | 0.59 | 175.93 | 60.70 |
| 8546 | 0.00470 | 0.88 | 0.77 | 70.19 | 19.40 |
| 8550 | 0.00100 | 1.20 | 0.78 | 241.90 | 91.00 |
| 1122 | 0.00200 | 2.10 | 0.80 | 69.12 | 45.50 |
| 1123 | 0.00230 | 1.30 | 0.85 | 97.09 | 39.60 |
| 1126 | 0.00090 | 1.50 | 0.91 | 215.03 | 101.10 |
| 1127 | 0.00150 | 1.70 | 0.74 | 113.84 | 60.70 |
| Mean | 0.00199 | 1.40 | 0.77 | 140.40 | 59.71 |
| SEM | 0.00049 | 0.15 | 0.04 | 26.60 | 10.83 |
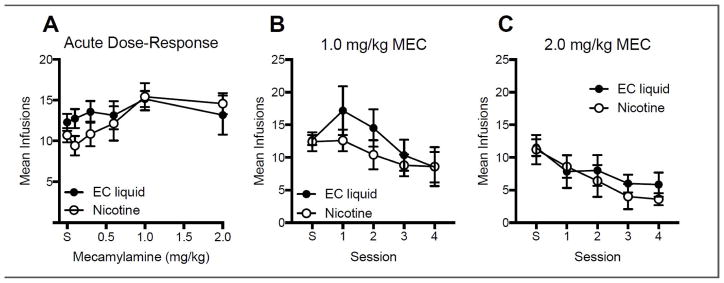
Fig. 8 shows the effects of acute (left panel) and repeated (right panel) MEC pretreatment on mean infusions per session. Although there was a tendency for infusion rates to be higher in the EC liquid group at low MEC doses, there were no statistically significant differences between formulations at any MEC dose, or any formulation x MEC dose interaction. During repeated MEC testing (right panel), there was a significant reduction in infusion rates across sessions (main effect: F(4,36) = 3.81, p < 0.001 and F(4,36) = 9.43, p < 0.0001 for the 1.0 and 2.0 mg/kg dose, respectively), but no significant difference between formulation or a formulation x session interaction.
Figure 8.
Mean (±SEM) infusions following acute pretreatment with the indicated MEC dose in each group (panel A) and following repeated pretreatment with the 1.0 mg/kg (panel B) or 2.0 mg/kg (panel C) MEC dose. Each point is the mean of 6–7 rats. “S” refers to saline.
4. DISCUSSION
Given the dramatic rise in EC use among adolescents and current smokers and the FDA CTP’s intention to regulate ECs, the present study begins to address an urgent need for preclinical research on the behavioral pharmacology of EC liquids. The main findings of the present study were that EC liquid administration decreased ICSS thresholds to a similar degree as nicotine alone, but was less potent than nicotine alone at increasing ICSS thresholds at high doses. In contrast, there were no differences between EC liquid and nicotine alone with regard to their reinforcing effects in a self-administration assay, blockade of behavioral effects by mecamylamine, nicotine pharmacokinetics, binding affinities at a range of nAChRs, or functional activity at α4β2 or α3β4 nAChRs. Taken together, the findings suggest that the relative abuse liability of this EC liquid is similar to that of nicotine alone in terms of its reinforcing and reinforcement-enhancing effects, but that it may have less aversive/anhedonic effects at high doses. These findings have several important implications for understanding the factors influencing EC use, and for future preclinical regulatory research on ECs.
The present findings help clarify the mechanisms through which non-nicotine constituents may contribute to the reinforcing effects of ECs. Some EC liquids like the one used in the present study contain non-nicotine tobacco alkaloids, flavorants, and other compounds in an attempt to simulate cigarette smoking. These compounds could contribute to the reinforcing effects of ECs through peripheral sensory mechanisms (e.g., improved taste, “throat hit”; Evans and Hoffman, 2014), CNS mechanisms (e.g., activation of mesolimbic dopamine pathways, Dwoskin et al., 1993, 1995), pharmacokinetic mechanisms (e.g., altered nicotine metabolism, Kramlinger et al., 2012; Stalhandske and Slanina, 1982) or a combination of these factors. Elucidating the relative roles of these mechanisms requires study of multiple routes of administration. To date, human studies have only examined ECs via the inhalation route, which doesn’t allow control of differences in peripheral sensory factors and absorption between products in order to isolate the influence of CNS factors. By using the intravenous route in the self-administration model and the subcutaneous route in the ICSS model, the present study suggests that systemic (i.e., post absorption) or parenteral exposure to the non-nicotine constituents in these ECs does not contribute to the direct CNS-mediated primary reinforcing or reinforcement-enhancing effects of ECs. Any influence of non-nicotine constituents on these aspects of EC abuse liability may reflect their peripheral sensory effects, influence on nicotine pharmacokinetics, or both. As such, the present findings suggest that the inhalational route is the key mode through which non-nicotine constituents impact abuse liability of ECs in humans.
The present study suggests a novel effect of non-nicotine EC constituents in moderating the behavioral effects of nicotine. Although the presence of non-nicotine constituents did not affect the primary reinforcing or reinforcement-enhancing effects of nicotine, they modestly attenuated the anhedonic or aversive effects of high nicotine doses in the ICSS assay. Because nicotine’s aversive effects limit its intake (Eissenberg and Balster, 2000; Fowler and Kenny, 2013; Fowler et al., 2011; Hu et al., 2006; Sartor et al., 2010), a reduction in these effects might increase consumption and, thus, the relative abuse liability of ECs compared to medicinal nicotine products (e.g., nicotine inhaler). However, intake of EC liquid was similar to nicotine alone in the SA model. This inconsistency between the ICSS and SA models was somewhat unexpected, as other manipulations that attenuate the ICSS threshold-elevating effects of nicotine (knockdown of brain alpha5 nAChRs) also increase SA of nicotine (Fowler et al., 2011). Indeed, the effects of this manipulation was most apparent at higher nicotine doses. The discrepancy in findings between models in the present study could be due to differences in EC liquid administration (e.g., dose, route, contingency, etc.). For instance, the cumulative level of nicotine intake in the SA experiment (0.72 mg/kg/session at the end of acquisition) may not have been sufficient to allow aversive effects to manifest. Studies examining SA at higher unit nicotine doses that maintain less robust SA, presumably due to aversive or other side effects (e.g., 0.09 mg/kg/infusion), might help clarify this issue. On the other hand, the serum nicotine concentrations produced by the 1.0–1.25 mg/kg nicotine doses in the ICSS study may be higher than what most rats could achieve with higher NSA unit doses (Craig et al., 2014; Donny et al., 2000; LeSage et al., 2002). If so, the ICSS findings may reflect an attenuation of nicotine’s toxic effects rather than its abuse liability. However, such an effect would still have important regulatory implications, as it could inform FDA CTP product standards to reduce toxicity of tobacco products.
Isolation and characterization of individual non-nicotine constituents in EC liquid will be important to better understand the extent to which these compounds could contribute to EC abuse liability. For example, we have previously shown that some isolated minor alkaloids (e.g., nornicotine, anabasine) that are normally present in EC liquid at very low concentrations can have significant effects on ICSS thresholds at much higher concentrations (Harris et al., 2015a). Therefore, although current levels of these constituents may not have influenced the reinforcing effects of the EC liquid used in the present study, higher levels in this or other EC liquids might impact abuse liability. Another constituent of interest is propylene glycol, an alcohol that is a primary ingredient in EC liquids and is pharmacologically and behaviorally active in both humans and animals (Forrest and Galletly, 1988; Lin et al., 1998; Singh et al., 1982). Given that levels of propylene glycol vary widely among EC products and that alcohol can enhance nicotine reinforcement and attenuate nicotine aversion (Deehan et al., 2015; Glautier et al., 1996; Griffiths et al., 1976; Kunin et al., 1999), relative propylene glycol content might be a determinant of relative abuse liability between EC products. Studies examining the interaction between propylene glycol and nicotine in the ICSS and SA models are needed to address this issue. Such work might also help clarify factors mediating the aversion-attenuating effects of EC liquids in the ICSS model.
Delivery of nicotine in EC liquid did not influence its binding affinity or function at any nAChR subtype studied. These data are consistent with prior reports that nicotine alone and tobacco smoke extract or smokeless tobacco extract produced a similar binding affinity profile at a range of nAChRs, including neuronal a 4β2 nAChRs (Costello et al., 2014; Harris et al., 2015b). Cigarette smoke and EC vapor at similar nicotine concentrations also produced equivalent upregulation of brain a 4β2 nAChRs in mice (Ponzoni et al., 2015). Together, these findings do not support alterations in nAChR binding affinity or α4β2 or α3β4 nAChR function as a mechanism by which non-nicotine tobacco constituents could contribute to EC or tobacco product use. Further research is needed to examine whether non-nicotine EC constituents alter the function of other nAChRs or the binding affinity and function of non-cholinergic receptors.
The finding that nicotine pharmacokinetics did not differ between formulations is similar to our prior study comparing serum and brain nicotine concentrations between nicotine alone and a smokeless tobacco extract 10 min after s.c. injection of equivalent nicotine doses (Harris 2012). The present study extends our prior findings by measuring nicotine concentrations at a wider range of time points and using a different route of administration and type of tobacco product. Although serum concentrations were only measured up to 60 min post administration, the strong similarity between formulations suggests that nicotine elimination is unaffected by the presence of non-nicotine constituents in EC liquid. These findings are consistent with others showing no effect of non-nicotine constituents on nicotine disposition (Cao et al., 2007). However, it will be important to examine the effects of menthol-flavored EC liquids, given recent demonstrations that menthol can alter nicotine pharmacokinetics and behavioral effects (Abobo et al., 2012; Alsharari et al., 2015; Benowitz et al., 2004; Wang et al., 2014).
Although this EC liquid is formulated with non-nicotine tobacco alkaloids to emulate cigarette smoking, the present findings contrast with studies showing greater reinforcing effects of smoke extracts compared to nicotine alone, as well as greater resistance of smoke extract SA to the effects of nAChR antagonists (Brennan et al., 2013a, 2013b; Costello et al., 2014). Similarly, cigarette smoke exposure has been shown to reduce body weight and food intake, while EC aerosol had no effect (Ponzoni et al., 2015). The severity of some signs of MEC-precipitated withdrawal was also greater in mice exposed to cigarette smoke compared to EC aerosol (Ponzoni et al., 2015). Together with our studies showing no differences in behavioral effects between smokeless tobacco extract and nicotine alone, these findings suggest that the non-nicotine constituents contributing to the greater reinforcing effects of smoke extracts might be specific to the smoke from combusted tobacco products (i.e., those not found or present at relatively low levels in non-combusted products). Direct comparisons of EC liquid and smoke extract SA are needed to examine this issue.
Future studies should address a number of limitations. First, EC exposure was modeled in the present study using dilutions of EC liquid. Using an extract of the aerosol produced by EC use would more closely approximate EC exposure in humans and may result in a different spectrum of behavioral effects. Second, it is unclear to what extent the relative blood levels of nicotine and non-nicotine constituents in rats in the present study are comparable to what occurs in EC users. The lower doses used in the ICSS model and the unit dose used in the SA model yield blood nicotine concentrations in the range of smokers or EC users (Benowitz et al., 1982; Craig et al., 2014; Farsalinos et al., 2015; LeSage et al., 2002). Therefore, to the extent that relative concentration of key constituents in blood is similar to that in EC aerosol, levels of non-nicotine constituents in our model would be comparable to that in humans. However, studies in humans on blood levels of non-nicotine constituents are needed to confirm this. Third, given the plethora of EC products on the market that vary significantly in their formulation, it will be essential to determine whether the present findings generalize to other EC products. Fourth, the experimental design in the present study lacked a positive control (e.g., cocaine) to indicate whether the assays were sufficiently sensitive to detect differences in reinforcing efficacy. However, similar assays have detected differences between nicotine alone and tobacco extracts or combinations of nicotine with other tobacco constituents (Brennan et al., 2013a; Clemens et al., 2009; Costello et al., 2014). Nonetheless, further research is needed to examine other approaches that might be more sensitive in detecting differences in abuse liability between nicotine formulations. Finally, only adult males of one rat strain were used in the present study. Adolescents, females, and other strains can be more sensitive to some behavioral effects of nicotine and/or non-nicotine tobacco constituents (Belluzzi et al., 2005; Brower et al., 2002; Donny et al., 2000; Grebenstein et al., 2013) and, therefore, may be more sensitive to differences in reinforcing effects between nicotine alone and extracts of tobacco products. Despite its limitations, the present study makes and important initial contribution to the preclinical regulatory science needed to better understand the behavioral pharmacology and toxicology of EC exposure. As such, our findings may be useful to the FDA CTP in evaluating any claim that this EC liquid is substantially equivalent to nicotine alone.
Highlights.
Nicotine and electronic cigarette (EC) liquid produced similar decreases in brain reinforcement threshold.
EC liquid produced less aversion/anhedonia at high nicotine doses.
Binding and activation of nAChRs were similar between nicotine and EC liquid.
Nicotine pharmacokinetics were similar between nicotine and EC liquid.
Nicotine and EC liquid self-administration did not differ in rats.
Acknowledgments
Role of funding source
Funding for this study was provided by NIH/NCI grant U19-CA157345 (Hatsukami DH and Shields P, MPI; LeSage MG, PL), NIDA training grant T32 DA007097 (Smethells, JR; Molitor T, PI), and a Career Development Award (MGL) and Translational Research Program (ACH) from the Minneapolis Medical Research Foundation. These funding institutions had no role in the study design, data collection, data analysis, interpretation of the data, manuscript preparation, or decisions to submit the manuscript for publication. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Food and Drug Administration.
The authors thank Danielle Burroughs, Laura Tally, Theresa Harmon, Clare Schmidt, Christine Egan, and Andrew Banal for their excellent technical assistance in conducting the experiment. The authors also thank Drs. Steven Hursh and Pete Roma from the Institutes for Behavior Resources (Baltimore, MD) and Johns Hopkins University School of Medicine for providing the software for demand curve analysis and their assistance with conducting the analysis. Ki determinations and agonist/antagonist functional data were generously provided by the National Institute of Mental Health's Psychoactive Drug Screening Program, Contract # HHSN-271-2008-00025-C (NIMH PDSP). The NIMH PDSP is Directed by Bryan L. Roth MD, PhD at the University of North Carolina at Chapel Hill and Project Officer Jamie Driscol at NIMH, Bethesda MD, USA. Preliminary data from this study were presented at the 22nd Annual Meeting of the Society for Research on Nicotine and Tobacco, Chicago, IL; March 2016.
Footnotes
Contributors
MGL and ACH designed and supervised conduct of the study. MS, PM, and JRS conducted the behavioral studies. IS conducted the alkaloid analysis. PRP advised the design and data analysis for the pharmacokinetic assessment. RIV conducted the statistical analyses. MGL and ACH wrote drafts of the manuscript. All authors contributed to and approved the final manuscript.
Conflict of interest
The authors have no conflicts to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abobo CV, Ma J, Liang D. Effect of menthol on nicotine pharmacokinetics in rats after cigarette smoke inhalation. Nicotine Tob Res. 2012;14:801–808. doi: 10.1093/ntr/ntr287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsharari SD, King JR, Nordman JC, Muldoon PP, Jackson A, Zhu AZ, Tyndale RF, Kabbani N, Damaj MI. Effects of menthol on nicotine pharmacokinetic, pharmacology and dependence in mice. PLoS One. 2015;10:e0137070. doi: 10.1371/journal.pone.0137070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrose V, Miller JH, Dickson SJ, Hampton S, Truman P, Lea RA, Fowles J. Tobacco particulate matter is more potent than nicotine at upregulating nicotinic receptors on SH-SY5Y cells. Nicotine Tob Res. 2007;9:793–799. doi: 10.1080/14622200701485117. [DOI] [PubMed] [Google Scholar]
- Arrazola RA, Singh T, Corey CG, Husten CG, Neff LJ, Apelberg BJ, Bunnell RE, Choiniere CJ, King BA, Cox S, McAfee T, Caraballo RS Centers for Disease C, Prevention. Tobacco use among middle and high school students - United States, 2011–2014. MMWR. 2015;64:381–385. [PMC free article] [PubMed] [Google Scholar]
- Bardo MT, Green TA, Crooks PA, Dwoskin LP. Nornicotine is self-administered intravenously by rats. Psychopharmacology (Berl) 1999;146:290–296. doi: 10.1007/s002130051119. [DOI] [PubMed] [Google Scholar]
- Bauco P, Wise RA. Potentiation of lateral hypothalamic and midline mesencephalic brain stimulation reinforcement by nicotine: examination of repeated treatment. J Pharmacol Exp Ther. 1994;271:294–301. [PubMed] [Google Scholar]
- Belluzzi JD, Wang R, Leslie FM. Acetaldehyde enhances acquisition of nicotine self-administration in adolescent rats. Neuropsychopharmacology. 2005;30:705–712. doi: 10.1038/sj.npp.1300586. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: A powerful and practical approach to multiple testing in behavior genetics research. JR Stat Soc Series B Methodol. 1995;57:289–300. [Google Scholar]
- Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann Stat. 2001;29:1165–1188. [Google Scholar]
- Benowitz NL, Herrera B, Jacob P., 3rd Mentholated cigarette smoking inhibits nicotine metabolism. J Pharmacol Exp Ther. 2004;310:1208–1215. doi: 10.1124/jpet.104.066902. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Kuyt F, Jacob P., 3rd Circadian blood nicotine concentrations during cigarette smoking. Clin Pharmacol Ther. 1982;32:758–764. doi: 10.1038/clpt.1982.233. [DOI] [PubMed] [Google Scholar]
- Biala G, Staniak N, Budzynska B. Effects of varenicline and mecamylamine on the acquisition, expression, and reinstatement of nicotine-conditioned place preference by drug priming in rats. Naunyn Schmiedebergs Arch Pharmacol. 2010;381:361–370. doi: 10.1007/s00210-010-0498-5. [DOI] [PubMed] [Google Scholar]
- Brandon TH, Goniewicz ML, Hanna NH, Hatsukami DK, Herbst RS, Hobin JA, Ostroff JS, Shields PG, Toll BA, Tyne CA, Viswanath K, Warren GW. Electronic nicotine delivery systems: a policy statement from the American Association for Cancer Research and the American Society of Clinical Oncology. J Clin Oncol. 2015;33:952–963. doi: 10.1200/JCO.2014.59.4465. [DOI] [PubMed] [Google Scholar]
- Breland AB, Spindle T, Weaver M, Eissenberg T. Science and electronic cigarettes: current data, future needs. J Addict Med. 2014;8:223–233. doi: 10.1097/ADM.0000000000000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan KA, Crowther A, Putt F, Roper V, Waterhouse U, Truman P. Tobacco particulate matter self-administration in rats: differential effects of tobacco type. Addict Biol. 2013a doi: 10.1111/adb.12099. [DOI] [PubMed] [Google Scholar]
- Brennan KA, Laugesen M, Truman P. Whole tobacco smoke extracts to model tobacco dependence in animals. Neurosci Biobehav Rev. 2014;47C:53–69. doi: 10.1016/j.neubiorev.2014.07.014. [DOI] [PubMed] [Google Scholar]
- Brennan KA, Putt F, Roper V, Waterhouse U, Truman P. Nicotine and tobacco particulate self-administration: effects of mecamylamine, SCH23390 and ketanserin pretreatment. Curr Psychopharmacol. 2013b;2:229–240. [Google Scholar]
- Brennan KA, Putt F, Truman P. Nicotine-, tobacco particulate matter- and methamphetamine-produced locomotor sensitisation in rats. Psychopharmacology (Berl) 2013c;228:659–672. doi: 10.1007/s00213-013-3071-3. [DOI] [PubMed] [Google Scholar]
- Brower VG, Fu Y, Matta SG, Sharp BM. Rat strain differences in nicotine self-administration using an unlimited access paradigm. Brain Res. 2002;930:12–20. doi: 10.1016/s0006-8993(01)03375-3. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Palmatier MI, Liu X, Chaudhri N, Sved AF. The role of nicotine in smoking: a dual-reinforcement model. Nebr Symp Motiv. 2009;55:91–109. doi: 10.1007/978-0-387-78748-0_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Belluzzi JD, Loughlin SE, Keyler DE, Pentel PR, Leslie FM. Acetaldehyde, a major constituent of tobacco smoke, enhances behavioral, endocrine, and neuronal responses to nicotine in adolescent and adult rats. Neuropsychopharmacology. 2007;32:2025–2035. doi: 10.1038/sj.npp.1301327. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF. Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology (Berl) 2006;184:353–366. doi: 10.1007/s00213-005-0178-1. [DOI] [PubMed] [Google Scholar]
- Clarke PBS, Kumar R. The effects of nicotine on locomotor activity in non-tolerant and tolerant rats. Br J Pharmacol. 1983;78:329–337. doi: 10.1111/j.1476-5381.1983.tb09398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens KJ, Caille S, Stinus L, Cador M. The addition of five minor tobacco alkaloids increases nicotine-induced hyperactivity, sensitization and intravenous self-administration in rats. Int J Neuropsychopharmacol. 2009:1355–1366. doi: 10.1017/S1461145709000273. [DOI] [PubMed]
- Cobb CO, Hendricks PS, Eissenberg T. Electronic cigarettes and nicotine dependence: evolving products, evolving problems. BMC Med. 2015;13:119. doi: 10.1186/s12916-015-0355-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello MR, Reynaga DD, Mojica CY, Zaveri NT, Belluzzi JD, Leslie FM. Comparison of the reinforcing properties of nicotine and cigarette smoke extract in rats. Neuropsychopharmacology. 2014;39:1843–1851. doi: 10.1038/npp.2014.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig EL, Zhao B, Cui JZ, Novalen M, Miksys S, Tyndale RF. Nicotine pharmacokinetics in rats is altered as a function of age, impacting the interpretation of animal model data. Drug Metab Dispos. 2014;42:1447–1455. doi: 10.1124/dmd.114.058719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva AL, Elisabetsky E. Interference of propylene glycol with the hole-board test. Braz J Med Biol Res. 2001;34:545–547. doi: 10.1590/s0100-879x2001000400016. [DOI] [PubMed] [Google Scholar]
- Deehan GA, Jr, Hauser SR, Waeiss RA, Knight CP, Toalston JE, Truitt WA, McBride WJ, Rodd ZA. Co-administration of ethanol and nicotine: the enduring alterations in the rewarding properties of nicotine and glutamate activity within the mesocorticolimbic system of female alcohol-preferring (P) rats. Psychopharmacology (Berl) 2015;232:4293–4302. doi: 10.1007/s00213-015-4056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Rowell PP, Gharib MA, Maldovan V, Booth S, Mielke MM, Hoffman A, McCallum S. Nicotine self-administration in rats: estrous cycle effects, sex differences and nicotinic receptor binding. Psychopharmacology (Berl) 2000;151:392–405. doi: 10.1007/s002130000497. [DOI] [PubMed] [Google Scholar]
- Donny EC, Taylor TG, LeSage MG, Levin M, Buffalari DM, Joel D, Sved AF. Impact of tobacco regulation on animal research: new perspectives and opportunities. Nicotine Tob Res. 2012;14:1319–1338. doi: 10.1093/ntr/nts162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwoskin LP, Buxton ST, Jewell AL, Crooks PA. S(-)-nornicotine increases dopamine release in a calcium-dependent manner from superfused rat striatal slices. J Neurochem. 1993;60:2167–2174. doi: 10.1111/j.1471-4159.1993.tb03502.x. [DOI] [PubMed] [Google Scholar]
- Dwoskin LP, Crooks PA, Teng L, Green TA, Bardo MT. Acute and chronic effects of nornicotine on locomotor activity in rats: altered response to nicotine. Psychopharmacology (Berl) 1999;145:442–451. doi: 10.1007/s002130051079. [DOI] [PubMed] [Google Scholar]
- Dwoskin LP, Teng L, Buxton ST, Ravard A, Deo N, Crooks PA. Minor alkaloids of tobacco release [3H]dopamine from superfused rat striatal slices. Eur J Pharmacol. 1995;276:195–199. doi: 10.1016/0014-2999(95)00077-x. [DOI] [PubMed] [Google Scholar]
- Eissenberg T, Balster RL. Initial tobacco use episodes in children and adolescents: current knowledge, future directions. Drug Alcohol Depend. 2000;59(Suppl 1):S41–60. doi: 10.1016/s0376-8716(99)00164-7. [DOI] [PubMed] [Google Scholar]
- Etter JF, Zather E, Svensson S. Analysis of refill liquids for electronic cigarettes. Addiction. 2013;108:1671–1679. doi: 10.1111/add.12235. [DOI] [PubMed] [Google Scholar]
- Evans SE, Hoffman AC. Electronic cigarettes: abuse liability, topography and subjective effects. Tob Control. 2014;23(Suppl 2):ii23–29. doi: 10.1136/tobaccocontrol-2013-051489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos KE, Spyrou A, Stefopoulos C, Tsimopoulou K, Kourkoveli P, Tsiapras D, Kyrzopoulos S, Poulas K, Voudris V. Nicotine absorption from electronic cigarette use: comparison between experienced consumers (vapers) and naive users (smokers) Sci Rep. 2015;5:11269. doi: 10.1038/srep11269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foddai M, Dosia G, Spiga S, Diana M. Acetaldehyde increases dopaminergic neuronal activity in the VTA. Neuropsychopharmacology. 2004;29:530–536. doi: 10.1038/sj.npp.1300326. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration. Draft Guidance For Industry: Assessment Of Abuse Potential Of Drug. 2010 Retrieved from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM198650.pdf.
- Forrest P, Galletly DC. A double-blind comparative study of three formulations of diazepam in volunteers. Anaesth Intensive Care. 1988;16:158–163. doi: 10.1177/0310057X8801600205. [DOI] [PubMed] [Google Scholar]
- Fowler CD, Kenny PJ. Habenular signaling in nicotine reinforcement. Neuropsychopharmacology. 2012;37:306–307. doi: 10.1038/npp.2011.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CD, Kenny PJ. Nicotine aversion: Neurobiological mechanisms and relevance to tobacco dependence vulnerability. Neuropharmacology. 2013 doi: 10.1016/j.neuropharm.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ. Habenular alpha5 nicotinic receptor subunit signalling controls nicotine intake. Nature. 2011;471:597–601. doi: 10.1038/nature09797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glautier S, Clements K, White JA, Taylor C, Stolerman IP. Alcohol and the reward value of cigarette smoking. Behav Pharmacol. 1996;7:144–154. [PubMed] [Google Scholar]
- Goniewicz ML, Knysak J, Gawron M, Kosmider L, Sobczak A, Kurek J, Prokopowicz A, Jablonska-Czapla M, Rosik-Dulewska C, Havel C, Jacob P, 3rd, Benowitz N. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014;23:133–139. doi: 10.1136/tobaccocontrol-2012-050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebenstein P, Burroughs D, Zhang Y, LeSage MG. Sex differences in nicotine self-administration in rats during progressive unit dose reduction: implications for nicotine regulation policy. Pharmacol Biochem Behav. 2013;114–115:70–81. doi: 10.1016/j.pbb.2013.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebenstein PE, Burroughs D, Roiko SA, Pentel PR, LeSage MG. Predictors of the nicotine reinforcement threshold, compensation, and elasticity of demand in a rodent model of nicotine reduction policy. Drug Alcohol Depend. 2015;151:181–193. doi: 10.1016/j.drugalcdep.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Bigelow GE, Liebson I. Facilitation of human tobacco self-administration by ethanol: a behavioral analysis. J Exp Anal Behav. 1976;25:279–292. doi: 10.1901/jeab.1976.25-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillem K, Vouillac C, Azar MR, Parsons LH, Koob GF, Cador M, Stinus L. Monoamine oxidase inhibition dramatically increases the motivation to self-administer nicotine in rats. J Neurosci. 2005;25:8593–8600. doi: 10.1523/JNEUROSCI.2139-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell PT, Simmons VN, Correa JB, Padhya TA, Brandon TH. Electronic nicotine delivery systems ("e-cigarettes"): review of safety and smoking cessation efficacy. Otolaryngol Head Neck Surg. 2014;151:381–393. doi: 10.1177/0194599814536847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AC, Burroughs D, Pentel PR, LeSage MG. Compensatory nicotine self-administration in rats during reduced access to nicotine: an animal model of smoking reduction. Exp Clin Psychopharmacol. 2008;16:86–97. doi: 10.1037/1064-1297.16.1.86. [DOI] [PubMed] [Google Scholar]
- Harris AC, Mattson C, LeSage MG, Keyler DE, Pentel PR. Comparison of the behavioral effects of cigarette smoke and pure nicotine in rats. Pharmacol Biochem Behav. 2010;96:217–227. doi: 10.1016/j.pbb.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AC, Pentel PR, Burroughs D, Staley MD, LeSage MG. A lack of association between severity of nicotine withdrawal and individual differences in compensatory nicotine self-administration in rats. Psychopharmacology (Berl) 2011;217:153–166. doi: 10.1007/s00213-011-2273-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AC, Pentel PR, LeSage MG. Correlates of individual differences in compensatory nicotine self-administration in rats following a decrease in nicotine unit dose. Psychopharmacology (Berl) 2009;205:599–611. doi: 10.1007/s00213-009-1567-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AC, Stepanov I, Pentel PR, LeSage MG. Delivery of nicotine in an extract of a smokeless tobacco product reduces its reinforcement-attenuating and discriminative stimulus effects in rats. Psychopharmacology (Berl) 2012;220:565–576. doi: 10.1007/s00213-011-2514-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AC, Tally L, Muelken P, Banal A, Schmidt CE, Cao Q, LeSage MG. Effects of nicotine and minor tobacco alkaloids on intracranial-self-stimulation in rats. Drug Alcohol Depend. 2015a;153:330–334. doi: 10.1016/j.drugalcdep.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AC, Tally L, Schmidt CE, Muelken P, Stepanov I, Saha S, Vogel RI, LeSage MG. Animal models to assess the abuse liability of tobacco products: effects of smokeless tobacco extracts on intracranial self-stimulation. Drug Alcohol Depend. 2015b;147:60–67. doi: 10.1016/j.drugalcdep.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison AA, Gasparini F, Markou A. Nicotine potentiation of brain stimulation reward reversed by DH beta E and SCH 23390, but not by eticlopride, LY 314582 or MPEP in rats. Psychopharmacology (Berl) 2002;160:56–66. doi: 10.1007/s00213-001-0953-6. [DOI] [PubMed] [Google Scholar]
- Hieda Y, Keyler DE, VanDeVoort JT, Niedbala RS, Raphael DE, Ross CA, Pentel PR. Immunization of rats reduces nicotine distribution to brain. Psychopharmacology (Berl) 1999;143:150–157. doi: 10.1007/s002130050930. [DOI] [PubMed] [Google Scholar]
- Hillman MG, Schneider CW. Voluntary selection of and tolerance to 1,2 propanediol (propylene glycol) by high and low ethanol-selecting mouse strains. J Comp Physiol Psychol. 1975;88:773–777. doi: 10.1037/h0076407. [DOI] [PubMed] [Google Scholar]
- Hu MC, Davies M, Kandel DB. Epidemiology and correlates of daily smoking and nicotine dependence among young adults in the United States. Am J Public Health. 2006;96:299–308. doi: 10.2105/AJPH.2004.057232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR, Silberberg A. Economic demand and essential value. Psychological Review. 2008;115(1):186–198. doi: 10.1037/0033-295X.115.1.186. [DOI] [PubMed] [Google Scholar]
- Huston-Lyons D, Kornetsky C. Effects of nicotine on the threshold for rewarding brain stimulation in rats. Pharmacol Biochem Behav. 1992;41:755–759. doi: 10.1016/0091-3057(92)90223-3. [DOI] [PubMed] [Google Scholar]
- Jacob P, 3rd, Wilson M, Benowitz NL. Improved gas chromatographic method for the determination of nicotine and cotinine in biologic fluids. J Chromatogr. 1981;222:61–70. doi: 10.1016/s0378-4347(00)81033-6. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Polis I, Koob GF, Markou A. Low dose cocaine self-administration transiently increases but high dose cocaine persistently decreases brain reward function in rats. Eur J Neurosci. 2003;17:191–195. doi: 10.1046/j.1460-9568.2003.02443.x. [DOI] [PubMed] [Google Scholar]
- Kornetsky C, Esposito RU. Euphorigenic drugs: effects on the reward pathways of the brain. Fed Proc. 1979;38:2473–2476. [PubMed] [Google Scholar]
- Kornetsky C, Esposito RU, McLean S, Jacobson JO. Intracranial self-stimulation thresholds: a model for the hedonic effects of drugs of abuse. Arch Gen Psychiatry. 1979;36:289–292. doi: 10.1001/archpsyc.1979.01780030055004. [DOI] [PubMed] [Google Scholar]
- Kosmider L, Sobczak A, Fik M, Knysak J, Zaciera M, Kurek J, Goniewicz ML. Carbonyl compounds in electronic cigarette vapors: effects of nicotine solvent and battery output voltage. Nicotine Tob Res. 2014;16:1319–1326. doi: 10.1093/ntr/ntu078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramlinger VM, von Weymarn LB, Murphy SE. Inhibition and inactivation of cytochrome P450 2A6 and cytochrome P450 2A13 by menthofuran, beta–nicotyrine and menthol. Chem Biol Interact. 2012;197:87–92. doi: 10.1016/j.cbi.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunin D, Smith BR, Amit Z. Nicotine and ethanol interaction on conditioned taste aversions induced by both drugs. Pharmacol Biochem Behav. 1999;62:215–221. doi: 10.1016/s0091-3057(98)00155-5. [DOI] [PubMed] [Google Scholar]
- Lauterstein D, Hoshino R, Gordon T, Watkins BX, Weitzman M, Zelikoff J. The changing face of tobacco use among United States youth. Curr Drug Abuse Rev. 2014;7:29–43. doi: 10.2174/1874473707666141015220110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeSage MG. Toward a nonhuman model of contingency management: effects of reinforcing abstinence from nicotine self-administration in rats with an alternative nondrug reinforcer. Psychopharmacology (Berl) 2009;203:13–22. doi: 10.1007/s00213-008-1362-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeSage MG, Burroughs D, Dufek M, Keyler DE, Pentel PR. Reinstatement of nicotine self-administration in rats by presentation of nicotine-paired stimuli, but not nicotine priming. Pharmacol Biochem Behav. 2004;79:507–513. doi: 10.1016/j.pbb.2004.09.002. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Keyler DE, Collins G, Pentel PR. Effects of continuous nicotine infusion on nicotine self-administration in rats: relationship between continuously infused and self-administered nicotine doses and serum concentrations. Psychopharmacology (Berl) 2003;170:278–286. doi: 10.1007/s00213-003-1539-2. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Keyler DE, Shoeman D, Raphael D, Collins G, Pentel PR. Continuous nicotine infusion reduces nicotine self-administration in rats with 23-h/day access to nicotine. Pharmacol Biochem Behav. 2002;72:279–289. doi: 10.1016/s0091-3057(01)00775-4. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Shelley D, Pravetoni M, Pentel PR. Enhanced attenuation of nicotine discrimination in rats by combining nicotine-specific antibodies with a nicotinic receptor antagonist. Pharmacol Biochem Behav. 2012;102:157–162. doi: 10.1016/j.pbb.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HQ, Burden PM, Johnston GA. Propylene glycol elicits anxiolytic-like responses to the elevated plus-maze in male mice. J Pharm Pharmacol. 1998;50:1127–1131. doi: 10.1111/j.2042-7158.1998.tb03323.x. [DOI] [PubMed] [Google Scholar]
- Markou A, Koob GF. Construct validity of a self-stimulation threshold paradigm: effects of reward and performance manipulations. Physiol Behav. 1992;51:111–119. doi: 10.1016/0031-9384(92)90211-j. [DOI] [PubMed] [Google Scholar]
- Myers WD, Ng KT, Singer G. Intravenous self-administration of acetaldehyde in the rat as a function of schedule, food deprivation and photoperiod. Pharmacol Biochem Behav. 1982;17:807–811. doi: 10.1016/0091-3057(82)90364-1. [DOI] [PubMed] [Google Scholar]
- Myers WD, Ng KT, Singer G. Effects of naloxone and buprenorphine on intravenous acetaldehyde self-injection in rats. Physiol Behav. 1984;33:449–455. doi: 10.1016/0031-9384(84)90168-9. [DOI] [PubMed] [Google Scholar]
- Negus SS, Miller LL. Intracranial self-stimulation to evaluate abuse potential of drugs. Pharmacol Rev. 2014;66:869–917. doi: 10.1124/pr.112.007419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orellana-Barrios MA, Payne D, Mulkey Z, Nugent K. Electronic cigarettes-a narrative review for clinicians. Am J Med. 2015;128:674–681. doi: 10.1016/j.amjmed.2015.01.033. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Balfour DJ, Markou A. Chronic bupropion differentially alters the reinforcing, reward-enhancing and conditioned motivational properties of nicotine in rats. Nicotine Tob Res. 2008;10:995–1008. doi: 10.1080/14622200802097571. [DOI] [PubMed] [Google Scholar]
- Ponzoni L, Moretti M, Sala M, Fasoli F, Mucchietto V, Lucini V, Cannazza G, Gallesi G, Castellana CN, Clementi F, Zoli M, Gotti C, Braida D. Different physiological and behavioural effects of e-cigarette vapour and cigarette smoke in mice. Eur Neuropsychopharmacol. 2015;25:1775–1786. doi: 10.1016/j.euroneuro.2015.06.010. [DOI] [PubMed] [Google Scholar]
- Porter L, Duke J, Hennon M, Dekevich D, Crankshaw E, Homsi G, Farrelly M. Electronic cigarette and traditional cigarette use among middle and high school students in Florida, 2011–2014. PLoS One. 2015;10:e0124385. doi: 10.1371/journal.pone.0124385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangiah K, Hwang WT, Mesaros C, Vachani A, Blair IA. Nicotine exposure and metabolizer phenotypes from analysis of urinary nicotine and its 15 metabolites by LC–MS. Bioanalysis. 2011;3:745–761. doi: 10.4155/bio.11.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roiko SA, Harris AC, LeSage MG, Keyler DE, Pentel PR. Passive immunization with a nicotine-specific monoclonal antibody decreases brain nicotine levels but does not precipitate withdrawal in nicotine-dependent rats. Pharmacol Biochem Behav. 2009;93:105–111. doi: 10.1016/j.pbb.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor CE, Lessov-Schlaggar CN, Scherrer JF, Bucholz KK, Madden PA, Pergadia ML, Grant JD, Jacob T, Xian H. Initial response to cigarettes predicts rate of progression to regular smoking: findings from an offspring-of-twins design. Addict Behav. 2010;35:771–778. doi: 10.1016/j.addbeh.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh PP, Junnarkar AY, Seshagirirao C, Kaushal R, Naidu MU, Varma RK, Tripathi RM, Shridhar DR. A pharmacological study of propane-1,2-diol. Arzneimittelforschung. 1982;32:1443–1446. [PubMed] [Google Scholar]
- Spiller K, Xi ZX, Li X, Ashby CR, Jr, Callahan PM, Tehim A, Gardner EL. Varenicline attenuates nicotine-enhanced brain-stimulation reward by activation of alpha4beta2 nicotinic receptors in rats. Neuropharmacology. 2009;57:60–66. doi: 10.1016/j.neuropharm.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalhandske T, Slanina P. Nicotyrine inhibits in vivo metabolism of nicotine without increasing its toxicity. Toxicol Appl Pharmacol. 1982;65:366–372. doi: 10.1016/0041-008x(82)90382-9. [DOI] [PubMed] [Google Scholar]
- Takayama S, Uyeno ET. Intravenous self-administration of ethanol and acetaldehyde by rats. Yakubutsu Seishin Kodo. 1985;5:329–334. [PubMed] [Google Scholar]
- Touiki K, Rat P, Molimard R, Chait A, de Beaurepaire R. Effects of tobacco and cigarette smoke extracts on serotonergic raphe neurons in the rat. Neuroreport. 2007;18:925–929. doi: 10.1097/WNR.0b013e32811d6d21. [DOI] [PubMed] [Google Scholar]
- Villegier AS, Lotfipour S, McQuown SC, Belluzzi JD, Leslie FM. Tranylcypromine enhancement of nicotine self-administration. Neuropharmacology. 2007;52:1415–1425. doi: 10.1016/j.neuropharm.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Walton KM, Abrams DB, Bailey WC, Clark D, Connolly GN, Djordjevic MV, Eissenberg TE, Fiore MC, Goniewicz ML, Haverkos L, Hecht SS, Henningfield JE, Hughes JR, Oncken CA, Postow L, Rose JE, Wanke KL, Yang L, Hatsukami DK. NIH electronic cigarette workshop: developing a research agenda. Nicotine Tob Res. 2015;17:259–269. doi: 10.1093/ntr/ntu214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Wang B, Chen H. Menthol facilitates the intravenous self-administration of nicotine in rats. Front Behav Neurosci. 2014;8:437. doi: 10.3389/fnbeh.2014.00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Addictive drugs and brain stimulation reward. Annu Rev Neurosci. 1996;19:319–340. doi: 10.1146/annurev.ne.19.030196.001535. [DOI] [PubMed] [Google Scholar]
- Wise RA. Brain reward circuitry: insights from unsensed incentives. Neuron. 2002;36:229–240. doi: 10.1016/s0896-6273(02)00965-0. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bauco P, Carlezon WA, Jr, Trojniar W. Self-stimulation and drug reward mechanisms. Ann N Y Acad Sci. 1992;654:192–198. doi: 10.1111/j.1749-6632.1992.tb25967.x. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Fan H, Musachio JL, Wei ZL, Chellappan SK, Kozikowski AP, Kellar KJ. Sazetidine-A, a novel ligand that desensitizes alpha4beta2 nicotinic acetylcholine receptors without activating them. Mol Pharmacol. 2006;70:1454–1460. doi: 10.1124/mol.106.027318. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Meyer EL, Thompson JM, Surin A, Wroblewski J, Kellar KJ. Rat alpha3/beta4 subtype of neuronal nicotinic acetylcholine receptor stably expressed in a transfected cell line: pharmacology of ligand binding and function. Mol Pharmacol. 1998;54:322–333. doi: 10.1124/mol.54.2.322. [DOI] [PubMed] [Google Scholar]
- Zaroslinski JF, Browne RK, Possley LH. Propylene glycol as a drug solvent in pharmacologic studies. Toxicol Appl Pharmacol. 1971;19:573–578. doi: 10.1016/0041-008x(71)90289-4. [DOI] [PubMed] [Google Scholar]