Abstract
Objective:
Imaging may be promising for colorectal cancer (CRC) screening, since it has test characteristics comparable with colonoscopy but is less invasive. We aimed to assess the potential of CT colonography (CTC) and MR colonography (MRC) in terms of (cost-effectiveness) using the Adenoma and Serrated pathway to Colorectal CAncer model.
Methods:
We compared several CTC and MRC strategies with 5- or 10-yearly screening intervals with no screening, 10-yearly colonoscopy screening and biennial faecal immunochemical test (FIT) screening. We assumed trial-based participation rates in the base-case analyses and varied the rates in sensitivity analyses. Incremental lifetime costs and health effects were estimated from a healthcare perspective.
Results:
The health gain of CTC and MRC was similar and ranged from 0.031 to 0.048 life-year gained compared with no screening, for 2–5 screening rounds. Lifetime costs per person for MRC strategies were €60–110 higher than those for CTC strategies with an equal number of screening rounds. All imaging-based strategies were cost-effective compared with no screening. FIT screening was the dominant screening strategy, leading to most LYG and highest cost-savings. Compared with three rounds of colonoscopy screening, CTC with five rounds was found to be cost-effective in an incremental analysis of imaging strategies. Assumptions on screening participation have a major influence on the ordering of strategies in terms of costs and effects.
Conclusion:
CTC and MRC have potential for CRC screening, compared with no screening and compared with three rounds of 10-yearly colonoscopy screening. When taking FIT screening as the reference, imaging is not cost-effective. Participation is an important driver of effectiveness and cost estimates.
Advances in knowledge:
This is the first study to assess the cost-effectiveness of MRC screening for CRC.
BACKGROUND
With over 1.3 million incident cases and almost 700,000 deaths worldwide in 2012, colorectal cancer (CRC) is an important health problem, especially in developed countries.1 Screening has been shown to reduce both CRC incidence and mortality,2–4 and a range of screening modalities are available. However, the choice for a screening test is difficult, which is reflected by the different primary tests used in screening programmes. Some countries have implemented stool-based tests, whereas other countries have implemented for endoscopy or a combination of both.5 Alternatively, as is for example performed in the USA, participants can choose between several screening modalities.6
The decision for a screening test is based, among other factors, on test characteristics, affordability, safety and acceptance by participants. If we focus purely on test characteristics, colonoscopy is the optimal test because of its high diagnostic accuracy. However, the procedure, especially the bowel preparation, is burdensome, which may lead to a low population uptake.7,8 Strategies to increase participation in colonoscopy screening are investigated8 as well as alternative screening modalities.
Alternatives to colonoscopy are CT colonography (CTC) and MR colonography (MRC). Both methods enable external inspection of the colon and are therefore less invasive than colonoscopy. Moreover, it is possible to conduct CTC with limited bowel preparation.9,10 Screening participants seem to favour colonography11,12 over colonoscopy. CTC has wider availability, concerns a more established technique and is less costly compared with MRC and is therefore more extensively studied. The sensitivity of CTC for detecting small adenomas is slightly lower than that of colonoscopy,13–15 whereas the sensitivity for detecting large adenomas and CRC is comparable.16,17
MRC, which in contrast to CTC is free from ionizing radiation, is evaluated in a limited number of studies only. These studies showed that MRC has a lower sensitivity for small and large adenomas than colonoscopy but has a similar sensitivity for CRC.18–21 The results on patient acceptance for MRC compared with colonoscopy are inconclusive.22,23 The general idea is that higher patient acceptance may be achievable because MRC can be conducted after limited bowel preparation with faecal tagging.23–25 Thus, full bowel preparation—which is an important barrier in colonoscopy screening8—may not be required.
We aimed to compare CTC and MRC screening with no screening and colonoscopy screening in terms of effectiveness, costs and cost-effectiveness using the Adenoma and Serrated pathway to Colorectal CAncer (ASCCA) model.26 We also included faecal immunochemical testing (FIT) as a comparator because many countries have implemented stool-based testing. Various strategies differing in screening interval and number of screening rounds were evaluated.
METHODS
Adenoma and Serrated pathway to Colorectal CAncer model
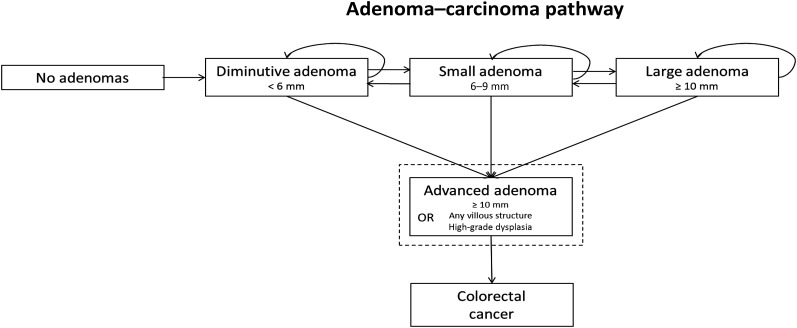
All analyses were conducted using the ASCCA model,26 which is described in more detail in the Appendix A. A flowchart of the adenoma–carcinoma pathway as simulated by the model is provided in Figure 1. In short, the ASCCA model simulates individuals' health trajectories from the age of 20 years until they are deceased or have reached the age of 90 years. Individuals in the model can develop up to 10 adenomas and 10 serrated lesions. The development of colorectal lesions is modelled as growth in size while taking lesion characteristics, e.g. location, morphology, dysplasia and villosity, into account.
Figure 1.
Flowchart of the adenoma–carcinoma pathway in the Adenoma and Serrated pathway to Colorectal CAncer model. It can be noted that advanced adenoma is a definition and not a state in the model.
We have excluded the serrated pathway to CRC to enable comparison of the study results with other modelling studies that did not consider this pathway. In addition, a recent study showed that inclusion of the serrated pathway to CRC hardly affected model predictions when serrated lesions were removed upon detection, which is clinical practice.27 Thus, in the model, all CRCs arise from adenomas and only advanced adenomas can progress to CRC. Advanced adenomas are defined as adenomas which are either 10 mm or larger, have any villous component or have high-grade dysplasia.28
CRC consists of four different stages according to the tumour-node-metastasis (TNM) classification system. Non-symptomatic tumours can progress to a more advanced stage or become detected, either by screening or by symptoms. The model satisfactorily reproduces the Dutch age- and sex-specific adenoma and serrated lesion prevalence as reported in the COlonoscopy vs COlonography Screening trial12 and Dutch CRC incidence and mortality rates.29
Screening strategies
We considered a treatment-only strategy without screening and 12 screening strategies that differed with respect to the primary screening instrument, screening interval and the number of screening rounds. The primary screening instruments were CTC, MRC, colonoscopy and FIT. For the imaging techniques, we considered four strategies with screening ages 55 and 65 years; 55, 65 and 75 years; 50, 60, 70 and 80 years; and 55, 60, 65, 70 and 75 years. For colonoscopy, three screening strategies were evaluated with screening at ages 55 and 65 years; 55, 65 and 75 years; and 50, 60, 70 and 80 years. For FIT testing, the Dutch programme was simulated consisting of biennial FIT screening in individuals aged 55–75 years.
For each strategy, we used observed participation rates from Dutch implementation trials. For CTC, colonoscopy and FIT, observed participation rates were 34,12 2212 and 63%.30 For MRC, participation was set at 34% because MRC may be expected to have comparable participation rates as CTC. Because data on individual participation patterns are lacking, we assumed for all screening modalities that the probability to attend screening is equal for each individual and is unrelated to participation in previous or future screening rounds.
Screening participants with a positive imaging test or FIT test are referred to diagnostic colonoscopy. CTC and MRC were considered positive when small (6–9 mm) or large adenomas (≥10 mm) or CRC were detected. For FIT testing, a cut-off point of 75 ng ml−1 was used. Adherence to diagnostic colonoscopy after a positive screening test was assumed to be 96%.31 Polypectomy was performed on all detected adenomas and a risk score was calculated based on the number, size and location of adenomas. This risk score determines the surveillance protocol, as specified in the Dutch surveillance guidelines.32 Participation for surveillance was set at 96%.31
Test characteristics
Table 1 provides an overview of the lesion-specific test characteristics for all evaluated screening tests. Test characteristics were based on meta-analyses on CTC,16,17,33 screening trials and a meta-analysis on MRC18–21 and a systematic review on colonoscopy.34 Because the literature does not provide lesion-specific FIT sensitivities, these were estimated via calibration of the model-predicted FIT positivity and detection rates for advanced adenomas and CRC to figures reported for a Dutch FIT screening trial.35 A detailed description of this calibration has been published previously.26
Table 1.
Test characteristics of CT colonography (CTC), MR colonography (MRC), colonoscopy and faecal immunochemical test (FIT)
| Test | Positivity rate per lesion |
||||||
|---|---|---|---|---|---|---|---|
| No adenoma (per person) (%) | Adenoma <6 mm (%) | Adenoma 6–9 mm (%) | Adenoma ≥10 mm (%) | CRC early stage (%) | CRC late stage (%) | References | |
| CTC | 9 | a | 76 | 86 | 96 | 96 | 16, 17 and 33 |
| MRC | 10 | a | 75 | 84 | 100 | 100 | 18–21 |
| Colonoscopy | 0 | 74 | 87 | 97.9 | 100 | 100 | 34 |
| FIT | |||||||
| Males | 4 | a | 12 | 30 | 50 | 85 | 26 and 35 |
| Females | 3 | a | 10 | 28 | 50 | 85 | |
CRC, colorectal cancer.
We assumed that the positivity rate in individuals with diminutive adenomas is equal to that in healthy individuals (i.e. individuals with no adenoma).
Costs
Costs related to screening and treatment of CRC are shown in Table 2, together with the source of each item. All costs were converted to 2014 Euros using the consumer price index for that year.36 Furthermore, we assumed a small risk of complications such as bowel perforations after colonography and colonoscopy.37,38 The costs of these complications are also taken into account. Since there are no data on costs of complications after colonography, we assumed these to be equal to the costs of complications after colonoscopy. Total costs were determined from a healthcare perspective.
Table 2.
Parameters and costs related to screening and colorectal cancer (CRC) treatment
| Variable | Value | Reference | |||
|---|---|---|---|---|---|
| Participation ratea | |||||
| CTC | 34% |
12 and 30 | |||
| MRC | 34% |
||||
| Colonoscopy | 22% |
||||
| FIT | 63% |
||||
| Compliance diagnostic colonoscopy | 96% |
31 | |||
| Compliance surveillance colonoscopy | 96% |
31 | |||
| Screen-detected CRC survival probabilities | Stage I | Stage II | Stage III | Stage IV | 29 and 54 |
| Year 1 | 0.9752 | 0.9380 | 0.9256 | 0.4700 | |
| Year 2 | 0.9936 | 0.9725 | 0.9366 | 0.5532 | |
| Year 3 | 0.9935 | 0.9784 | 0.9451 | 0.5769 | |
| Year 4 | 0.9934 | 0.9776 | 0.9570 | 0.7333 | |
| Year 5 | 0.9934 | 0.9845 | 0.9630 | 0.8182 | |
| Year 6 | 0.9933 | 0.9921 | 0.9606 | 0.8889 | |
| Year 7 | 0.9865 | 0.9876 | 0.9628 | 0.8750 | |
| Year 8 | 0.9938 | 0.9876 | 0.9628 | 0.8750 | |
| Year 9 | 0.9938 | 0.9876 | 0.9628 | 0.8750 | |
| Year 10 | 0.9938 | 0.9876 | 0.9628 | 0.8750 | |
| CTC costs | |||||
| Invitational costsb | €5.91 |
37 and 55 | |||
| Positive scan (organization, personnel, analysis)c | €161.62 |
||||
| Negative scan (organization, personnel, analysis)c | €152.12 |
||||
| Complications after CTC (1 per 1500) | €1373.93 |
||||
| MRC costs | |||||
| Invitational costsb | €5.91 |
55 and 56 | |||
| Costs per scan (organization, personnel, analysis)c | €269.77 |
||||
| Complications after MRC (1 per 1500) | €1373.93 |
||||
| Colonoscopy costs | |||||
| Invitational costsb | €5.29 |
38 and 56–59 | |||
| Without polypectomyc | €209.13 |
||||
| With polypectomyc | €356.07 |
||||
| Complications after colonoscopy (1 per 1000)d | €1373.93 |
||||
| FIT costs | |||||
| Testkitb | €1.37 |
58 | |||
| Organizationb | €14.96 |
||||
| Analysisc | €4.80 |
||||
| CRC treatment costs |
50 | ||||
| Stage I | €26,345 |
||||
| Stage II | €41,355 |
||||
| Stage III | €54,320 |
||||
| Stage IV | €40,610 | ||||
CTC, CT colonography; FIT, faecal immunochemical test; MRC, MR colonography.
All costs are presented in 2014 Euros.
At each round, each individual has the same probability to attend screening.
Costs per invitee.
Costs per participant.
Cost-effectiveness analyses
For each strategy, a cohort of 10,000,000 individuals was simulated. Results of each strategy included the number of colonoscopies due to the screening programme, the proportion of negative colonoscopies, number of CRC deaths, total lifetime costs per person and number of life-years (LY) lived per person. For the cost-effectiveness analyses, model predictions for total costs and LY from age 50 years onwards were used. Both were discounted using a discount rate of 3%.39 The outcomes of each screening strategy were compared with no screening by calculating the incremental cost-effectiveness ratio (ICER), which is the difference in costs divided by the difference in LY. An ICER less than the Dutch gross domestic product per capita in 2013, i.e. €35,916/life-year gained (LYG), was considered cost-effective.36,40 A strategy is considered dominant (also called strong dominance) when it is equally or more effective and less costly than another strategy or combination of other strategies.
Sensitivity analyses
We repeated all base-case analyses with a natural history parameter set representing low and high adenoma prevalence (Appendix A) to assess the impact of natural history assumptions. Furthermore, we assessed the impact of the following changes in one-way sensitivity analyses: (1) increasing and decreasing the costs of CRC treatment by both 25% and 50% to account for country-specific treatment costs as well as new treatment regimes, (2) increasing and decreasing the positivity rate of imaging techniques for small and large adenomas by 5%, (3) increasing and decreasing the positivity rate of imaging techniques in healthy individuals by 5%, (4) increasing and decreasing the costs per imaging test by €50 and (5) lowering the costs of complications due to imaging tests to €1000 and €500 because these complications may be less severe, and thus less costly, than complications due to colonoscopy. Finally, we set the participation rates of all screening modalities to 20, 40, 60, 80 and 100%.
RESULTS
Base-case analyses
Effect of screening on the number of colorectal cancer deaths and colonoscopies
Table 3 shows for each screening modality and for no screening the number of diagnostic and surveillance colonoscopies due to the screening programme as well as the number of CRC deaths. Without screening, the ASCCA model predicted 28.3 CRC deaths per lifetime of 1000 individuals. CTC and MRC led to comparable reductions in CRC deaths per 1000 individuals of around 25, 31, 35 and 45% for two, three, four and five screening rounds, respectively. For colonoscopy, reductions in CRC deaths for two, three, and four screening rounds were similar to the imaging strategies with an equal number of screening rounds. To achieve this reduction, 80–94 colonoscopies per prevented death were required in colonoscopy screening, and 35–38 colonoscopies per prevented death were required in CTC and MRC screening. FIT screening led to the highest reduction in CRC deaths, i.e. 57%, and required 34 colonoscopies to prevent 1 CRC death.
Table 3.
Number of diagnostic and surveillance colonoscopies due to the screening programme per 1000 individuals, proportion of negative colonoscopies, colorectal cancer (CRC) deaths per 1000 individuals, number of colonoscopies per death prevented, discounted life-years (LY) per individual and discounted total costs per individual
| Strategy | Per 1000 individuals |
Per individual |
||||||
|---|---|---|---|---|---|---|---|---|
| Number of screening rounds | Screening interval | Colonoscopies | Proportion of negative colonoscopies | CRC deaths | Colonoscopies/death prevented | Discounted LY | Discounted total costs (in €) | |
| No screening | NA | NA | NA | NA | 28.3 | NA | 19.4116 | 1423 |
| CTC | 2 | 10 | 250 | 37.0% | 21.1 | 35 | 19.4421 | 1253 |
| 3 | 10 | 305 | 32.5% | 19.5 | 35 | 19.4449 | 1234 | |
| 4 | 10 | 366 | 34.9% | 18.4 | 37 | 19.4531 | 1229 | |
| 5 | 5 | 471 | 35.2% | 15.5 | 37 | 19.4594 | 1157 | |
| MRC | 2 | 10 | 254 | 39.3% | 21.1 | 35 | 19.4425 | 1310 |
| 3 | 10 | 311 | 34.5% | 19.4 | 35 | 19.4450 | 1304 | |
| 4 | 10 | 374 | 37.0% | 18.4 | 38 | 19.4533 | 1323 | |
| 5 | 5 | 481 | 37.3% | 15.5 | 38 | 19.4597 | 1266 | |
| Colonoscopy | 2 | 10 | 579 | 72.5% | 21.1 | 80 | 19.4396 | 1220 |
| 3 | 10 | 725 | 69.9% | 19.9 | 86 | 19.4416 | 1202 | |
| 4 | 10 | 905 | 72.4% | 18.7 | 94 | 19.4500 | 1177 | |
| FIT | 11 | 2 | 545 | 42.1% | 12.2 | 34 | 19.4752a | 981a |
CTC, CT colonography; FIT, faecal immunochemical test; MRC, MR colonography; NA, not applicable.
This strategy dominates all other screening strategies in an incremental analysis.
Besides requiring the highest number of colonoscopies to prevent one death, colonoscopy screening also had the highest proportion of negative colonoscopies, i.e. 70–73% (Table 3). Using another primary screening instrument to preselect individuals at increased risk for referral to colonoscopy, the proportion of negative colonoscopies was considerably lower. CTC had the lowest proportion of negative colonoscopies (33–37%). Please note that the proportion of negative colonoscopies is related to the number of individuals with false-positive findings on the FIT or imaging test.
Cost-effectiveness analyses
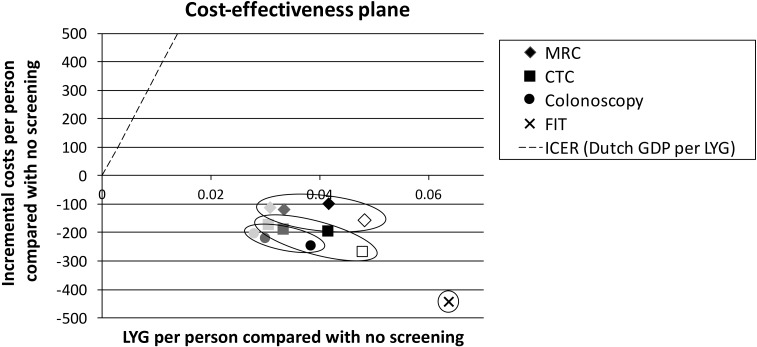
Figure 2 shows a cost-effectiveness plane with the incremental costs of each screening modality plotted against the number of LYG compared with no screening. In addition, from the origin, representing no screening, a dotted line is plotted with a slope corresponding to an ICER of €35,916/LYG, i.e. the Dutch gross domestic product per capita. Strategies located right from this line can be considered cost-effective compared with no screening. Strategies that are located in the lower right quadrant are dominant, i.e. they are both more effective and less costly than no screening. CTC strategies led to similar LYG as MRC strategies with an equal number of screening rounds. The health gain of CTC and MRC ranged between 0.031 and 0.048 LYG per person compared with no screening, for 2–5 screening rounds. However, MRC strategies cost €60–110 per person more than CTC strategies with an equal number of screening rounds. This is mainly due to the higher costs per test. The imaging strategies with a 10-year interval were slightly more effective (0.003 LY per person) than colonoscopy screening with an equal number of screening rounds, due to the difference in trial-based participation rates. When the screening interval for the imaging strategies was set at 5 years, imaging was more effective than all colonoscopy strategies. When comparing this 5-yearly strategy to the most effective colonoscopy strategy, i.e. four 10-yearly rounds of colonoscopy screening, imaging led to 0.01 more LY per person. FIT screening was the most effective strategy with 0.064 LYG compared with no screening.
Figure 2.
Cost-effectiveness plane depicting incremental life-year gained (LYG) (x-axis) against incremental costs (y-axis) for CT colonography (CTC), MR colonography (MRC), colonoscopy and faecal immunochemical test (FIT) screening to no screening (origin). Darker colours indicate more screening rounds (two, three and four rounds with a 10-year screening interval) and the open symbol indicates the imaging strategies with five screening rounds with a 5-year interval. GDP, gross domestic product; ICER, incremental cost-effectiveness ratio.
When taking no screening as the reference, all screening strategies were cost-effective. Each strategy led to more LY at lower costs than no screening (Table 3). Being the most effective screening strategy and saving most costs, FIT screening dominated all other screening strategies.
We also calculated ICERs of the imaging strategies using three rounds of colonoscopy screening as the reference, as this is one of the recommended CRC screening strategies in the USA (Table 4).41 CTC and MRC strategies with three, four or five screening rounds were cost-effective alternatives for three rounds of colonoscopy screening. Five rounds of CTC screening was even more effective at lower costs. In an incremental analysis, CTC with five screening rounds was found to be cost-effective. When FIT screening was taken as the comparator, none of the strategies were cost-effective alternatives; all strategies were less effective and more costly.
Table 4.
The discounted life-years (LY) and total costs per individual for three rounds of colonoscopy screening (reference) and eight imaging strategies
| Strategy | Discounted total costs (in €) | Discounted LY | Difference in costs (in €) | LYG | ICER 1 | ICER 2 |
|---|---|---|---|---|---|---|
| Colonoscopy, 3 rounds | 1202 | 19.4416 | Reference | Reference | Reference | Reference |
| CTC, 5 rounds | 1157 | 19.4594 | −45 | 0.0178 | Dominant | Dominant |
| CTC, 4 rounds | 1229 | 19.4531 | 27 | 0.0115 | 2346 | Dominated |
| CTC, 3 rounds | 1234 | 19.4449 | 32 | 0.0033 | 9691 | Dominated |
| CTC, 2 rounds | 1253 | 19.4421 | 51 | 0.0005 | 101,320 | Dominated |
| MRC, 5 rounds | 1266 | 19.4597 | 63 | 0.0181 | 3498 | €363,333a |
| MRC, 3 rounds | 1304 | 19.4450 | 102 | 0.0034 | 29,917 | Dominated |
| MRC, 2 rounds | 1307 | 19.4425 | 107 | 0.0009 | 119,333 | Dominated |
| MRC, 4 rounds | 1323 | 19.4533 | 120 | 0.0117 | 10,278 | Dominated |
CTC, CT colonography; ICER, incremental cost-effectiveness ratio; LYG, life-year gained; MRC, MR colonography.
ICERs in bold indicate strategies that are cost-effective compared with three rounds of colonoscopy screening according to a cost-effectiveness threshold of €35,916/LYG.
The imaging strategies are ordered according to increasing costs. The ICER of each imaging strategy compared with three rounds of colonoscopy screening is indicated by ICER 1, whereas ICER 2 is calculated using the nearest cheaper strategy that is not dominated as the reference.
ICER compared with five rounds of CTC screening.
Sensitivity analyses
Appendix B shows the results of the sensitivity analyses concerning natural history assumptions, treatment costs, test characteristics of imaging strategies, costs per imaging test and costs of complications due to imaging. Model predictions were robust to changes in these parameters. In all sensitivity analyses, screening remained more effective than no screening (Supplementary Table B1). In most analyses, screening was also less costly than no screening. Only when the treatment costs decreased by 50%, MRC strategies with 2–5 screening rounds cost an additional €31–91 per person compared with no screening. Nevertheless, compared with no screening, the maximum ICER was 11,946 €/LYG, meaning that all screening strategies were still cost-effective compared with no screening.
In direct comparison with three rounds of colonoscopy screening (Supplementary Table B2), CTC screening with three, four or five rounds and MRC screening with four or five rounds were cost-effective in all sensitivity analyses. Three rounds of MRC screening was no longer cost-effective when the positivity rate for small and large adenomas was decreased by 5% or when the costs per test were increased by €50. Two rounds of CTC became a cost-effective alternative when the positivity rate for small and large adenomas was increased by 5%, whereas two rounds of MRC was a cost-effective alternative for colonoscopy screening when the costs per test were decreased by €50. When we assumed a high adenoma prevalence parameter set, all imaging strategies were cost-effective compared with colonoscopy screening.
In an incremental analysis, CTC with five rounds remained the only cost-effective strategy. Only when the treatment costs decreased by 50% or when the costs of complications were set at €500, CTC with three rounds and CTC with four rounds were also cost-effective alternatives, respectively. When FIT screening was considered the reference strategy, none of the imaging strategies were cost-effective in any of the sensitivity analyses.
Finally, we assessed the impact of setting the participation rates of all screening strategies to 20, 40, 60, 80 and 100%, as shown in Table 5. When participation rates of screening strategies change substantially, the ordering in terms of cost-savings and effects may also change considerably. In the base-case analysis, FIT screening dominated all other screening strategies under evaluation. Table 5 shows that under specific, and unlikely, circumstances, imaging could lead to more LYG and lower costs than FIT screening. For example, four rounds of CTC at 60% participation resulted in 0.038 LYG and cost-savings of €96 compared with FIT screening at 20% participation.
Table 5.
Results of sensitivity analyses regarding participation rates. Discounted life-years (LY) and discounted total costs per individual for all screening strategies
| Strategy | Number of screening rounds | Participation rate |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 20% |
40% |
60% |
80% |
100% |
|||||||
| LY | Costs (in €) | LY | Costs (in €) | LY | Costs (in €) | LY | Costs (in €) | LY | Costs (in €) | ||
| CTC | 2 | 19.4301 | 1321 | 19.4474 | 1228 | 19.4627 | 1147 | 19.4768 | 1081 | 19.4921 | 1008 |
| 3 | 19.4318 | 1305 | 19.4501 | 1206 | 19.4664 | 1127 | 19.4809 | 1065 | 19.4951 | 1003 | |
| 4 | 19.4372 | 1300 | 19.4596 | 1201 | 19.4788 | 1128 | 19.4956 | 1074 | 19.5129 | 1022 | |
| 5 | 19.4424 | 1239 | 19.4652 | 1132 | 19.4812 | 1081 | 19.4928 | 1067 | 19.5038 | 1059 | |
| MRC | 2 | 19.4302 | 1352 | 19.4470 | 1292 | 19.4632 | 1245 | 19.4771 | 1211 | 19.4922 | 1170 |
| 3 | 19.4322 | 1345 | 19.4504 | 1287 | 19.4661 | 1248 | 19.4807 | 1225 | 19.4956 | 1201 | |
| 4 | 19.4377 | 1355 | 19.4594 | 1313 | 19.4793 | 1291 | 19.4958 | 1290 | 19.5130 | 1289 | |
| 5 | 19.4431 | 1304 | 19.4653 | 1260 | 19.4819 | 1262 | 19.4930 | 1303 | 19.5046 | 1346 | |
| Colonoscopy | 2 | 19.4371 | 1237 | 19.4596 | 1083 | 19.4780 | 963 | 19.4901 | 873 | 19.5072 | 816 |
| 3 | 19.439 | 1221 | 19.4612 | 1067 | 19.4800 | 957 | 19.4957 | 885 | 19.5077 | 849 | |
| 4 | 19.4464 | 1196 | 19.4746 | 1033 | 19.4971 | 929 | 19.5148 | 872 | 19.5278 | 857 | |
| FIT | 11 | 19.4413 | 1224 | 19.4601 | 1089 | 19.4737 | 993 | 19.4830 | 924 | 19.4926 | 857 |
CTC, CT colonography; FIT, faecal immunochemical test; MRC, MR colonography.
When comparing strategies at the same participation rate, the ordering in terms of incremental health effects and cost-savings remained fairly similar. At equal participation, in general, colonoscopy was the most effective strategy with the highest cost-savings.
DISCUSSION
In this study, we assessed the cost-effectiveness of several strategies of CTC and MRC screening for CRC compared with no screening, colonoscopy screening and FIT screening. According to our model predictions, all imaging strategies were cost-effective compared with no screening. Although MRC strategies led to similar health gains as CTC strategies with an equal number of screening rounds, the cost-savings were lower, which was mainly due to the higher costs per test.
Since this is the first study to assess the cost-effectiveness of MRC screening for CRC, it is not possible to compare our results for MRC screening with other studies. However, we also included CTC, colonoscopy and FIT screening, which are evaluated by various other modelling studies. A review of these studies42 concluded that CRC screening is cost-effective compared with no screening for all screening modalities. In addition, some of the studies included in the review reported that colonoscopy, CTC and FIT screening were more effective and less costly than no screening. This is in line with our results.
Because several countries have already implemented a CRC screening programme, we also compared imaging-based screening with colonoscopy screening and FIT screening. Especially the comparison with colonoscopy screening, which is for example implemented in Austria, Greece and Poland,43 is interesting because CTC and MRC have comparable test characteristics for large adenomas and CRC compared with colonoscopy screening13–21 but are less invasive. Also, bowel preparation, which is an important barrier in colonoscopy screening,7,44 is likely to be less burdensome for imaging techniques.24,45 Therefore, higher participation rates may be achievable. This is underlined by the findings in a Dutch trial comparing colonoscopy and CTC screening in which participation rates were 22% and 34%, respectively.12
Our study showed that imaging can be an attractive alternative to colonoscopy screening. An incremental analysis of imaging strategies showed that CTC with five rounds was the preferred imaging alternative. Besides being cost-effective, imaging can lead to a considerable health gain as well as a decrease in screening burden. A health gain of 0.018 LY, corresponding with a 22% reduction in CRC deaths, can be achieved with five rounds of imaging. Furthermore, compared with colonoscopy screening, the number of colonoscopies required to prevent one CRC death can be reduced by 55% when imaging is the primary screening test. Also, the number of negative colonoscopies can be markedly reduced with imaging. Nevertheless, in imaging strategies, still over 30% of colonoscopies are negative, which leads to considerable screening burden.
Although negative colonoscopies in imaging strategies are mainly due to false-positive imaging tests, they can also be the consequence of imperfect detection of colorectal lesions during colonoscopy itself. In clinical practice, these discordant test results may lead to additional referrals to either CTC or colonoscopy. This would lead to additional healthcare costs that were not included in our analyses.
In our base-case analysis, the participation rates for FIT, colonoscopy and CTC were based on trials.12,30 However, participation in the context of a trial does not necessarily reflect participation rates that are achievable in a screening programme. For example, participation in the first round of the Dutch FIT screening programme was 8% higher than the participation in pilot studies.30,46 Furthermore, the communication of information to screen-eligible individuals also influences participation in a screening programme.47 Thus, it is possible that higher participation rates for CTC can be achieved than the rates we assumed in our base-case analysis. Therefore, we carried out extensive sensitivity analyses in which we assumed participation rates of 20, 40, 60, 80 and 100% for all screening strategies. We found that different assumptions for participation rates of the screening strategies could change the ordering of the screening strategies in terms of costs and effects considerably. Nevertheless, only under unlikely assumptions for participation rates of FIT and CTC screening, CTC can be more effective with higher cost-savings than FIT screening. Thus, none of the imaging strategies are likely to be a cost-effective alternative for FIT screening. Instead, CTC screening may be offered to individuals who are unwilling or not able to undergo diagnostic colonoscopy.48
An important difference between imaging and other CRC screening modalities is the ability of imaging to visualize extracolonic structures. This enables the detection of, for example, aneurysms and extracolonic cancers. However, it is questioned whether this is an advantage or disadvantage of screening by imaging.49 On the one hand, earlier detection of cancer and other abnormalities could potentially lead to health gains. On the other hand, most extracolonic findings are clinically unimportant and therefore, detection of these lesions may only cause futile distress. More research is required to evaluate the impact of extracolonic findings on the cost-effectiveness of CTC and MRC screening for CRC.
A limitation of the present study is that the participation rate of CTC was derived from a study using limited bowel preparation,12 whereas the test characteristics of CTC were derived from studies using full bowel preparation.16,17,33 We assessed the impact of these assumptions in one-way sensitivity analyses in which we lowered positivity rates of CTC. Results did not change considerably. Furthermore, we based the participation rate of MRC on the participation rate of CTC owing to limited evidence on MRC participation. However, the procedures and bowel preparation differ, which could in practice lead to differences in the participation rate. In a sensitivity analysis, we both increased and decreased the participation rate of MRC. This analysis showed that MRC participation should be markedly higher than CTC participation to be a cost-effective alternative.
In addition, costs of CRC treatment were based on a study reporting treatment costs in Ireland in 2008.50 Although we converted these costs to 2014 Euros based on the consumer price index, it is possible that these costs do not reflect current treatment costs in Netherlands. Firstly, treatment regimens can differ between countries. Secondly, new, mostly expensive, treatments have become available for CRC treatment since 2008. Therefore, CRC treatment costs were changed in a sensitivity analysis. Screening led to even higher cost-savings when treatment costs were increased, whereas the opposite holds for decreasing treatment costs. Nevertheless, all screening strategies remained cost-effective compared with no screening.
The parameters in the ASCCA model are mainly derived from Dutch data. Nevertheless, model predictions can also be meaningful for other Western countries. Firstly, the natural history of CRC, on which the structure of the ASCCA model is based, is considered to be universal. Furthermore, test characteristics of colonoscopy, CTC and MRC are based on international systematic reviews and meta-analyses and therefore also apply for other countries. Thirdly, the Dutch surveillance guideline is comparable with the surveillance protocol of other Western countries. On the other hand, adenoma prevalence in the ASCCA model is higher than that in several other countries.51–53 Furthermore, participation rates for each screening modality are also country specific. If the prevalence and participation rates in a country differ substantially from the rates used in this study, reparameterization of the model is required to obtain more accurate cost-effectiveness estimates.
A strength of the present study is the extensive evaluation of the impact of changing key assumptions. The results of our sensitivity analyses did not change our main conclusion that all screening strategies lead to more LY at lower costs compared with no screening. Furthermore, CTC was still preferable over MRC owing to the lower costs per test. If computationally feasible, it would be valuable for future modelling studies to conduct a probabilistic sensitivity analysis in order to assess the joint effect of parameter uncertainty.
In conclusion, this study assessed the potential of imaging techniques as a screening tool for CRC. Our results showed that imaging-based CRC screening is cost-effective compared with no screening. CTC screening is favoured over MRC screening owing to the lower costs per test. Furthermore, an incremental analysis of imaging strategies showed that CTC screening with five rounds is a cost-effective alternative for three rounds of colonoscopy screening. Compared with FIT screening, imaging-based screening is unlikely to be ever cost-effective. Participation is an important driver of effectiveness and cost estimates.
Acknowledgments
ACKNOWLEDGMENTS
This research was performed within the framework of the Center for Translational Molecular Medicine, project DeCoDe (Grant 03O-101).
APPENDIX A
THE ADENOMA AND SERRATED PATHWAY TO COLORECTAL CANCER MODEL
Model description
The ASCCA model is a Markov microsimulation model, which means that individual health trajectories are simulated. Individuals are modelled from the age of 20 years to the age of 90 years or death, whichever comes first. During their lifetime, individuals can transition through a specific set of mutually exclusive health states. Each cycle, which has a length of 1 year, an individual has the probability to remain in the current health state or progress or regress to an adjacent health state. The model is programmed in C++.
Natural history of colorectal cancer
The ASCCA model describes the natural history of CRC, i.e. the development of colorectal lesions to cancer. Two pathways to CRC are included: the adenoma–carcinoma pathway as postulated by Morson et al in 1974A1,A2 and the more recently described serrated pathway. All individuals start in the model as healthy and are assigned a personal risk index. Together with an age-specific baseline risk, this risk index determines an individual's risk of developing colorectal lesions. This risk index ensures that some individuals develop multiple lesions, whereas the majority will not develop adenomas during their lifetime.
In the model, individuals can develop up to 10 adenomas and 10 serrated lesions. The development of each adenoma is modelled independently. When an individual develops an adenoma, this is allocated several characteristics such as location, morphology, dysplasia and villosity. Each cycle, an adenoma has the probability to grow or regress in size. However, we did not allow for complete regression.A3–A5 Also, dysplasia and villosity can progress each cycle to a more severe stage. This risk of developing malignant features increases with adenoma size.
When an adenoma is an advanced adenoma, i.e. has high-grade dysplasia, has any villous component or is ≥10 mm, it can progress to CRC. In the present study, we assumed that all CRCs arise via the adenoma–carcinoma pathway; that is, serrated lesions are considered innocuous. This enables comparison of model results with other CRC screening models, which do not include the serrated pathway to CRC. The ASCCA model includes four non-symptomatic and four symptomatic CRC stages. Each round, a tumour can progress to a more advanced stage or can become detected by symptoms.
Screening component
The ASCCA model is supplemented with a flexible screening component so that numerous screening strategies can be simulated. The screening component consists of three parts: (1) primary screening, (2) diagnostic colonoscopy and (3) surveillance. For primary screening, a screening age range, screening interval, participation rate and test characteristics need to be specified. It is also possible to specify two primary screening tests in order to evaluate co-testing or a primary test plus triage test. Also, the decision rule for referral to diagnostic colonoscopy needs to be defined.
For diagnostic colonoscopy, the model requires input on the proportion of individuals that is compliant with referral to diagnostic colonoscopy, test characteristics and the risk of complications and deaths during the procedure. Also, the polypectomy strategy needs to be defined: that is, it needs to be specified which colorectal lesions, e.g. adenomas, small serrated lesions in the rectosigmoid etc., are removed upon detection.
Surveillance in the model is based on the findings during diagnostic colonoscopy. Individuals can be either referred back to primary screening or enter the surveillance programme. For the surveillance programme, the proportion of individuals that complies with the surveillance protocol, the test characteristics of the surveillance test, the interval between subsequent surveillance colonoscopies, an ending rule which determines when an individual is referred back to screening and a maximum age need to be specified.
A cancer can become detected by screening during diagnostic colonoscopy or during surveillance colonoscopy. Screen-detected CRCs are assigned different survival probabilities compared with symptom-detected CRCs because the former have a better prognosis.A6
It is possible to run the ASCCA model without the screening component. In that case, a scenario without screening is simulated. When a specific screening strategy is specified in the screening component, the impact of that screening strategy can be evaluated. In that case, screening can, when dependent on the screening input, disrupt the natural history; that is, adenomas have the probability to become detected and removed by means of polypectomy in order to prevent the development of CRC. The model keeps track of the underlying health status of an individual as well as the health status as determined by screening and colonoscopy. Consequently, the number of true positives, true negatives, false positives and false negatives in a specific screening strategy can be determined.
Model parameters
An important data source for the ASCCA model was the Dutch COlonoscopy vs COlonography Screening (COCOS) trial.A7 In this trial, previously unscreened individuals at average risk aged 50–75 years were subjected to either colonoscopy or CTC. For the model parameters, data from only the colonoscopy arm were used.
First, we calibrated the age- and sex-specific adenoma incidence and personal risk index to prevalence rates observed in the COCOS trial. For age categories that were not included in this trial, we used results from Rutter et al.A8 Subsequently, the progression rates and regression rates between the states diminutive adenoma, small adenoma and large adenoma were calibrated against the age-, sex- and size-specific prevalence rates found in the COCOS trial. During this calibration procedure, we used studies on adenoma growth rates to guide model input.A4,A5 It should be noted that we assumed that complete regression, i.e. from “diminutive adenoma” to “healthy”, is not possible and that only adenomas without malignant features can regress in size.
Size-specific transition probabilities to acquire malignant features (“low-grade dysplasia” to “high-grade dysplasia” and “tubular” to “‘tubulovillous/villous”) were fitted against the proportion of adenomas with that specific malignant feature in each size category. Other adenoma characteristics, namely location distribution and morphology, were directly derived from the COCOS data.
The sex-specific progression rate from advanced adenoma to CRC is defined by a Weibull distribution. The shape and scale of this distribution were calibrated against Dutch age- and sex-specific CRC incidence rates as reported by the Dutch Cancer Registry.A10 For the stage-specific transition rates to a subsequent CRC stage, we used dwell times reported by Brenner et al,A11 whereas stage-specific detection rates were fitted against the stage distribution of detected CRC.A12
10-yearly stage-specific survival rates for CRC were derived from the Dutch Cancer Registry.A10 The age- and sex-specific risk of dying owing to other causes was based on observations of the Dutch Central Bureau of Statistics.A13
After calibration, the ASCCA model satisfactorily reproduced data from the COCOS trial as well as the Dutch CRC incidence and mortality rates. Multiple parameter sets, i.e. a low, mean and high prevalence set, were obtained as a result of calibrating against the lower limit, mean and upper limit of the data. An overview of important model parameters as used in the base-case analyses of the present study is provided in Supplementary Table A1. In addition, Supplementary Table A2 shows the model-predicted age- and sex-specific prevalence of adenomas and advanced adenomas in the situation without screening.
Cost-effectiveness analyses
The cost-effectiveness of a specific screening strategy compared with the reference strategy is determined by dividing the difference in costs by the number of LYG. This results in the ICER. For this study, an ICER below €35,916/LYG, i.e. the Dutch GDP per capita, was considered cost-effective.
To determine the costs of each strategy, we assigned costs to certain procedures as well as certain health states. These costs are shown in Supplementary Table A2. For every individual, the model keeps a record of the procedures the individual has underwent as well as the health states. When the life trajectory of an individual is completed, total costs for that individual are calculated. This is repeated for all simulated individuals. All costs are summed up which results in the total costs of that specific strategy.
The number of LY for each strategy consists of all LY lived by the individuals simulated in that strategy. In order to calculate LY, the age of death is recorded for every individual. The age at which individuals start in the model, i.e. 20 years, is deducted from the age of death to determine the number of LY lived for a specific individual. Then, the LY for all individuals in a specific strategy are summed.
Besides determining the total costs and total number of LY, discounted total costs and discounted total number of LY are also estimated. Costs and LY are discounted using the international discount rate of 3%, starting at age 50 years.A14
REFERENCES
- A1. Morson BC. Evolution of cancer of the colon and rectum. Cancer 1974; 34: 845–9. [DOI] [PubMed]
- A2. Muto T, Bussey HJ, Morson BC. The evolution of cancer of the colon and rectum. Cancer 1975; 36: 2251–70. [DOI] [PubMed]
- A3. Bersentes K, Fennerty MB, Sampliner RE, Garewal HS. Lack of spontaneous regression of tubular adenomas in two years of follow-up. Am J Gastroenterol 1997; 92: 1117–20. [PubMed]
- A4. Hoff G, Foerster A, Vatn MH, Sauar J, Larsen S. Epidemiology of polyps in the rectum and colon. Recovery and evaluation of unresected polyps 2 years after detection. Scand J Gastroenterol 1986; 21: 853–62. [DOI] [PubMed]
- A5. Hofstad B, Vatn MH, Andersen SN, Huitfeldt HS, Rognum T, Larsen S, et al. Growth of colorectal polyps: redetection and evaluation of unresected polyps for a period of three years. Gut 1996; 39: 449–56. [DOI] [PMC free article] [PubMed]
- A6. Mapp TJ, Hardcastle JD, Moss SM, Robinson MH. Survival of patients with colorectal cancer diagnosed in a randomized controlled trial of faecal occult blood screening. Br J Surg 1999; 86:1286–91. [DOI] [PubMed]
- A7. Stoop EM, de Haan MC, de Wijkerslooth TR, Bossuyt PM, van Ballegooijen M, Nio CY, et al. Participation and yield of colonoscopy versus non-cathartic CT colonography in population-based screening for colorectal cancer: a randomised controlled trial. Lancet Oncol 2012; 13: 55–64. [DOI] [PubMed]
- A8. Rutter CM, Yu O, Miglioretti DL. A hierarchical non-homogenous Poisson model for meta-analysis of adenoma counts. Stat Med 2007; 26: 98–109. [DOI] [PMC free article] [PubMed]
- A9. van Rijn JC, Reitsma JB, Stoker J, Bossuyt PM, van Deventer SJ, Dekker E. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol 2006; 101: 343–50. [DOI] [PubMed]
- A10. IKNL. Cijfers over kanker [Updated 2011; cited 2012 Feb 6] Available from: http://www.cijfersoverkanker.nl/
- A11. Brenner H, Altenhofen L, Katalinic A, Lansdorp-Vogelaar I, Hoffmeister M. Sojourn time of preclinical colorectal cancer by sex and age: estimates from the German national screening colonoscopy database. Am J Epidemiol 2011; 174: 1140–46. [DOI] [PubMed]
- A12. Visser O, van Leeuwen FE. Stage-specific survival of epithelial cancers in North-Holland/Flevoland, Netherlands. Eur J Cancer 2005; 41: 2321–30. [DOI] [PubMed]
- A13. Central Bureau for Statistics [Updated 2012. Cited April 2012]. Available from: http://www.cbs.nl
- A14. Siegel JE, Torrance GW, Russell LB, Luce BR, Weinstein MC, Gold MR. Guidelines for pharmacoeconomic studies. Recommendations from the panel on cost effectiveness in health and medicine. Panel on cost effectiveness in health and medicine. Pharmacoeconomics 1997; 11: 159–68. [DOI] [PubMed]
Contributor Information
Marjolein J E Greuter, Email: mj.greuter@vumc.nl.
Johannes Berkhof, Email: h.berkhof@vumc.nl.
Remond J A Fijneman, Email: r.fijneman@nki.nl.
Erhan Demirel, Email: erhan.demirel@gmail.com.
Jie-Bin Lew, Email: jiebin.lew@nswcc.org.au.
Gerrit A Meijer, Email: g.meijer@nki.nl.
Jaap Stoker, Email: j.stoker@amc.uva.nl.
Veerle M H Coupé, Email: v.coupe@vumc.nl.
REFERENCES
- 1.International Agency for Research on Cancer. GLOBOCAN 2012 [Updated 2012; cited 25 March 2014]. Available from: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx [Google Scholar]
- 2.Shaukat A, Mongin SJ, Geisser MS, Lederle FA, Bond JH, Mandel JS, et al. Long-term mortality after screening for colorectal cancer. N Engl J Med 2013; 369: 1106–14. doi: 10.1056/NEJMoa1300720 [DOI] [PubMed] [Google Scholar]
- 3.Elmunzer BJ, Hayward RA, Schoenfeld PS, Saini SD, Deshpande A, Waljee AK. Effect of flexible sigmoidoscopy-based screening on incidence and mortality of colorectal cancer: a systematic review and meta-analysis of randomized controlled trials. Plos Med 2012; 9: e1001352. doi: 10.1371/journal.pmed.1001352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zauber AG, Winawer SJ, O'Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med 2012; 366: 687–96. doi: 10.1056/NEJMoa1100370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.International Agency for Research on Cancer. Cancer screening in the European Union. Report on the implementation of the Council Recommendation on cancer screening. First Report. 2008. [Google Scholar]
- 6.U.S. Preventive Services Task Force. Screening for Colorectal Cancer [Updated 2008; cited September 2014]. Available from: www.uspreventiveservicetaskforce.org/uspst/uspcolo.htm [Google Scholar]
- 7.Harewood GC, Wiersema MJ, Melton LJ, 3rd. A prospective, controlled assessment of factors influencing acceptance of screening colonoscopy. Am J Gastroenterol 2002; 97: 3186–94. doi: 10.1111/j.1572-0241.2002.07129.x [DOI] [PubMed] [Google Scholar]
- 8.Jones RM, Devers KJ, Kuzel AJ, Woolf SH. Patient-reported barriers to colorectal cancer screening: a mixed-methods analysis. Am J Prev Med 2010; 38: 508–16. doi: 10.1016/j.amepre.2010.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liedenbaum MH, Denters MJ, Zijta FM, van Ravesteijn VF, Bipat S, Vos FM, et al. Reducing the oral contrast dose in CT colonography: evaluation of faecal tagging quality and patient acceptance. Clin Radiol 2011; 66: 30–7. doi: 10.1016/j.crad.2010.06.016 [DOI] [PubMed] [Google Scholar]
- 10.Liedenbaum MH, de Vries AH, Gouw CI, van Rijn AF, Bipat S, Dekker E, et al. CT colonography with minimal bowel preparation: evaluation of tagging quality, patient acceptance and diagnostic accuracy in two iodine-based preparation schemes. Eur Radiol 2010; 20: 367–76. doi: 10.1007/s00330-009-1570-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin OS, Kozarek RA, Gluck M, Jiranek GC, Koch J, Kowdley KV, et al. Preference for colonoscopy versus computerized tomographic colonography: a systematic review and meta-analysis of observational studies. J Gen Intern Med 2012; 27: 1349–60. doi: 10.1007/s11606-012-2115-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stoop EM, de Haan MC, de Wijkerslooth TR, Bossuyt PM, van Ballegooijen M, Nio CY, et al. Participation and yield of colonoscopy versus non-cathartic CT colonography in population-based screening for colorectal cancer: a randomised controlled trial. Lancet Oncol 2012; 13: 55–64. doi: 10.1016/S1470-2045(11)70283-2 [DOI] [PubMed] [Google Scholar]
- 13.Johnson CD, Chen MH, Toledano AY, Heiken JP, Dachman A, Kuo MD, et al. Accuracy of CT colonography for detection of large adenomas and cancers. N Engl J Med 2008; 359: 1207–17. doi: 10.1056/NEJMoa0800996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaparro M, Gisbert JP, Del Campo L, Cantero J, Maté J. Accuracy of computed tomographic colonography for the detection of polyps and colorectal tumors: a systematic review and meta-analysis. Digestion 2009; 80: 1–17. doi: 10.1159/000215387 [DOI] [PubMed] [Google Scholar]
- 15.Rosman AS, Korsten MA. Meta-analysis comparing CT colonography, air contrast barium enema, and colonoscopy. Am J Med 2007; 120: 203–10. e4. doi: 10.1016/j.amjmed.2006.05.061 [DOI] [PubMed] [Google Scholar]
- 16.Pickhardt PJ, Hassan C, Halligan S, Marmo R. Colorectal cancer: CT colonography and colonoscopy for detection—systematic review and meta-analysis. Radiology 2011; 259: 393–405. doi: 10.1148/radiol.11101887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Haan MC, van Gelder RE, Graser A, Bipat S, Stoker J. Diagnostic value of CT-colonography as compared to colonoscopy in an asymptomatic screening population: a meta-analysis. Eur Radiol 2011; 21: 1747–63. doi: 10.1007/s00330-011-2104-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hartmann D, Bassler B, Schilling D, Adamek HE, Jakobs R, Pfeifer B, et al. Colorectal polyps: detection with dark-lumen MR colonography versus conventional colonoscopy. Radiology 2006; 238: 143–9. doi: 10.1148/radiol.2381041756 [DOI] [PubMed] [Google Scholar]
- 19.Kuehle CA, Langhorst J, Ladd SC, Zoepf T, Nuefer M, Grabellus F, et al. Magnetic resonance colonography without bowel cleansing: a prospective cross sectional study in a screening population. Gut 2007; 56: 1079–85. doi: 10.1136/gut.2006.109306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graser A, Melzer A, Lindner E, Nagel D, Herrmann K, Stieber P, et al. Magnetic resonance colonography for the detection of colorectal neoplasia in asymptomatic adults. Gastroenterology 2013; 144: 743–50. e2. doi: 10.1053/j.gastro.2012.12.041 [DOI] [PubMed] [Google Scholar]
- 21.Zijta FM, Bipat S, Stoker J. Magnetic resonance (MR) colonography in the detection of colorectal lesions: a systematic review of prospective studies. Eur Radiol 2010; 20: 1031–46. doi: 10.1007/s00330-009-1663-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kinner S, Kuehle CA, Langhorst J, Ladd SC, Nuefer M, Zoepf T, et al. MR colonography vs. optical colonoscopy: comparison of patients' acceptance in a screening population. Eur Radiol 2007; 17: 2286–93. doi: 10.1007/s00330-007-0643-9 [DOI] [PubMed] [Google Scholar]
- 23.Florie J, Birnie E, van Gelder RE, Jensch S, Haberkorn B, Bartelsman JF, et al. MR colonography with limited bowel preparation: patient acceptance compared with that of full-preparation colonoscopy. Radiology 2007; 245: 150–9. doi: 10.1148/radiol.2451061244 [DOI] [PubMed] [Google Scholar]
- 24.van der Paardt MP, Stoker J. Magnetic resonance colonography for screening and diagnosis of colorectal cancer. Magn Reson Imaging Clin N Am 2014; 22: 67–83. doi: 10.1016/j.mric.2013.07.006 [DOI] [PubMed] [Google Scholar]
- 25.van der Paardt MP, Boellaard TN, Zijta FM, Baak LC, Depla AC, Dekker E, et al. Magnetic resonance colonography with a limited bowel preparation and automated carbon dioxide insufflation in comparison to conventional colonoscopy: patient burden and preferences. Eur J Radiol 2015; 84: 19–25. doi: 10.1016/j.ejrad.2014.10.006 [DOI] [PubMed] [Google Scholar]
- 26.Greuter MJ, Xu XM, Lew JB, Dekker E, Kuipers EJ, Canfell K, et al. Modeling the Adenoma and Serrated pathway to Colorectal Cancer (ASCCA). Risk Anal 2014; 34: 889–910. doi: 10.1111/risa.12137 [DOI] [PubMed] [Google Scholar]
- 27.Greuter MJ, Demirel E, Lew JB, Berkhof J, Xu XM, Canfell K, et al. Long-term impact of the Dutch colorectal cancer screening program on cancer incidence and mortality-model-based exploration of the serrated pathway. Cancer Epidemiol Biomarkers Prev 2016; 25: 135–44. doi: 10.1158/1055-9965.EPI-15-0592 [DOI] [PubMed] [Google Scholar]
- 28.Winawer SJ, Zauber AG. The advanced adenoma as the primary target of screening. Gastrointest Endosc Clin N Am 2002; 12: 1–9, v. doi: 10.1016/S1052-5157(03)00053-9 [DOI] [PubMed] [Google Scholar]
- 29. IKNL. Cijfers over kanker [Updated 2011; cited 2013 Feb]. Available from: http://www.cijfersoverkanker.nl/
- 30.Kapidzic A, Grobbee EJ, Hol L, van Roon AH, van Vuuren AJ, Spijker W, et al. Attendance and yield over three rounds of population-based fecal immunochemical test screening. Am J Gastroenterol 2014; 109: 1257–64. doi: 10.1038/ajg.2014.168 [DOI] [PubMed] [Google Scholar]
- 31.van Roon AH, Goede SL, van Ballegooijen M, van Vuuren AJ, Looman CW, Biermann K, et al. Random comparison of repeated faecal immunochemical testing at different intervals for population-based colorectal cancer screening. Gut 2013; 62: 409–15. doi: 10.1136/gutjnl-2011-301583 [DOI] [PubMed] [Google Scholar]
- 32.Nederlandse Vereniging van Maag-, Darm- en Leverartsen. Nederlandse Richtlijn Coloscopie Surveillance; 2013.
- 33.Mulhall BP, Veerappan GR, Jackson JL. Meta-analysis: computed tomographic colonography. Ann Intern Med 2005; 142: 635–50. doi: 10.7326/0003-4819-142-8-200504190-00013 [DOI] [PubMed] [Google Scholar]
- 34.van Rijn JC, Reitsma JB, Stoker J, Bossuyt PM, van Deventer SJ, Dekker E. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol 2006; 101: 343–50. doi: 10.1111/j.1572-0241.2006.00390.x [DOI] [PubMed] [Google Scholar]
- 35.van Rossum LG, van Rijn AF, Laheij RJ, van Oijen MG, Fockens P, van Krieken HH, et al. Random comparison of guaiac and immunochemical fecal occult blood tests for colorectal cancer in a screening population. Gastroenterology 2008; 135: 82–90. doi: 10.1053/j.gastro.2008.03.040 [DOI] [PubMed] [Google Scholar]
- 36. Central Bureau for Statistics [Updated 2015; cited 2015 May]. Available from: http://www.cbs.nl.
- 37.Atalla MA, Rozen WM, Niewiadomski OD, Croxford MA, Cheung W, Ho YH. Risk factors for colonic perforation after screening computed tomographic colonography: a multicentre analysis and review of the literature. J Med Screen 2010; 17: 99–102. doi: 10.1258/jms.2010.010042 [DOI] [PubMed] [Google Scholar]
- 38.Lüning TH, Keemers-Gels ME, Barendregt WB, Tan AC, Rosman C. Colonoscopic perforations: a review of 30,366 patients. Surg Endosc 2007; 21: 994–7. doi: 10.1007/s00464-007-9251-7 [DOI] [PubMed] [Google Scholar]
- 39.Siegel JE, Torrance GW, Russell LB, Luce BR, Weinstein MC, Gold MR. Guidelines for pharmacoeconomic studies. Recommendations from the panel on cost effectiveness in health and medicine. Panel on cost Effectiveness in health and medicine. PharmacoEconomics 1997; 11: 159–68. doi: 10.2165/00019053-199711020-00005 [DOI] [PubMed] [Google Scholar]
- 40. WHO. WHO-CHOICE: WHO [Updated 2013; cited 2014 Jan]. Available from: http://www.who.int/choice/en/
- 41. Final Update Summary: Colorectal Cancer: Screening—US Preventive Services Task Force. [Updated 2015; cited 2016 April 22]. Available from: http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/colorectal-cancer-screening.
- 42.Lansdorp-Vogelaar I, Knudsen AB, Brenner H. Cost-effectiveness of colorectal cancer screening. Epidemiol Rev 2011; 33: 88–100. doi: 10.1093/epirev/mxr004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schreuders EH, Ruco A, Rabeneck L, Schoen RE, Sung JJ, Young GP, et al. Colorectal cancer screening: a global overview of existing programmes. Gut 2015; 64: 1637–49. doi: 10.1136/gutjnl-2014-309086 [DOI] [PubMed] [Google Scholar]
- 44.de Wijkerslooth TR, de Haan MC, Stoop EM, Bossuyt PM, Thomeer M, Essink-Bot ML, et al. Burden of colonoscopy compared to non-cathartic CT-colonography in a colorectal cancer screening programme: randomised controlled trial. Gut 2012; 61: 1552–9. doi: 10.1136/gutjnl-2011-301308 [DOI] [PubMed] [Google Scholar]
- 45.Rockey DC. Computed tomographic and magnetic resonance colonography: challenge for colonoscopy. Dig Dis 2012; 30(Suppl. 2): 60–7. doi: 10.1159/000341895 [DOI] [PubMed] [Google Scholar]
- 46.Rijksinstituut voor Volksgezondheid en Milieu. Factsheet Bevolkingsonderzoek Darmkanker; 2015.
- 47.D'Hauwers KW, Gadet PF, Donders AR, Tjalma W. Impact of medical education on knowledge and attitudes regarding the human papilloma virus and vaccination: comparison before and 6 years after the introduction of the vaccines. Vaccine 2013; 31: 5843–7. doi: 10.1016/j.vaccine.2013.09.068 [DOI] [PubMed] [Google Scholar]
- 48. Rijksinstituut voor Volksgezondheid en Milieu. Bevolkingsonderzoek darmkanker [Updated 2013; cited 2014 April 28]. Available from: http://www.rivm.nl/Onderwerpen/B/Bevolkingsonderzoek_darmkanker/
- 49.de Haan MC, Halligan S, Stoker J. Does CT colonography have a role for population-based colorectal cancer screening? Eur Radiol 2012; 22: 1495–503. doi: 10.1007/s00330-012-2449-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tilson L, Sharp L, Usher C, Walsh C, Whyte S, O'Ceilleachair A, et al. Cost of care for colorectal cancer in Ireland: a health care payer perspective. Eur J Health Econ 2012; 13: 511–24. doi: 10.1007/s10198-011-0325-z [DOI] [PubMed] [Google Scholar]
- 51.Ferlitsch M, Reinhart K, Pramhas S, Wiener C, Gal O, Bannert C, et al. Sex-specific prevalence of adenomas, advanced adenomas, and colorectal cancer in individuals undergoing screening colonoscopy. JAMA 2011; 306: 1352–8. doi: 10.1001/jama.2011.1362 [DOI] [PubMed] [Google Scholar]
- 52.Bokemeyer B, Bock H, Hüppe D, Duffelmeyer M, Rambow A, Tacke W, et al. Screening colonoscopy for colorectal cancer prevention: results from a German online registry on 269000 cases. Eur J Gastroenterol Hepatol 2009; 21: 650–5. doi: 10.1097/MEG.0b013e32830b8acf [DOI] [PubMed] [Google Scholar]
- 53.Boursi B, Halak A, Umansky M, Galzan L, Guzner-Gur H, Arber N. Colonoscopic screening of an average-risk population for colorectal neoplasia. Endoscopy 2009; 41: 516–21. doi: 10.1055/s-0029-1214757 [DOI] [PubMed] [Google Scholar]
- 54.Mapp TJ, Hardcastle JD, Moss SM, Robinson MHE. Survival of patients with colorectal cancer diagnosed in a randomized controlled trial of faecal occult blood screening. Br J Surg 1999; 86: 1286–91. doi: 10.1046/j.1365-2168.1999.01229.x [DOI] [PubMed] [Google Scholar]
- 55.de Haan MC, Thomeer M, Stoker J, Dekker E, Kuipers EJ, van Ballegooijen M. Unit costs in population-based colorectal cancer screening using CT colonography performed in university hospitals in Netherlands. Eur Radiol 2013; 23: 897–907. doi: 10.1007/s00330-012-2689-6 [DOI] [PubMed] [Google Scholar]
- 56. Nederlandse Zorgautoriteit [Updated 2013; cited 2013 May]. Available from: www.nza.nl/regelgeving/tarieven.
- 57.Stoop E, de Wijkerslooth T, Bossuyt FF, van Leerdam ME, Stoker J. The colonoscopy unit costs of population-based screening for colorectal cancer. In submission.
- 58.Health Council of Netherlands. Population Screening Act: national population screening programme for bowel cancer. The Hague: Health Council of Netherlands; 2009: Publication no. 2009.13. [Google Scholar]
- 59.Rex DK, Petrini JL, Baron TH, Chak A, Cohen J, Deal SE, et al. Quality indicators for colonoscopy. Gastrointest Endosc 2006; 63(Suppl. 4): S16–28. doi: 10.1016/j.gie.2006.02.021 [DOI] [PubMed] [Google Scholar]