Abstract
The anterolateral thigh (ALT) flap has widespread use throughout the body because of the many engineering options. The ALT has a complex local vasculature, which can be of importance for the surgical approach. In general, the flap receives its perfusion from branches of the lateral circumflex femoral artery (LCFA). The LCFA, however, has a large anatomic variance. CT angiography can guide the surgeon in the selection of the most suitable site and aid in the surgical approach.
INTRODUCTION
The use of the anterolateral thigh (ALT) soft tissue to create a versatile flap was first introduced in 1984 by Song et al.1 This soft-tissue flap has become an increasingly popular option for complex reconstructions in the head and neck region. The tissue flap is known for its low donor site morbidity, can be quickly harvested with a large skin paddle and has the possibility for a long pedicle length.2 The ALT flap can be used in a versatile way for example as a myocutaneous, fasciocutaneous, adipofascial flap, as a suprafascial flap or as a free flap. There is a possibility to preserve the lateral femoral cutaneous nerve with the possibility of providing flap sensation.3 The cutaneous perforators originating from the lateral circumflex femoral artery (LCFA) provide vascular supply to the ALT region. The midpoint of the line lies between the anterior superior iliac spine and the lateral border of the patella. 85–100% of the perforators can then be found in an area of 5–6 cm around this midpoint (Figure 1).
Figure 1.
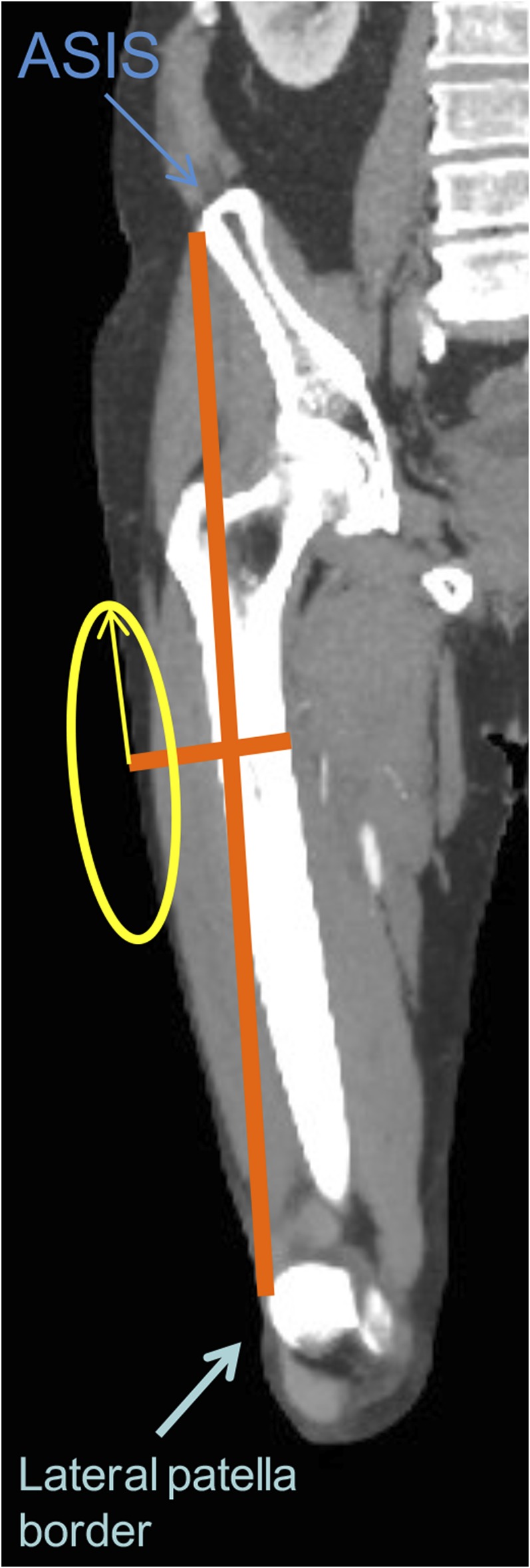
Arterial CT angiography of the lower limb after coronal reformatting. The orange line is connecting the anterior superior iliac spine (ASIS) and the lateral patellar border. The midpoint of the line is calculated; the perforators are generally found in the 5–6-cm area around this midpoint (yellow circle). This is the same method as the clinically used method pre-operatively. For colour image see online.
One of the potential difficulties is the wide anatomical variation of the vascular supply, with 4% of blood flow arising from a single perforator of the LCFA and 4% of blood flow arising directly from the deep femoral artery.4 These variations can create the possibility or necessity for an alternative surgical approach. The limitations of the ALT flap are manly this potential variance in vascular supply5 and a large variation in flap thickness. These variants augment the risk of flap loss or increase the donor site morbidity6 respectively.
Vascular supply of the anterolateral thigh
The vascular supply of the ALT flap skin is maintained by perforators, which pass through an intermuscular septum or through a muscular tissue (the vastus lateralis muscle or rectus femoris muscle), creating septocutaneous and musculocutaneous perforators, respectively.4 These perforators originate from the LCFA, which has a variable anatomic origin from the femoral artery7 (Figure 2). Most frequently, the LCFA originates directly from the deep femoral artery and second most frequently from the common femoral artery (10–25%) or even more proximally from the external iliac artery (6%). Pre-operative knowledge of these variations can result in other surgical approaches, with the possible creation of a longer vascular pedicle at the time of flap harvesting. The LCFA bifurcates into three branches: the ascending branch, the descending branch and a transverse branch. In 35% of patients, an oblique branch can be identified. Most commonly, the perforators for the ALT flap originate from the descending branch of the LCFA, making the descending branch, in general, the most important branch to search for. The descending branch can originate in seldom cases as a single branch directly from the circumflex femoral artery (CFA), leaving a smaller LCFA from the deep femoral artery. This can be of importance owing to the possibility of a longer vascular pedicle on harvest. In a small number (4%), the perforators can directly originate from the superficial femoral artery and in 1% directly from the CFA.
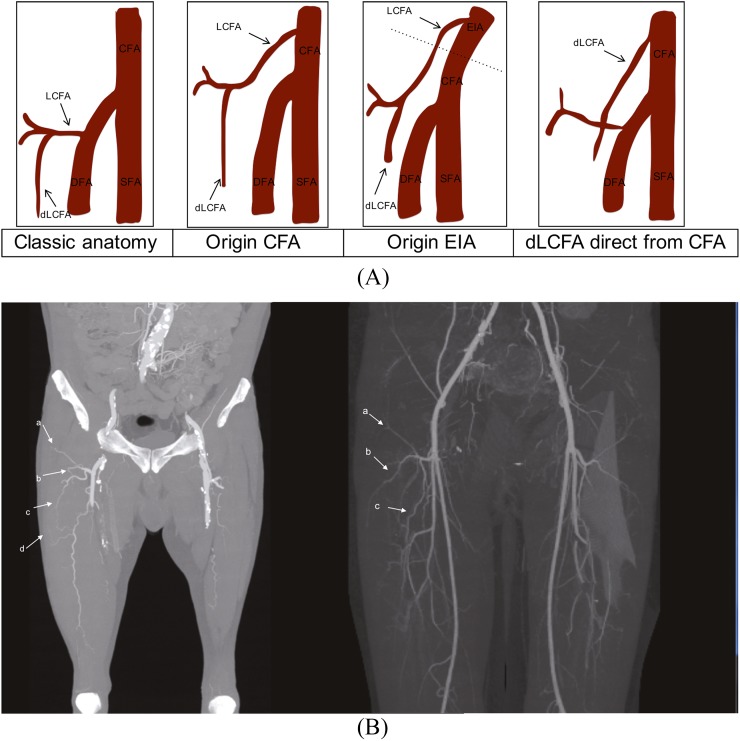
Figure 2.
(A) A schematic drawing of the anatomical variations of the lateral circumflex femoral artery (LCFA). (From left to right) In the classic anatomical situation, the LCFA originates proximally from the deep femoral artery, most commonly with a larger descending branch. Usually, the descending branch of the LCFA gives rise to the biggest perforators for the anterolateral thigh flap region. A common important variant is a more proximal origin of the LCFA from the common femoral artery; this creates the potential for a longer vascular pedicle. A higher origin of the LCFA directly from the external iliac artery and a more seldom, but important, variant with a solitary origin for the descending branch of the LCFA directly from the circumflex femoral artery (CFA). (B) The maximum intensity projection (MIP) reconstruction showing the standard anatomy with the normal course of the ascending branch (a), transverse branch (b) and the important descending branch (c) in two different patients. The left patient has a small perforator branch visible on the MIP image (d). DFA, deep femoral artery; dlCFA, descending ramus of lateral circumflex artery; EIA, external iliac artery; SFA, superficial femoral artery.
LCFA variants arise mostly from the CFA or the external iliac artery and in a few cases directly from a dominant descending ramus from the CFA in this case a longer vascular can be harvested directly.8 However, attention must be given in these cases because of the elevated risk of lower leg ischaemia and ulcers when harvesting this long pedicle from the common femoral artery in a patient with a lot of collaterals and arterial insufficiency.9 Knowledge of the variant pre-operatively can help the surgeon to decide, for example, whether to leave a little stump of the artery or to search for an alternative site for flap harvest.
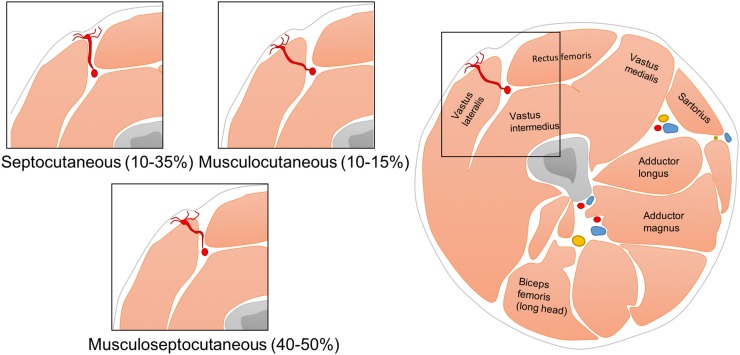
The perforators can have a course through the septum providing the skin flap in a direct way or they can have a course through the adjoining muscle with short muscular branches. A mixed version exists where perforators have a combined course partially through the septum and with in the muscle. These are the musculoseptocuteneous branches. The determination of the course of these perforators can aid in the surgeon's decision in branch selection. A septocutaneous perforator has the potential to be easier and faster to dissect, resulting in a lower donor site morbidity, shorter operation time and shorter anaesthesia. 10–35% of the perforators have a septocutaneous course, 10–15% of the perforators are musculocutaneous and in 40–50% of patients, the perforators have a mixed course4 (Figure 3).
Figure 3.
A schematic drawing of the potential variation in the perforator course ranging from a pure septocutaneous tract with the potential of easier and faster dissection to a purely musculocutaneous trajectory.4
Procedure details
Standard CT angiography (CTA) of the lower extremities from the pelvis to just below the knee is performed at our institution. Reconstructions are made in the axial range from the iliac crest to the patella in thin slice and most importantly, a maximum intensity projection reconstruction (thickness of 7–10 mm with overlap of 3 mm) is made in the axial and coronal directions. This maximum intensity projection reconstruction provides a good visibility of the small perforators.
In a second step, the zone of maximal perforators is determined to provide a similar zone, which the surgeon uses to determine the perforators clinically. This is performed with the use of the bony landmarks used by the surgeon. The midpoint of the line between the anterior superior iliac spine and the superior lateral border of the ipsilateral patella is determined. In the 5–6-cm oval area (along the leg axis) from the midpoint, 85–100% of perforators to the ALT flap can be found (yellow circle in Figure 1). The coordinates of the perforator origin at the muscular or fascial level can be calculated by projection of the perforators on the skin. The distance from the midpoint can then be determined for the surgeon.
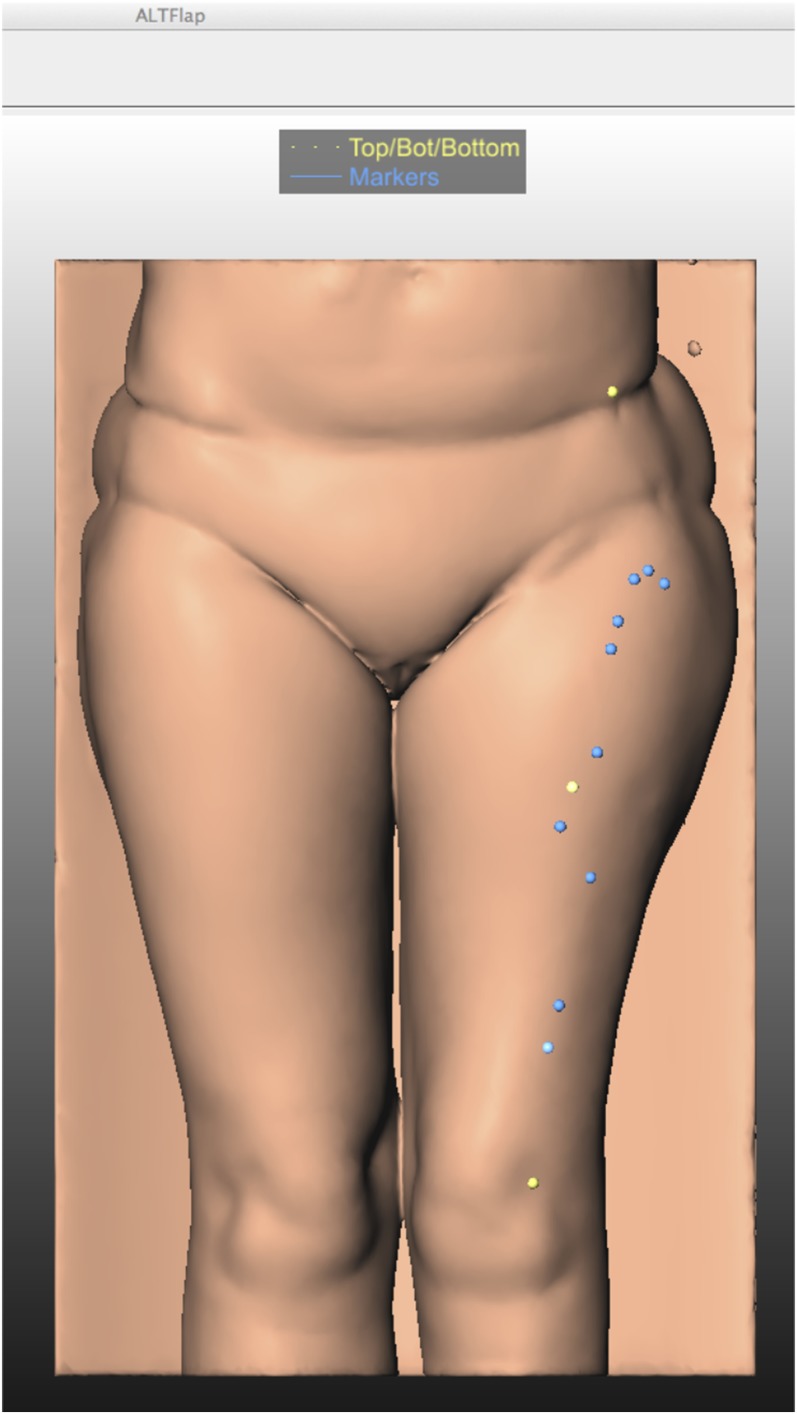
At our institution, a program to calculate a three-dimensional model was created with Mevislab® (MeVis Medical solutions AG, Fraunhofer MEVIS, Bremen, Germany). This program calculates the previously mentioned midpoint after manually marking the ASIS and the lateral patellar border. The perforator origin has to be determined in the axial plain at the muscular level (Figure 4). Mevislab calculates the lateral and craniocaudal distance from the reference point (Figures 5 and 6). These points can be marked on the skin pre-operatively to guide the surgeon (Figure 7).
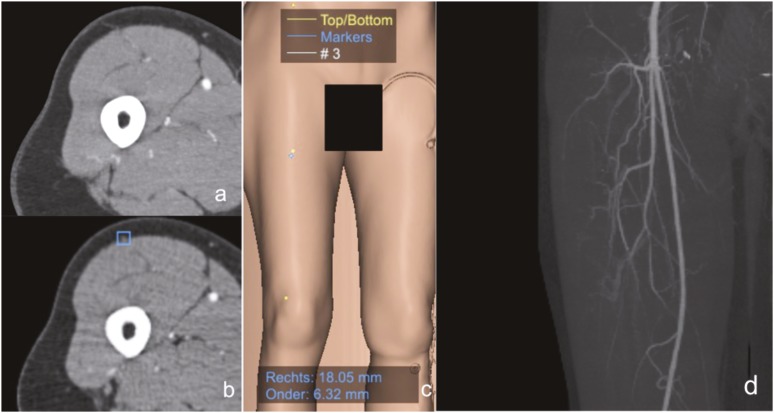
Figure 4.
Co-ordinate markings starting with a three-dimensional model reconstruction in Mevislab® (MeVis Medical solutions AG, Fraunhofer MEVIS, Bremen, Germany). The anterior superior iliac spine and lateral patellar border are located (yellow dots) with a calculation of the midpoint. This is the starting point for surgical dissection. The blue dot is showing the perforator origin at the fascia. Mevislab calculates the distance from the reference point to the perforator: lateral/medial and craniocaudal. The (a) septocutaneous perforator, (b) manual annotation on the axial image in Mevislab, (c) the projected and calculated lateral and inferior distance from the midpoint and (d) the maximum intensity projection reconstruction are showing the anatomical proportions. For colour image see online.
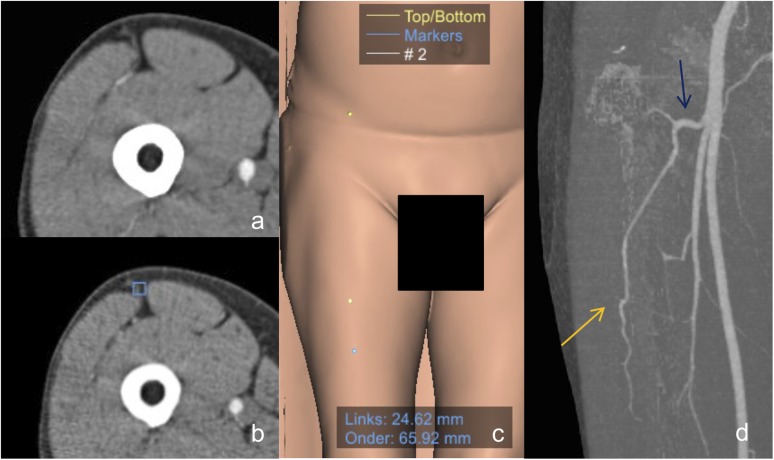
Figure 5.
The descending branch variant: (a) the septocutaneous perforator, (b) the manual annotation on the axial image in Mevislab® (MeVis Medical solutions AG, Fraunhofer MEVIS, Bremen, Germany) and (c) the projected and calculated lateral and inferior distance from the midpoint. Blue dot shows the perforator origin and yellow dots show the reference points. (d) The descending branch (orange arrow) of the lateral circumflex femoral artery (blue arrow) is the most common origin of the perforators. For colour image see online.
Figure 6.
The three-dimensional projection in Mevislab® (MeVis Medical solutions AG, Fraunhofer MEVIS, Bremen, Germany), with the blue dots showing the perforators and the yellow dots showing the reference points.
Figure 7.
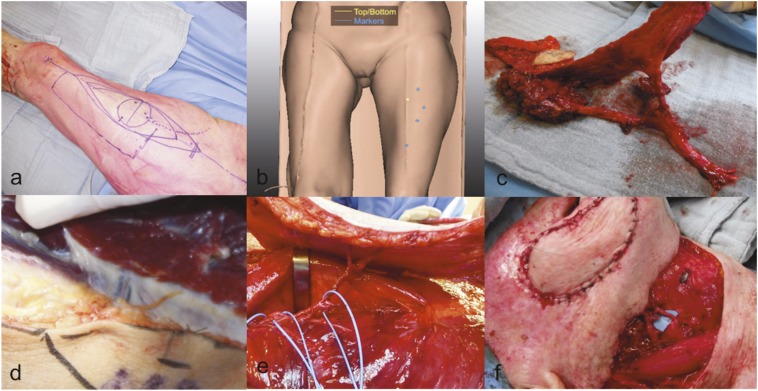
Markings of the anterolateral thigh flap on the thigh. (a) Perforators from the lateral descending artery and vein most frequently, but not exclusively, entering the skin in a circle of 3-cm radius half way a line between the anterior superior iliac spine and the upper lateral border of the patella. This line serves as the guideline to map the perforators on CT angiography. (b) The line largely corresponding with a fat pad or a septum between the rectus femoris (RF) and vastus lateralis (VL) muscles. Blue dots show the perforator origin and yellow dot shows reference points. (c) Perforators located in this septum are “septocutaneous perforators” and lie on the VL or RF muscles. However, the thigh perforators that surgeons clinically need most frequently “perforate” the VL in a short or long intramuscular course originating from the descending vascular pedicle. (d) Two perforators joining to form one perforator 2 cm from their insertion in the skin paddle and have been dissected in the intermuscular fat pad between the RF (hook) and VL. The blue vessel loops are used to gently lift the fragile vascular pedicle during dissection. (e) Based on the number of available perforators, a “chimera flap” can be procured with multiple separate units, such as a muscle or fascia segment and one or more skin paddles. The latter is very useful after oncologic resections in the head and neck area, when both an internal and external skin island is required for the reconstruction. (f) The final reconstruction and microanastomosis in a patient. For colour image see online.
The course of the perforators can be of importance for the surgeon because it can make the dissection easier and faster. This is certainly the case when the perforator has a septocutaneous course, which also allows for minimum donor site morbidity. Musculocutaneous perforators are more valuable in case of musculocutaneous flap transfers.4
CONCLUSION
The ALT flap is a versatile skin flap owing to its unique properties. The flap is known for its possible large skin paddle, potential sensory innervation, long vascular pedicle and minimum donor site morbidity. The ALT flap is now commonly used in reconstructions after large excisions in head and neck cancers. There are some disadvantages; the flap has a large variation in the perforator anatomy and thus in the vascular supply to the tissue. In general, there are one to three perforators providing the flap zone, originating from the descending branch of the LCFA. However, multiple variations of the LCFA exist, with a high origin from the external iliac artery to the solitary origin of the descending branch. These variations can create opportunities and possible drawbacks: a potential long vascular pedicle, a preferred perforator for harvesting or a higher risk of ischaemia. Hence, knowing these vascular variations in advance with imaging can be of great aid in the operative room for the reconstructive surgeon. Secondly, knowing the potential flap thickness can be important for site selection and approach. When there is not enough material to work with, the flap can be useless for a larger reconstruction.
CTA can aid the surgeon in the determination of these variations and locating the ideal perforator. The surgeon can select the optimal flap, perforator, flap design and thickness as well as prevent the rare but ischaemic complication of the lower limb. The radiologist report can aid the surgeon in providing the ideal perforator and location from the point of reference, determined on CTA. The perforator trajectory and thickness is important for the surgeon to decide between a musculocutaneous or cutaneous flap. Reporting the variation of the LCFA can be useful in guiding the pre-operative search for the longest possible pedicle; for example, in case of a solitary descending branch. Last but not least, the flap thickness can be reported to make sure the flap harvest creates a flap with enough working capabilities.
In summary, the key points for the radiologist to report are the location of the perforator from the reference point, the perforator thickness and course, the flap thickness and the anatomical variation of the LCFA to aid in the determination of the longest pedicle.
Contributor Information
Tom De Beule, Email: tom.debeule@gmail.com.
Wouter Van Deun, Email: wouter.vandeun@gmail.com.
Jan Vranckx, Email: Jan.Vranckx@uzleuven.be.
Bart de Dobbelaere, Email: Bart.dedobbelaere@uzleuven.be.
Geert Maleux, Email: geert.maleux@uzleuven.be.
Sam Heye, Email: sam.heye@uzleuven.be.
REFERENCES
- 1.Song YG, Chen GZ, Song YL. The free thigh flap: a new free flap concept based on the septocutaneous artery. Br J Plast Surg 1984; 37: 149–59. doi: 10.1016/0007-1226(84)90002-X [DOI] [PubMed] [Google Scholar]
- 2.Wei FC, Jain V, Celik N, Chen HC, Chuang DC, Lin CH. Have we found an ideal soft-tissue flap? An experience with 672 anterolateral thigh flaps. Plast Reconstr Surg 2002; 109: 2219–26; discussion 2227–30. doi: 10.1097/00006534-200206000-00007 [DOI] [PubMed] [Google Scholar]
- 3.Luo S, Raffoul W, Luo J, Luo L, Gao J, Chen L, et al. Anterolateral thigh flap: a review of 168 cases. Microsurgery 1999; 19: 232–8. doi: [DOI] [PubMed] [Google Scholar]
- 4.Seth R, Manz RM, Dahan IJ, Nuara MJ, Meltzer NE, McLennan G, et al. Comprehensive analysis of the anterolateral thigh flap vascular anatomy. Arch Facial Plast Surg 2011; 13: 347–54. doi: 10.1001/archfacial.2011.16 [DOI] [PubMed] [Google Scholar]
- 5.Gedebou TM, Wei FC, Lin CH. Clinical experience of 1284 free anterolateral thigh flaps. Handchir Mikrochir Plast Chir 2002; 34: 239–44. doi: 10.1055/s-2002-36290 [DOI] [PubMed] [Google Scholar]
- 6.Kimata Y, Uchiyama K, Ebihara S, Sakuraba M, Iida H, Nakatsuka T, et al. Anterolateral thigh flap donor-site complications and morbidity. Plast Reconstr Surg 2000; 106: 584–9. doi: 10.1097/00006534-200009010-00009 [DOI] [PubMed] [Google Scholar]
- 7.Rozen WM, Ashton MW, Pan WR, Kiil BJ, McClure VK, Grinsell D, et al. Anatomical variations in the harvest of anterolateral thigh flap perforators: a cadaveric and clinical study. Microsurgery 2009; 29: 16–23. doi: 10.1002/micr.20550 [DOI] [PubMed] [Google Scholar]
- 8.Lakhiani C, Lee MR, Saint-Cyr M. Vascular anatomy of the anterolateral thigh flap: a systematic review. Plast Reconstr Surg 2012; 130: 1254–68. doi: 10.1097/PRS.0b013e31826d1662 [DOI] [PubMed] [Google Scholar]
- 9.Hage JJ, Woerdeman LA. Lower limb necrosis after use of the anterolateral thigh free flap: is preoperative angiography indicated? Ann Plast Surg 2004; 52: 315–18. doi: 10.1097/01.sap.0000100422.66597.13 [DOI] [PubMed] [Google Scholar]