Abstract
Objective:
Peritumoral oedema (PTO) is commonly observed on MRI in malignant brain tumours including brain metastasis (bMET) and glioblastoma multiforme (GBM). This study aimed to differentiate bMET from GBM by comparing the volume ratio of PTO to tumour lesion (Rvol).
Methods:
56 patients with solitary bMET or GBM were enrolled, and MRI was analyzed by a semi-automatic methodology based on MATLAB (Mathworks, Natick, MA). The PTO volume (Voedema) was segmented for quantification using T2 fluid-attenuated inversion-recovery images, while the tumour volume was quantified with enhanced T1 images. The quantitative volume of the tumour, PTO and the ratio of PTO to tumour were interpreted using SPSS® (IBM Corp., New York, NY; formerly SPSS Inc., Chicago, IL) by considering different locations and pathologies.
Results:
The tumour volumes of supratentorial GBM, supratentorial bMET (supra-bMET) and infratentorial bMET were 32.22 ± 21.9, 18.45 ± 17.28 and 11.40 ± 5.63 ml, respectively. The corresponding Voedema were 44.08 ± 25.84, 73.20 ± 40.35 and 23.74 ± 7.78 ml, respectively. The Voedema difference between supratentorial and infratentorial lesions is significant (p-value = 0.002). Supra-bMET has a smaller tumour volume (p-value = 0.032), but a larger PTO (p-value = 0.007). The ratio of Voedema to the tumour volume in bMET is statistically higher than that in GBM (p-value = 0.015). The cut-off ratio for identifying bMET from GBM is 3.9, with a specificity and sensitivity of 90.0% and 68.8%, respectively.
Conclusion:
Segmentation is an efficient method to quantify irregular PTO. bMET possesses more extensive oedema with smaller tumour volume than does GBM. The Rvol is a valuable index to distinguish bMET from GBM.
Advances in knowledge:
This study presents a new method for the quantitation of PTO to differentiate bMET from GBM.
INTRODUCTION
Peritumoral oedema (PTO) is a common clinical feature during the physiopathological processes of malignant brain tumours, cerebral inflammation, stroke and traumatic brain injuries. PTO usually induces compression of the surrounding brain tissues leading to clinical manifestation and dynamically changes along with the evolution of the disease.1 In addition, the extent of PTO has been demonstrated to be associated with malignant brain tumour growth patterns2 and also with patient survival.2,3 However, quantitative approaches for describing the radiological feature of PTO has not been well studied.
PTO is an important indicator of tumour malignancy when clinicians are evaluating radiological images. Cerebral oedema around malignant brain tumours, such as brain metastasis (bMET) and glioblastoma multiforme (GBM), mainly comes from both vasogenic and cytotoxic factors. Vascular endothelial growth factor (VEGF) could weaken the tight junctions of the blood–brain barrier (BBB).4 Meanwhile, some inflammatory mediators such as the tumour necrosis factor-α, interleukin-1β, interleukin-4 and transforming growth factor-β would affect BBB and increase its permeability.5,6 As a result, PTO is consistently involved with the pathological pattern of malignant brain tumours. In most previous studies, PTO was evaluated by empirical qualitative findings or linear-based measurement on MRI or CT. Instead, quantitative three-dimensional volume calculation offers a more accurate interpretation of PTO.2,7
With the advancement of graphics and image processing, the quantification and visualization of MR data have been widely used in cancer imaging,8,9 especially in brain tumours.10,11 Many open-source software are available. Various imaging techniques of transformation, filtering and segmentation11,12 allow us to analyze MRI in a comprehensive approach beyond eyesight. Jones et al13 applied whole-brain diffusion tensor imaging segmentation to delineate tumour volume and improved the accuracy of diagnosis, while Chow et al14 used reliable semi-automated segmentation to monitor tumour progression in patients with GBM. Furthermore, computer-assisted assessment could identify GBM molecular subtypes even without biopsy.15 Therefore, utilizing MR data with the new silicon technique can bring more important information for clinical use. Here, we conducted a study to quantify the volumes of malignant brain tumours and PTO. A semi-automatic method based on MATLAB (Mathworks, Natick, MA) was proposed to segment brain tumours and PTO. Tumour size and the extent of PTO were compared between bMET and GBM. In addition, the volume ratio of PTO to tumour lesion was presented as a valuable marker for brain tumour imaging.
METHODS AND MATERIALS
Patients
54 patients with a solitary malignant brain tumour from Huashan Hospital between 2010 and 2014 were selected for this retrospective study. Inclusion criteria were as follows: (1) at least 18 years old, (2) diagnosed with a solitary brain tumour of bMET or GBM confirmed by neuropathologists and (3) complete clinical data with full neuroimages. Patients who underwent pre-operative radiation, chemotherapy or had recurrent tumours were excluded.
All patients with GBM (n = 23) were confirmed by neuropathologists based on the World Health Organization classification of central nervous system tumours.16 The 31 patients with bMET received brain tumour resection or biopsy. All the patients were reviewed by the tumour board with certified neuroradiologists, neurosurgeons and neuropathologists. Collection and review of patient data in the present study were approved by the Huashan Committee on Human Research at Fudan University, and informed consent was obtained from each subject.
MR images
Pre-operative MR images were obtained on 3.0-T MR scanners (SIEMENS, Munich, Germany, or GE Healthcare, Barrington, IL) using a standard eight-channel phased-array radiofrequency coil and receiver. Typical sequences were included, such as T1 [echo time (TE): 3–4 ms; repetition time (TR): 8–10 ms], T2 (TE: 140–150 ms; TR: 10–14 ms) and T2 fluid-attenuated inversion recovery (FLAIR) (TE: 140–150 ms; TR: 8400–9000 ms; inversion time: 2000 ms). Enhanced T1 (T1 + C, same parameters as above) was acquired immediately following gadolinium agent injection (gadopentetate dimeglumine) (BeiLu Pharmaceutical Co., Ltd, Beijing, China). The scanning parameters were as follows: 6-mm slice thickness, 250 × 250-mm2 field of view and 256 × 256 pixel matrix.
Image process
The images in the format of digital imaging and communications in medicine were obtained from picture archiving and communication systems (GE Medical System). The enhanced area on T1 + C images was defined as active tumour tissue. The high signal area around the tumour lesion on T2 FLAIR was taken as PTO.17,18 Cystic change was defined as >50% proportion of cyst to tumour lesion by qualification analysis.19,20 The cyst contained fluid with a signal similar to cerebrospinal fluid (CSF) on T2 weighted MR images and a contrast-enhancing rim on T1 weighted MR images, with a clear interface between the cyst and the surrounding brain tissue. Multicysts were examined as single ones when the walls of the compartments were thin. We manually selected the region of interest of the major cyst in the tumour around the inner edge of the contrast-enhancing rim on T1 weighted MR images and calculated the volume of cyst.
A MATLAB software (MATLAB 2010b) was launched to process the MR images. The digital data were imported and stored in matrix form. The images of enhanced T1 were used for tumour lesion volume (Vtumour) measurements. Manual delineation was performed by tracing the border of the tumours on each slice. The images of T2 FLAIR were applied for PTO volume (Voedema) measurements. The region of interest was set manually by covering the PTO region on each slice. The Gaussian high-pass filter and median-pass filter were applied for image sharpening and noise reduction. Otsu's method21,22 was consequently applied to define the appropriate global threshold for the segmentation of PTO from normal brain tissues and tumours. The analysis was conducted independently by two teams of neurosurgeons and neuroradiologists under the guidance of a senior neuroradiologist.
Data process
The total study population was divided into three subgroups, GBM (all supratentorial), supratentorial bMET (supra-bMET) and infratentorial bMET (infra-bMET). Vtumour and Voedema were summarized from all slices. Each slice area was the product of segmented pixel number and pixel size or spacing. Volume ratio of PTO to tumour lesion (Rvol) was calculated as an additional marker for differentiating the tumour types. Vtumour, Voedema and Rvol were compared between the three subgroups.
Statistical analysis
SPSS® v. 19.0 (IBM Corp., New York, NY; formerly SPSS Inc., Chicago, IL) was used for all statistical calculations.
Continuous variables such as age, Voedema, Vtumour and Rvol were compared using t test or analysis of variance. Discrete variables such as gender were compared using χ2 test. A multiple linear regression model was used for the analysis of relationships between Voedema and other variables. In addition, receiver-operating characteristic (ROC) analysis was performed for Rvol to determine the sensitivity and specificity for differentiating malignant brain tumours (bMET and GBM). The area under the curve (AUC) was used as a measure of ROC performance. For all tests, a two-sided p-value of <0.05 was considered as statistically significant.
RESULTS
Clinical characteristics
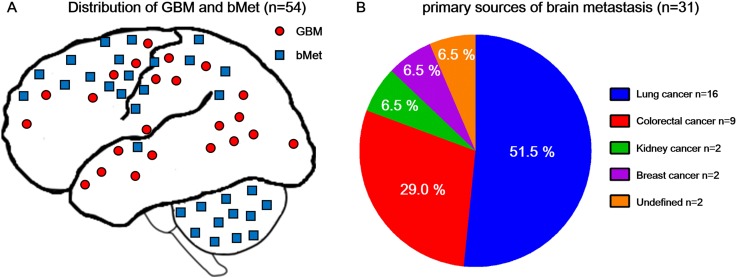
54 patients were enrolled in this study, including 31 patients with bMET and 23 patients with GBM confirmed clinically and by pathology. The median age was 58 years (range from 33 to 74 years), and no difference was recognized according to age and gender distribution between bMET and GBM subgroups (Table 1). The location distribution is shown in Figure 1a. All GBMs were primary and supratentorial, while bMET included 19 supratentorial lesions and 12 infratentorial lesions. As for bMET, 16 lesions originated from lung cancer, 9 lesions from colorectal cancers, 2 lesions from breast cancers, 2 lesions from kidney cancers and 2 lesions from carcinomas (Figure 1b).
Table 1.
Clinical data and corresponsive radiological volume. The main patient characteristics including the age, gender, tumour characteristics, peritumoral oedema volume, tumour volume and ratio
| Parameter | GBMa | bMET |
p-valueb | |
|---|---|---|---|---|
| Supra | Infra | |||
| Age (median, range) (years) | 57, 33–73 | 58, 45–71 | 57.5, 42–74 | 0.138 |
| Gender (male/female) | 14/9 | 9/10 | 7/5 | 0.665 |
| Tumour characteristics (non-cystic/cystic) |
20/3 | 15/4 | 12/0 | 0.682 |
| Voedema (mean±SD) (ml) | 44.08 ± 25.84 | 73.20 ± 40.35 | 23.74 ± 7.78 | 0.007 |
| Vtumour (mean±SD) (ml) | 32.22 ± 21.92 | 18.45 ± 17.28 | 11.40 ± 5.63 | 0.032 |
| Rvol (median) | 1.9 | 3.1 | 2.5 | 0.015 |
bMET, brain metastasis; GBM, glioblastoma multiforme; Rvol, volume ratio of peritumoral oedema to tumour lesion; SD, standard deviation; Vtumour, tumour lesion volume; Voedema, peritumoral oedema volume.
No infratentorial GBM included in this study.
Comparison between GBM and supra-bMET.
Figure 1.
(a) The distribution of glioblastoma multiforme (GBM) and brain metastasis (bMET): all GBM are located in the supratentorial and there is no infratentorial GBM in our study. bMET is more predisposed to scatter in the field of middle artery supply. (b) Primary sources of bMET: lung cancer (51.5%) is the most common bMET in our study. The rest are colorectal cancer (29.0%), breast cancer (6.5%) and kidney cancer (6.5%). 2 (6.5%) cases are undefined cancer (also called cancer of unknown primary), which have a definite brain tumour pathology of carcinoma, but the origin of the cancer could not be found even through positron emission tomography/CT scanning.
A larger tumour volume with glioblastoma multiforme and more extensive oedema with brain metastases was observed
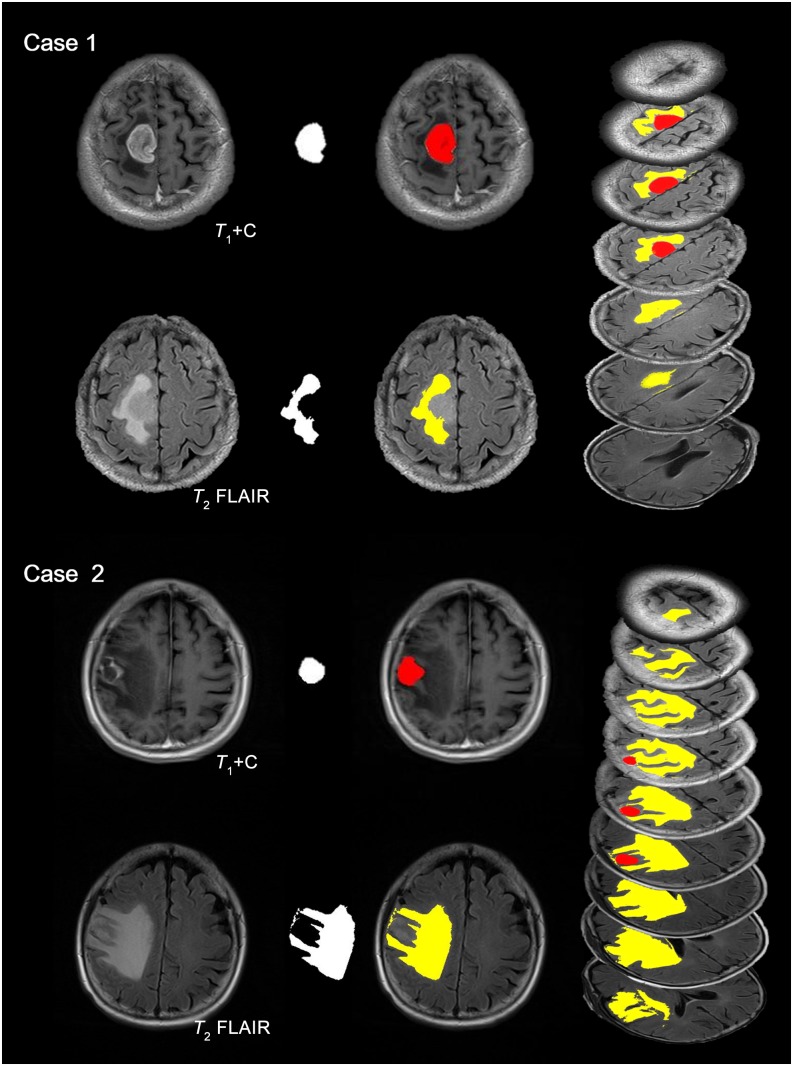
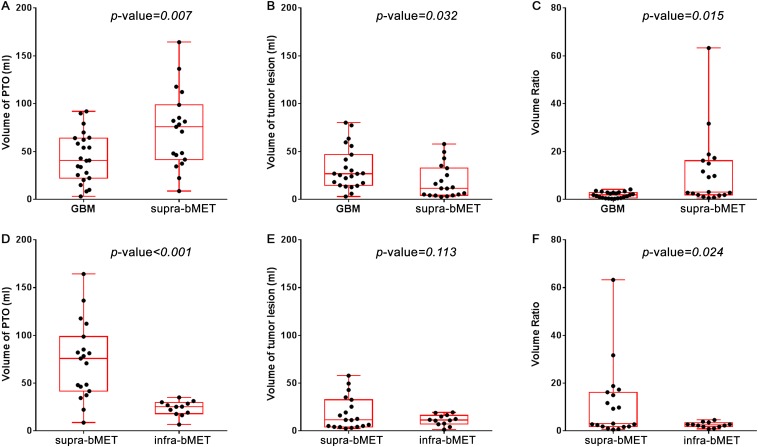
Segmentation and quantification for the volume of PTO and tumour lesion were successfully obtained in all 54 MR data sets. Typical cases of GBM and bMET are presented in Figure 2. Case 1 is a 63-year-old male with right frontal–parietal GBM and Case 2 is a 60-year-old female with solitary right frontal metastasis from lung cancer. Tumours extracted from T1 contrast images are labelled in red and PTO segmented from T2 FLAIR images in yellow. All slices were analyzed and summarized. The mean Voedema of GBM, supra-bMET and infra-bMET was 44.08, 73.20 and 23.74 ml (Table 1). The oedema around supra-bMET was more extensive than the oedema around GBM (p-value = 0.007) (Figure 3a). The mean volume of tumour (Vtumour) was 22.75 ml (ranging from 1.46 to 80.21 ml) and the mean volume of PTO (Voedema) was 49.81 ml (ranging from 3.11 to 164.32 ml). Interobserver difference of volume measurement was <3% (data not shown). The mean Vtumour of GBM, supra-bMET and infra-bMET was 32.22, 18.45 and 11.40 ml, respectively. GBM had a larger tumour volume than bMET (p-value = 0.032) (Figure 3b). Furthermore, analysis showed that supra-bMET has a significantly larger Vtumour than infra-bMET (p-value < 0.01) (Figure 3d).
Figure 2.
Segmentation of tumour lesion and peritumoral oedema. Typical cases of glioblastoma multiforme (GBM) and brain metastasis are presented here. Case 1 is a patient with right frontal–parietal GBM (63 years old, male). Case 2 is a patient with single right frontal metastasis from lung cancer (60 years old, female). Enhanced T1 has been applied to identify the tumour lesion and T2 fluid-attenuated inversion recovery (FLAIR) for peritumoral oedema (first column). Segmentation results (second column) are enhanced with pseudocolour (third column). Tumour with necrosis is coloured red, while peritumoral oedema is in yellow. All slices are segmented and transformed to pseudo-three-dimensional images (fourth column). For colour image see online.
Figure 3.
The comparison of volume and ratio in glioblastoma multiforme (GBM) and brain metastasis (bMET). (a) Volume of peritumoral oedema (PTO) for supratentorial GBM and supratentorial bMET (supra-bMET) shows significantly higher PTO volume (Voedema) in supra-bMET (p-value = 0.007). (b) Volume of tumour lesion for supratentorial GBM and supra-bMET shows significantly higher tumour lesion volume (Vtumour) in supratentorial GBM (p-value = 0.032). (c) Ratio of Voedema to Vtumour in supratentorial GBM and supra-bMET shows significantly higher volume ratio of PTO to tumour lesion (Rvol) in supra-bMET (p-value = 0.015). (d) Volume of PTO for supra-bMET and infratentorial bMET (infra-bMET) shows significantly higher Voedema in supra-bMET (p-value < 0.001). (e) Volume of tumour lesion for supra-bMET and infra-bMET shows no significant difference (p-value = 0.113). (f) Ratio of Voedema to Vtumour in supra-bMET and infra-bMET shows significantly higher Rvol in supra-bMET (p-value = 0.024).
Tumour location and cystic degeneration could influence peritumoral oedema
Pathology could definitely affect PTO, as shown above; however, other factors were taken into consideration. Multiple linear regression was conducted and it revealed that Voedema was related to location (standard coefficient = 0.681, p-value < 0.001), cystic degeneration (standard coefficient = 0.270, p-value = 0.045) and pathology (standard coefficient = −0.392, p-value = 0.010) (Table 2). Supratentorial tumours (including GBM and supra-bMET) had more extensive oedema than infratentorial tumours (infra-bMET only, 57.25 vs 23.74 ml, p-value = 0.002) Supplementary Figure A). Although many tumours showed cystic degeneration, only seven tumours (three GBM and four supra-bMET) met the criteria of >50%. Non-cystic tumours had a tendency to have more extensive oedema than cystic tumours (Supplementary Figure B), although p-values are not significant, probably owing to limited sample size.
Table 2.
Multiple linear regression model of the volume of peritumoral oedema (PTO). Analysis of multiple factors including the age, gender, volume of tumour lesion, tumour location, tumour characteristics, tumour type and linear regression of volume of PTO
| Variable | Standardized coefficients | p-value |
|---|---|---|
| Age (years) | 0.19 | 0.144 |
| Gender (male = 1, female = 0) | −0.03 | 0.821 |
| Volume of tumour lesion | −0.025 | 0.857 |
| Tumour location (supra = 1, infra = 0) | 0.681 | <0.001 |
| Tumour characteristics (non-cystic = 1, cystic = 0) | 2.059 | 0.045 |
| tumour type (GBM = 1, bMET = 0) | −0.392 | 0.01 |
bMET, brain metastasis; GBM, glioblastoma multiforme.
Voedema was related to location (standard coefficient = 0.681, p-value < 0.001), cystic degeneration (standard coefficient = 0.270, p-value = 0.045) and pathology (standard coefficient = −0.392, p-value = 0.010).
The volume ratio of peritumoral oedema to tumour lesion could serve as a diagnostic value for differentiating brain tumours
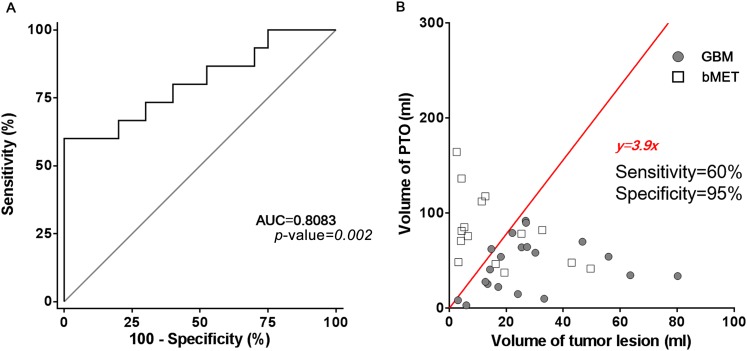
The Rvol from GBM and supra-bMET subgroups was 1.8 and 11.2, and a significant difference was observed (p-value = 0.015) (Figure 3c). ROC analysis suggested that Rvol could serve as a predictor for differentiating GBM vs supra-bMET (AUC = 0.779, 95% confidence interval: 0.634–0.924, p-value = 0.003) (Figure 4a), which is more effective than Vtumour (AUC = 0.719) or Voedema (AUC = 0.721). When Rvol is >3.9, the sensitivity of the differentiation between GBM and bMET could reach 90.0%, while the specificity could reach 68.75% (Figure 4b).
Figure 4.
Receiver-operating characteristic curve and linear regression of volume of peritumoral oedema (PTO) and lesion. (a) The receiver-operating characteristic curve shows the area under the curve (AUC) = 0.8083 with a significant difference (p-value = 0.002). (b) Linear regression shows that when PTO volume = 3.9 × tumour volume, glioblastoma multiforme (GBM) and brain metastasis (bMET) can be separated with a sensitivity of 60% and specificity of 95%. The ratio between volume of PTO and volume of tumour lesion can be a promising factor for separating GBM and bMET.
DISCUSSION
Data mining of images with new techniques could offer more information about tumours and provide diagnostic or even prognostic values.23 It is widely accepted that malignant brain tumours are related to severe PTO. However, an accurate assessment of oedema and tumour was not tried to differentiate bMET and GBM. Segmentation with Otsu's method will traverse all values of pixels from the digital images and an optimal threshold will be calculated to minimize the intraclass variance and maximize the interclass variance. In this study, we presented an easy and feasible semi-automatic method to quantify the volume of tumour lesion and PTO and attempted to identify brain tumours with their image phenotypes.
Solitary bMET and glioblastoma may appear similar on conventional MRI. Differentiation remains ambiguous even with the development of MRS combining with diffusion and perfusion.24 Our results revealed that bMET had relatively smaller tumour lesions and more extensive oedema volume than GBM, which is consistent with clinical experience. So, quantitative optimization of images could offer us more information and provide diagnostic or even prognostic values.23 Both GBM and bMET could be assessed with enhanced T1 images to define the tumour volume and with T2 FLAIR to define PTO as suggested by response assessment in neuro-oncology (RANO) criteria.17,18 Tumour infiltration and space-occupying effect may cause PTO, which presents hyperintensity on T2 or FLAIR images, while FLAIR will suppress normal CSF signals to intensify PTO. Combing the data from the tumour and PTO, a tendency of bMET to have a higher ratio of Voedema to Vtumour was observed. Using a cut-off of 3.9, this index of Rvol had a sensitivity of 90% and specificity of 68.75% for differentiating bMET from GBM. These results suggest that a volume ratio of PTO to tumour lesion may be a promising and non-invasive index for clinically differentiating supra-bMET from GBM. A previous study presented novel whole-brain diffusion tensor imaging segmentation for delineating tumour volumes of interest as a biomarker that may provide a valuable adjunct in non-invasive brain tumour diagnosis with an overall accuracy of 94.7%. The sensitivity and specificity of tumour classification was 90% and 97%, respectively, for all tumour types except cystic glioblastoma.13 Compared with this research, our method provided a more brief and efficient way to measure PTO and tumour, which is more useful for clinical application.
Many factors could affect brain oedema. PTO was demonstrated to be related to the breakdown of BBB, usually aggravated by the secretion of VEGF. These tumour-derived VEGF could increase the local blood flow, impair tight junctions such as claudins and occludin25 and induce the redistribution of aquaporin 4.2,26,27 Here, we further analyzed the possible related clinical factors. The multiple linear regression model showed that cystic degeneration could influence PTO besides histological type, which needs further exploration. Interestingly, tumour location also contributed to the extent of PTO. Cerebellar GBM is quite rare, and no extensive PTO in cerebellar GBM was observed based on an empirical result in a previous report.28 Supra-bMET and infra-bMET were compared, and infratentorial lesions had less extensive oedema, which might be explained by the space limitation of the infratentorial structure. The prediction of Rvol could not be applied in multiple bMET (data not shown), although multiple bMET could easily be distinguished in clinics.
The limitation in this study is the heterogeneity of bMET and it is hard to discriminate the bias. Metastases from different primary cancers with differing degrees of malignant cellularity, necrosis, intratumoral haemorrhage, cystic degeneration and invasiveness could produce various PTO patterns and trigger bias. A larger population-based study is needed to confirm our conclusion in the future.
Brain segmentation is an active and fast-growing field of computer graphics and image processing. Currently, it comes into focus by providing image phenotype data to refine brain tumour classification. Image processing provides a powerful tool for extracting more information beyond eyesight. Texture analysis presented by 1p/19q in oligodendroglioma and O-6-methylguanine-DNA methyltransferase promoter methylation in GBM remains in the spotlight. Our preliminary survey attempted to offer relationships between imaging phenotypes and tumour characteristics, which may contribute to the texture analysis of brain tumours. Moreover, with the progress of radioinformatics, more comprehensive biomedical knowledge about brain tumours will be accessible for clinicians and scientists. This basic analysis of tumour lesion and PTO could offer a constituent for future comprehensive analysis. In conclusion, threshold-based segmentation could help us define PTO quantitatively, and bMET possessed more extensive PTO and smaller tumour volume than GBM. The volume ratio of PTO to tumour could serve as a promising index for differentiating bMET from GBM.
FUNDING
This work was supported by the International S&T Cooperation Program of China (2014DFA31470), China National Funds for Distinguished Young Scientists (81025013), China National Natural Science Foundation (81001115, 81572483) and the fund of Science and Technology Commission of Shanghai (12ZR1404600).
Contributor Information
Chengcheng Zhou, Email: philip_zg@hotmail.com.
Zixiao Yang, Email: 13788995626@139.com.
Zhengwei Yao, Email: zwyao@fudan.edu.cn.
Bo Yin, Email: yinbo7@163.com.
Jiawei Pan, Email: panjiawei_1987@qq.com.
Yang Yu, Email: yy_0324@126.com.
Wei Zhu, Email: drzhuwei@fudan.edu.cn.
Wei Hua, Email: hs_glioma@126.com.
Ying Mao, Email: yingmao67@163.com.
REFERENCES
- 1.Roth P, Regli L, Tonder M, Weller M. Tumor-associated edema in brain cancer patients: pathogenesis and management. Expert Rev Anticancer Ther 2013; 13: 1319–25. doi: 10.1586/14737140.2013.852473 [DOI] [PubMed] [Google Scholar]
- 2.Spanberger T, Berghoff AS, Dinhof C, Ilhan-Mutlu A, Magerle M, Hutterer M, et al. Extent of peritumoral brain edema correlates with prognosis, tumoral growth pattern, HIF1a expression and angiogenic activity in patients with single brain metastases. Clin Exp Metastasis 2013; 30: 357–68. doi: 10.1007/s10585-012-9542-9 [DOI] [PubMed] [Google Scholar]
- 3.Schoenegger K, Oberndorfer S, Wuschitz B, Struhal W, Hainfellner J, Prayer D, et al. Peritumoral edema on MRI at initial diagnosis: an independent prognostic factor for glioblastoma? Eur J Neurol 2009; 16: 874–8. doi: 10.1111/j.1468-1331.2009.02613.x [DOI] [PubMed] [Google Scholar]
- 4.Harhaj NS, Antonetti DA. Regulation of tight junctions and loss of barrier function in pathophysiology. Int J Biochem Cell Biol 2004; 36: 1206–37. doi: 10.1016/j.biocel.2003.08.007 [DOI] [PubMed] [Google Scholar]
- 5.Easton AS. Regulation of permeability across the blood-brain barrier. Adv Exp Med Biol 2012; 763: 1–19. [DOI] [PubMed] [Google Scholar]
- 6.da Fonseca AC, Matias D, Garcia C, Amaral R, Geraldo LH, Freitas C, et al. The impact of microglial activation on blood-brain barrier in brain diseases. Front Cell Neurosci 2014; 8: 362. doi: 10.3389/fncel.2014.00362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zinn PO, Mahajan B, Sathyan P, Singh SK, Majumder S, Jolesz FA, et al. Radiogenomic mapping of edema/cellular invasion MRI-phenotypes in glioblastoma multiforme. PLoS One 2011; 6: e25451. doi: 10.1371/journal.pone.0025451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duncan JS, Ayache N. Medical image analysis: progress over two decades and the challenges ahead. IEEE Trans Pattern Anal Mach Intell 2000; 22: 85–106. [Google Scholar]
- 9.Eadie LH, Taylor P, Gibson AP. A systematic review of computer-assisted diagnosis in diagnostic cancer imaging. Eur J Radiol 2012; 81: e70–6. doi: 10.1016/j.ejrad.2011.01.098 [DOI] [PubMed] [Google Scholar]
- 10.Ahmed R, Oborski MJ, Hwang M, Lieberman FS, Mountz JM. Malignant gliomas: current perspectives in diagnosis, treatment, and early response assessment using advanced quantitative imaging methods. Cancer Manag Res 2014; 6: 149–70. doi: 10.2147/CMAR.S54726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bauer S, Wiest R, Nolte LP, Reyes M. A survey of MRI-based medical image analysis for brain tumor studies. Phys Med Biol 2013; 58: R97–129. doi: 10.1088/0031-9155/58/13/R97 [DOI] [PubMed] [Google Scholar]
- 12.Uchida S. Image processing and recognition for biological images. Dev Growth Differ 2013; 55: 523–49. doi: 10.1111/dgd.12054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones TL, Byrnes TJ, Yang G, Howe FA, Bell BA, Barrick TR. Brain tumor classification using the diffusion tensor image segmentation (D-SEG) technique. Neuro Oncol 2015; 17: 466–76. doi: 10.1093/neuonc/nou159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chow DS, Qi J, Guo X, Miloushev VZ, Iwamoto FM, Bruce JN, et al. Semiautomated volumetric measurement on postcontrast MR imaging for analysis of recurrent and residual disease in glioblastoma multiforme. AJNR Am J Neuroradiol 2014; 35: 498–503. doi: 10.3174/ajnr.A3724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naeini KM, Pope WB, Cloughesy TF, Harris RJ, Lai A, Eskin A, et al. Identifying the mesenchymal molecular subtype of glioblastoma using quantitative volumetric analysis of anatomic magnetic resonance images. Neuro Oncol 2013; 15: 626–34. doi: 10.1093/neuonc/not008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 2007; 114: 97–109. doi: 10.1007/s00401-007-0243-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 2010; 28: 1963–72. doi: 10.1200/JCO.2009.26.3541 [DOI] [PubMed] [Google Scholar]
- 18.Lin NU, Lee EQ, Aoyama H, Barani IJ, Barboriak DP, Baumert BG, et al. Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol 2015; 16: e270–8. doi: 10.1016/S1470-2045(15)70057-4 [DOI] [PubMed] [Google Scholar]
- 19.Kaur G, Bloch O, Jian BJ, Kaur R, Sughrue ME, Aghi MK, et al. A critical evaluation of cystic features in primary glioblastoma as a prognostic factor for survival. J Neurosurg 2011; 115: 754–9. doi: 10.3171/2011.5.JNS11128 [DOI] [PubMed] [Google Scholar]
- 20.Sarmiento JM, Nuno M, Ortega A, Mukherjee D, Fan X, Black KL, et al. Cystic glioblastoma: an evaluation of IDH1 status and prognosis. Neurosurgery 2014; 74: 71–5: discussion 75–6. doi: 10.1227/NEU.0000000000000200 [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez RC, Woods RE, Eddins SL. Digital image processing using MATLAB. Upper Saddle River, NJ: Prentice Hall; 2004. [Google Scholar]
- 22.Artzi M, Aizenstein O, Jonas-Kimchi T, Myers V, Hallevi H, Ben Bashat D. FLAIR lesion segmentation: application in patients with brain tumors and acute ischemic stroke. Eur J Radiol 2013; 82: 1512–8. doi: 10.1016/j.ejrad.2013.05.029 [DOI] [PubMed] [Google Scholar]
- 23.Parmar C, Rios Velazquez E, Leijenaar R, Jermoumi M, Carvalho S, Mak RH, et al. Robust Radiomics feature quantification using semiautomatic volumetric segmentation. PLoS One 2014; 9: e102107. doi: 10.1371/journal.pone.0102107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bauer AH, Erly W, Moser FG, Maya M, Nael K. Differentiation of solitary brain metastasis from glioblastoma multiforme: a predictive multiparametric approach using combined MR diffusion and perfusion. Neuroradiology 2015; 57: 697–703. doi: 10.1007/s00234-015-1524-6 [DOI] [PubMed] [Google Scholar]
- 25.Argaw AT, Gurfein BT, Zhang Y, Zameer A, John GR. VEGF-mediated disruption of endothelial CLN-5 promotes blood-brain barrier breakdown. Proc Natl Acad Sci U S A 2009; 106: 1977–82. doi: 10.1073/pnas.0808698106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang L, Wang X, Zhen S, Zhang S, Kang D, Lin Z. Aquaporin-4 upregulated expression in glioma tissue is a reaction to glioma-associated edema induced by vascular endothelial growth factor. Oncol Rep 2012; 28: 1633–8. doi: 10.3892/or.2012.1973 [DOI] [PubMed] [Google Scholar]
- 27.Yamahara T, Numa Y, Oishi T, Kawaguchi T, Seno T, Asai A, et al. Morphological and flow cytometric analysis of cell infiltration in glioblastoma: a comparison of autopsy brain and neuroimaging. Brain Tumor Pathol 2010; 27: 81–7. doi: 10.1007/s10014-010-0275-7 [DOI] [PubMed] [Google Scholar]
- 28.Occhiogrosso M, Spada A, Merlicco G, Vailati G, De Benedictis G. Malignant cerebellar astrocytoma. Report of five cases. J Neurosurg Sci 1985; 29: 43–50. [PubMed] [Google Scholar]