Abstract
Purpose
To determine whether postoperative radiation therapy (PORT) is associated with an overall survival benefit in patients with completely resected Masaoka or Masaoka-Koga stage II and III thymoma.
Patients and Methods
All patients with completely resected (R0) stage II–III thymoma were identified in a large database of the International Thymic Malignancy Interest Group (ITMIG). Clinical, pathologic, treatment, and follow up information were extracted. Overall survival (OS) was the primary endpoint. A univariate analysis using log-rank test and a multivariate Cox model were created to identify factors associated with OS.
Results
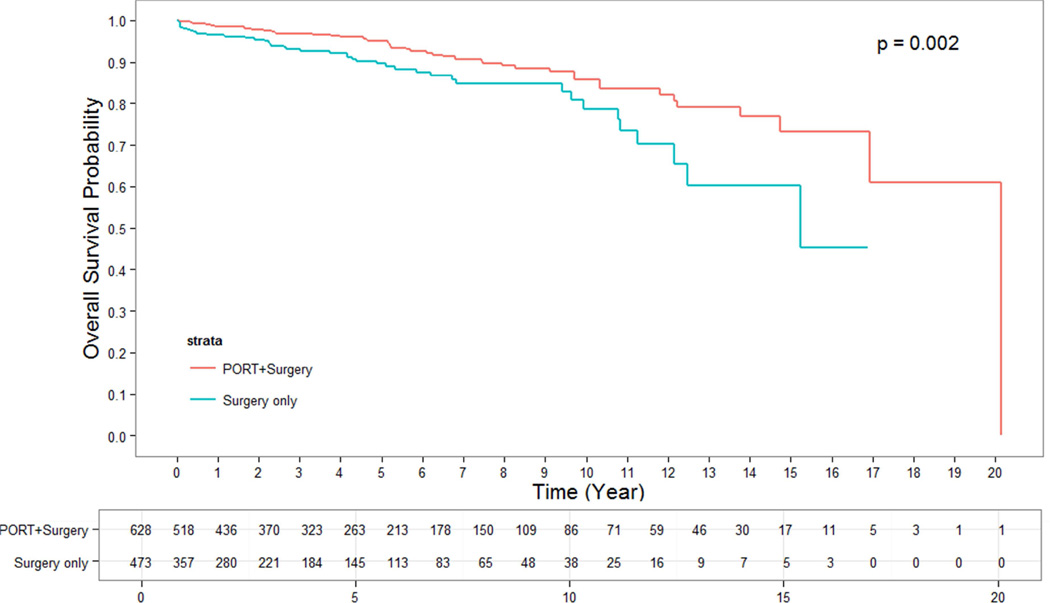
In 1263 patients meeting the selection criteria 870 (69%) patients had stage II thymoma. The WHO histologic subtype was A/AB in 360 (30%) and B1/B2/B3 in 827 (70%) patients. PORT was given to 55% (n=689) of patients, 15% (n=180) received chemotherapy, and 10% (n=122) both. The 5- and 10-year OS rates for patients having undergone surgery + PORT were 95% and 86%, respectively, compared to 90% and 79% for patients with surgery alone(p = 0.002). This OS benefit remained significant when separately analyzing patients with stage II (p= 0.02) and stage III thymoma (p=0.0005). On multivariate analysis, earlier stage, younger age, absence of paraneoplastic syndrome and PORT were significantly associated with improved OS.
Conclusions
We observed an OS benefit with the use of PORT in completely resected stage II and III thymoma. In the absence of a randomized trial, this represents the most comprehensive individual patient data analysis and strong evidence in favor of PORT in this patient population.
Keywords: thymoma, complete resection, postoperative radiation therapy
Introduction
The benefit of postoperative radiation therapy (PORT) in patients with completely resected stage II and III thymoma has been unclear. Many studies have not demonstrated a significant survival benefit of PORT after complete resection of early stage thymomas,1–3 but excellent mediastinal and local control have been reported after surgical resection and PORT.4,5 Thus, the use of PORT in stage II–III disease with an R0 resection remains controversial, particularly given the limitations of most prior studies such as small patient size and heterogeneous patient cohorts. Indeed, population-based studies frequently lack patient-specific data that is necessary for a more detailed analysis.6–9
The International Thymic Malignancy Interest Group (ITMIG) has built two large, multi-institutional international databases of thymic malignancies (retrospective and prospective). The former is the largest of this nature, and we utilized it to examine the role of PORT in patients with completely resected stage II and III thymomas. Our objectives were to: 1) examine the impact of PORT on survival outcomes for patients with completely resected Masaoka stage II and III thymoma, and 2) to determine the role of other potential prognostic factors such as tumor size, patient age, gender, histologic subtype, treatment regimen, and the presence of a paraneoplastic syndrome.
Materials and Methods
This study does not constitute Human Subjects Research, and Institutional Review Board approval was waived, as only completely de-identified data were used.
Patient and Treatment Characteristics
The ITMIG retrospective database is a multi-institutional international database of thymic malignancies, further details of which are described elsewhere.10 Thymoma cases from 39 participating institutions were included in this analysis. Contributing centers are listed in Appendix A. De-identified data from each institution were extracted, and multiple rounds of requests for missing data were performed in order to obtain a dataset that was as complete as possible.
We identified 1263 patients with completely resected (R0) Masaoka or Masaoka-Koga stage II or III thymoma who were treated with either surgery alone or surgery followed by postoperative RT (PORT). All patients were treated between 1990 and 2012. Patients with thymic carcinomas, neuroendocrine tumors of the thymus and preoperative or palliative RT were excluded.
Demographics and clinical tumor, patient and treatment characteristics were collected. These included gender, presence of paraneoplastic syndrome, pathologic stage, tumor size, WHO histology subtypes, use of chemotherapy and PORT. These characteristics are listed in Table 1. Note that various centers reported tumor stage using either Masaoka11 or Masaoka-Koga12,13 systems. Initial analyses of the ITMIG/IASLC database revealed a lack of difference in outcomes between subsets of stage II patients and between the Masaoka and Masaoka-Koga staging systems. Therefore cases staged using the Masaoka and Masaoka-Koga staging systems were combined together (stages IIA, IIB, and II were all categorized as stage II).
Table 1.
Patient and disease characteristics.
| All patients (n=1263) |
Surgery alone (n=574) |
Surgery + PORT (n=689) |
p-value | |
|---|---|---|---|---|
| Median age at diagnosis | 54 | 59 | 51 | <0.0001 |
| [years] | (10 to 88) | (10 to 88) | (15 to 82) | |
| Range | ||||
| Gender | <0.0001 | |||
| Male | 634 (50.2%) | 253 (39.9%) | 381 (60.1%) | |
| Female | 629 (49.8%) | 321 (51.0%) | 308 (49.0%) | |
| Paraneoplastic Syndrome | ||||
| Myasthenia gravis | 464 (37.2%) | 159 (34.3%) | 305 (65.7%) | <0.0001 |
| None | 745 (59.7%) | 389 (52.2%) | 356 (47.8%) | |
| Other | 38 (3.1%) | 15 (39.5%) | 23 (60.5%) | |
| Pathological Stage | ||||
| II | 870 (68.9%) | 449 (51.6%) | 421 (48.3%) | <0.0001 |
| III | 393 (31.1%) | 125 (31.8%) | 268 (68.1%) | |
| Median tumor size [cm] | 5.8 | 5.5 | 6 | 0.046 |
| Range | (0.7 to 22) | (1 to 20.5) | (0.7 to 22) | |
| Histology | ||||
| A/AB | 360 (30.3%) | 225 (62.5%) | 135 (37.5%) | <0.0001 |
| B1/B2/B3 | 827 (69.7%) | 321 (38.8%) | 506 (61.2%) | |
| Chemotherapy | 180 (14.9%) | 58 (32.2%) | 122 (67.8%) | 0.0003 |
Statistical Analysis
All analyses were performed using SAS 9.3 (SAS Institute Inc, Cary, NC) and R version 3.1.2 (R Foundation for Statistical Computing, Vienna, Austria). For patient clinical characteristics, continuous variables are presented as median (range) and categorical ones as frequency with relative percentage. Group comparisons were performed with the use of Fisher’s exact test or chi-square tests for categorical variables and Mann–Whitney U tests for continuous variables, as appropriate. Overall survival (OS) was the primary outcome, measured from the date of surgery until either the date of death or the last known date that the patient was alive. The association of overall survival with clinical and prognostic factors was tested using the log-rank test. Prognostic factors that were significantly associated with survival on univariate analysis and clinically relevant factors were included in a Cox proportional-hazards model for multivariate analysis. These factors include age at diagnosis, sex, paraneoplastic syndrome, pathologic stage, tumor size, histology subtype, chemotherapy, as well as treatment group (Surgery alone vs. Surgery + PORT). Recurrence-free survival was performed using a competing risk model with death as the competing event. A p-value of <0.05 was considered statistically significant.
Results
Patient and disease characteristics
A total of 1263 patients who had undergone a complete resection for stage II and III thymomas with or without PORT were identified in the ITMIG retrospective database. Among 1263 patients, there were 473 patients (37%) from Asia, 486 patients (39%) from Europe and 304 patients (24%) from North/South America. All cases were diagnosed and treated between 1990 and 2012.
The patient and disease characteristics are described in Table 1. Patients who received PORT had a younger median age, were more likely of male gender, had a higher rate of myasthenia gravis, higher proportion of stage III disease, larger tumors, more likely WHO type B1, B2 or B3 histology than A or AB and less likely to having received chemotherapy.
Postoperative Radiation Therapy is Associated with Improved Overall Survival in Patients with Stage II and III Thymoma
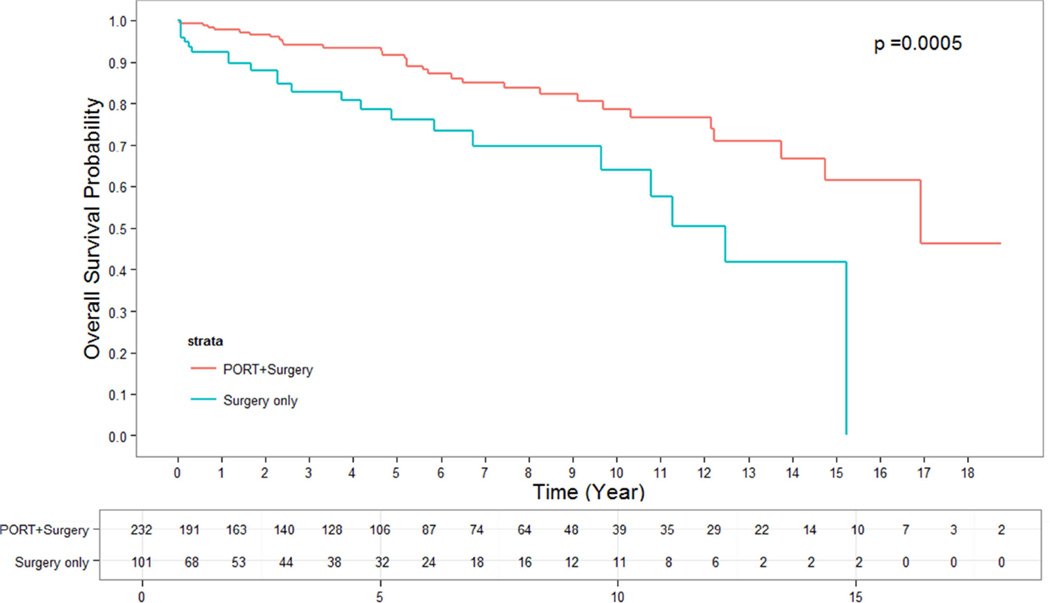
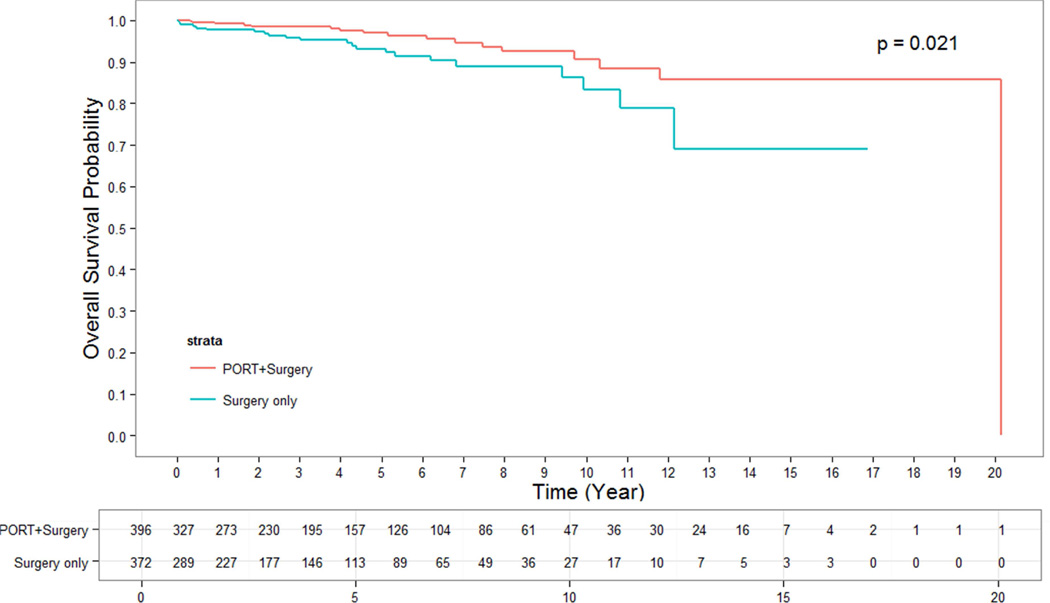
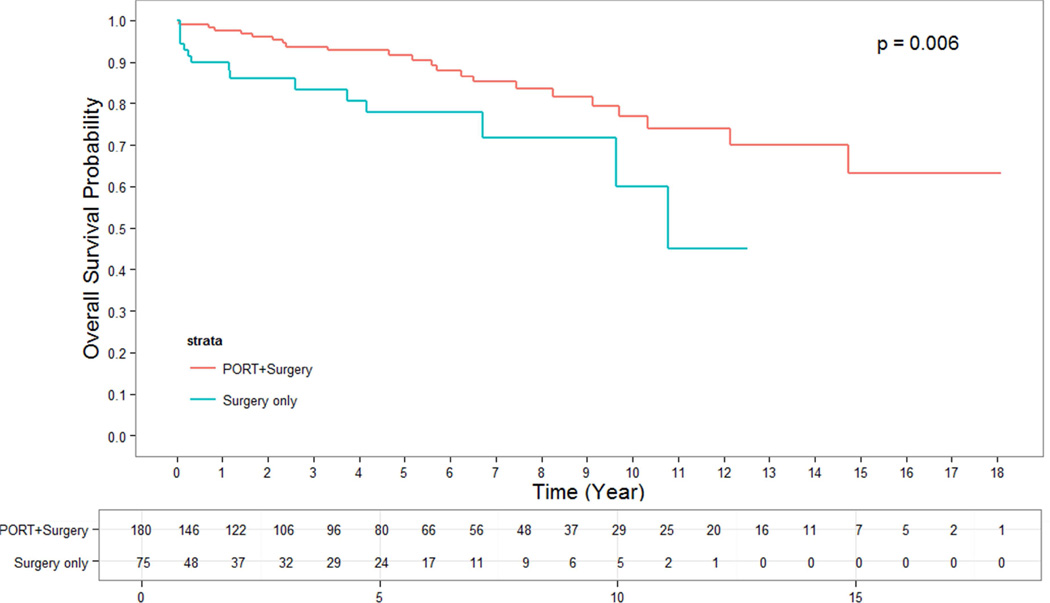
The median follow up time for alive patients who underwent surgery alone was 2.66 years. The median follow up time for alive patients who underwent surgery + PORT was 4.05 years. OS for patients who were treated with surgery + PORT were significantly longer than for those undergoing surgery alone. The 5- and 10-year OS rates for patients having undergone surgery + PORT were 95% and 86%, while for patients with surgery alone they were 90% and 79%, respectively (p = 0.002) (Figure 1). This effect remained strongly significant for the subgroup of patients with stage III thymoma in whom surgery alone versus surgery + PORT resulted in a 5-and 10-year OS of 76% and 64% versus 92% and 79%, respectively (p = 0.0005) (Figure 3). In the subgroup of patients with stage II thymoma, the OS benefit of the addition of PORT to surgical resection was confirmed and remained significant. The 5- and 10-year OS rates for stage II patients having undergone surgery alone versus surgery + PORT were 93% and 83% versus 97% and 91%, respectively (p = 0.02) (Figure 2). When analyzing subgroups based on histologic subtypes (WHO type A or AB versus B1, B2 or B3) in stage II and III thymoma, PORT was associated with a trend towards better OS in all subgroups. The greatest and most statistically significant survival advantage with PORT was seen in the subgroup of patients with stage III, WHO type B1, B2 or B3 thymoma (Figure 4). Recurrence-free survival was not significantly different with surgery + PORT compared to surgery alone (data not shown).
Figure 1.
OS of PORT vs surgery alone for all stages
Figure 3.
OS of PORT vs surgery alone for stage III thymoma
Figure 2.
OS of PORT vs surgery alone for stage II thymoma
Figure 4.
OS of PORT vs surgery alone for stage III, WHO type B1–B3, thymoma
Predictors of OS for Patients with Resected Stage II and III Thymoma
Factors associated with longer OS in the entire patient population on univariate analysis included less advanced pathologic stage (p<0.0001), use of PORT (p = 0.002), no treatment with chemotherapy (p = 0.006), female gender (p = 0.007) and younger age (p <0.0001). Tumor size was borderline significant (p = 0.06).
On multivariate analysis younger age, female gender, absence of paraneoplastic syndrome (not myasthenia gravis only), stage II versus stage III thymoma, and use of PORT were associated with longer OS (Table 2). Age and pathologic stage were the most significant factors (both p <0.0001). The addition of PORT remained significant with a hazard ratio of 0.58 (95% CI 0.34 to 0.97; p = 0.037). The absence of chemotherapy was no longer significant on multivariate analysis.
Table 2.
Multivariate analysis of factors predictive for OS
| Multivariate analysis | |||
|---|---|---|---|
| Characteristic | Strata | HR (95%CI) | p-value* |
| Age at diagnosis | 1.07 (1.05 – 1.09) | <0.0001 | |
| Gender | Male vs Female | 1.61 (1.002 – 2.57) |
0.049 |
| Paraneoplastic syndrome |
Myasthenia gravis vs none Other vs none |
1.60 (0.96 – 2.66) 2.93 (1.13 – 7.56) |
0.035 0.07# 0.027# |
| Pathologic Stage | II vs III | 0.36 (0.22– 0.60) | <0.0001 |
| Tumor size | 1.04 (0.97 – 1.11) | 0.29 | |
| Histology subtype | A/AB vs B1/B2/B3 | 0.61 (0.36 – 1.06) | 0.077 |
| Chemotherapy | Yes vs No | 0.65 (0.37 – 1.13) | 0.13 |
| Treatment type | Surgery vs Surgery + PORT |
0.58 (0.34 – 0.97) | 0.037 |
Likelihood ratio P value from a Cox model.
Wald P values.
Discussion
This study represents the largest dataset on the role of PORT in completely resected Masaoka stage II and III thymoma. It even exceeds the datasets of several SEER analyses.7–9,14 While the ITMIG database is a retrospective database and therefore no amount of covariance adjustment using multivariable statistical models can overcome a lack of group equivalence, its large size allowed us to perform a detailed and well-powered multivariate analysis that included many known prognostic factors. We found that the addition of PORT to a complete surgical resection of Masaoka stage II and III thymoma resulted in a significant OS benefit. When examining patients by individual stage, this improvement was significant not only for patients with stage III, but also with stage II thymoma. This effect remained significant on multivariate analysis with a HR of 0.58 (p=0.037), Indeed, the current study significantly adds to the currently available literature by providing the largest retrospective individual patient dataset on the controversial question of the role of PORT in patients with completely resected stage II and III thymomas. In addition, through combining the large size and multi-institutional nature of the dataset with patient, disease, and treatment characteristics that are not typically included in population-based studies, the results of the study are strengthened further.
Previous reports had been conflicted about the benefit of PORT in patients with completely resected thymomas. In general, most studies are limited in their interpretability and conclusiveness by their small sample size. Many studies include patients with thymomas of various stages (I to IV), variable resection status (R0, R1, R2), and other histologies such as thymic carcinomas or neuroendocrine tumors of the thymus which are likely associated with a different biologic behavior and potentially different response to therapies.15–19
To demonstrate the mixed results that have been reported on this question in the past in single institution studies, Curran et al. found that there was no mediastinal recurrence (0/5) after surgery + PORT compared to 6/18 recurrences with surgery alone (5-year relapse rate 47%) in patients with stage II or III thymoma.4 Similarly, Wu et al. described a 10-year local control rate of 96.8% after surgical resection and postoperative irradiation for stage II thymomas.20 Ogawa et al. also found no recurrences in the irradiated field of 103 patients with completely resected stage I–III thymomas, suggesting a highly effective strategy by surgical resection + PORT.5 Chang et al. reported that in 76 patients with completely resected stage II or III thymomas there was a significant difference in 5- and 10-year DFS (98% vs 80% and 92.7% vs 70%, respectively).21 PORT was associated with improved DFS on multivariate analysis (p=0.043). On a subgroup analysis for patients with stage II thymomas (n=65; 15 patients received PORT) the benefit of PORT did not reach statistical significance.
In contrast, many studies have shown little to no benefit for postoperative RT, especially in patients with stage II thymomas. This was likely due to statistical limitations because of small patient numbers. Utsumi et al. found no significant difference in 10-year disease specific survival with the use of PORT after a complete resection of various stages (I–IV).3 Similarly, at least three other single institution retrospective studies observed no difference with or without PORT.2,22,23 Berman et al. reported that at the University of Pennsylvania the local recurrence rate of completely resected stage II thymoma patients was 3.2% (8% with surgery alone and 0% with surgery + PORT; p=0.15).1 While surgical resection + PORT again appeared to be highly effective to prevent recurrences, the recurrence rate was quite low and thus limited the ability to detect a significant difference with the addition of PORT. Mangi et al. did not detect a decrease in local recurrence or DFS with the addition of PORT in patients with stage III thymoma.24 Some series have described WHO type B3 histologic subtype as a negative prognostic factor on multivariate analysis.2
Indeed, the current analysis is more aptly compared to larger, population-based analyses that have examined this question. To this end, Weksler et al. reported on 476 patients with stage III thymoma in the SEER database, of which 68% were treated with PORT.6 On univariate analysis patients who were treated with PORT had improved OS and DSS. On multivariate analysis PORT was associated with improved DSS, but no longer improved OS. Patel et al. described in another SEER analysis a significant OS benefit and marginal improvement in cause-specific survival with the addition of RT in patients with stage II–III thymomas.8 Forquer et al. analyzed the role of PORT in a mixed SEER population of thymomas and thymic carcinomas and found a possible OS benefit for stage II–III, but no benefit in patients with stage I thymic malignancies after complete resection.9 Consistent with most findings to date Fernandes et al. found a clear benefit of PORT in patients with stage III or IV thymoma and a marginal benefit for stage IIB thymomas.7 A review and meta-analysis of the current literature by Korst et al. including 592 patients with completely resected stage II or III thymic tumors revealed no statistically significant reduction in recurrence with the use of PORT (OR 1.05; p=0.84).25 Most recently, a propensity-score matched SEER analysis on 592 patients found that PORT was associated with better OS and DSS in patients with stage III or IV thymoma, but did not reach statistical significance in stage IIB thymomas.14 Taken together the prior literature appeared to provide stronger support for a role of PORT in patients with stage III or locoregional stage IV thymoma, with unclear potential but still undefined role for stage II thymoma and likely no role for stage I thymomas. However, we believe that this large international dataset with detailed patient characteristics provides the strongest evidence to date of the utility of PORT in either stage II or III disease, even when completely resected.
There are constraints on the interpretation of the results in this study. First, in a retrospective patient dataset there is likely an unfavorable bias in patient referral patterns for PORT, as patients will generally more likely be treated with PORT when they are considered at a higher risk for recurrence due to concerns about residual disease or unfavorable tumor characteristics. Indeed, in our dataset we found that patients receiving PORT had a slightly greater tumor diameter, greater proportion of histologic subtypes deemed to be more aggressive (B1 to B3), and a higher rate of paraneoplastic syndromes, all of which have been previously reported to be potentially adverse prognostic factors. When analyzing subgroups with different histologic subtypes, the patient group with stage III, WHO type B1, B2 or B3, thymomas had the greatest benefit with PORT. Most other subgroups all revealed the same trend favoring PORT but did not reach statistical significance, possibly due to limited patient numbers. On the other hand, the median age of patients receiving PORT was 8 years younger than those undergoing surgery alone which may have an impact on overall survival as the primary endpoint. Indeed, age at diagnosis remained a highly significant factor on multivariate analysis, even though the hazard ratio was only 1.07. As recurrent thymoma can be observed for extended periods of time without developing life-threatening symptoms or may be effectively treated with salvage therapies, age-related comorbidities may affect overall survival in this patient population. However, it also implies that PORT was not associated with excess deaths from intercurrent disease such as long-term cardiac effects or secondary malignancies.
Second, while the database was centralized in terms of setting systematic criteria for providing data, as well as storing and analyzing results, quality assurance at each institution remained dependent on each institution and could not be assessed centrally to preserve patient identifiers.
Finally, our dataset was limited in that we were not able to perform a detailed failure pattern analysis to determine how many recurrences occurred within the radiation field. We also did not have detailed radiation records available to explore the role of the radiation dose, field design, technique etc. Given the time period in which our patient population was treated a majority of patients was likely treated with 3D conformal radiation therapy. We and others have reported on patients with thymomas treated with IMRT,26,27 and the improved target coverage and organ sparing with IMRT would likely have an impact on local control and short- and long-term toxicities, as shown in multiple other disease sites. Proton therapy has emerged as a promising treatment technique that has the potential to spare surrounding lung and the posterior mediastinum even more effectively. Early data from a prospective study at the University of Pennsylvania appears very promising with low esophagitis (7%) and pneumonitis (4%) rates.28 It should be noted that PORT to the tumor bed, typically in the mediastinum, is assumed not to affect the risk of pleural recurrences. As previously described pleural recurrences are the most common site of failure in patients treated with surgical resection + PORT.26,27 The survival benefit seen with PORT to the mediastinum or resection bed may be related to differences in effective salvage strategies depending on site of failure. A failure in the mediastinum, near the heart, great vessels, and central airways is more likely going to lead to potentially life-threatening complications than a peripheral pleural failure. Pleural masses may be more amenable to re-resection, additional radiation therapy or other pleura-targeting treatment strategies.
In conclusion, through an analysis of a multi-institutional, international database of approximately 1200 patients, we found that PORT was correlated with improved OS in patients with completely resected stage II and III thymomas, and that this effect held when accounting for several other patient, disease, and treatment related factors. These analyses suggest an OS benefit provided it is assumed that the intervention groups are equivalent. It represents the best data available in the absence of randomized level 1 evidence, and as such we would recommend strong consideration of PORT in patients with either stage II or III disease even when complete resection is achieved. Further studies are needed to analyze optimal dosing, radiation field design, timing of radiation, and impact of radiation techniques such as IMRT or proton therapy to determine patient subsets that may particularly benefit from PORT. To this end, a prospective database that includes all of these details was established by ITMIG in 2013, and studies addressing these questions are currently underway. While a prospective randomized trial would serve to definitively answer this question, in the context of this data we recommend that providers consider PORT in completely resected stage II and III thymomas.
Supplementary Material
List of contributing institutions
Acknowledgments
Dr. Rimner reports grants, personal fees and non-financial support from Varian Medical Systems, grants from Boehringer Ingelheim, personal fees and non-financial support from Bristol Myers-Squibb, outside the submitted work.
Dr. Huang reports grants from Bristol Myers-Squibb, outside the submitted work.
Dr. Antonicelli reports grants and non-financial support from Medela, outside the submitted work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented in part at the Annual Meeting of the International Thymic Malignancy Interest Group (ITMIG), Shanghai, 2015
Disclaimers: None related to this publication.
DISCLOSURES
Dr. Detterbeck has nothing to disclose.
Dr. Yao has nothing to disclose.
Dr. Ahmad has nothing to disclose.
Dr. Korst has nothing to disclose.
Dr. Gomez has nothing to disclose.
References
- 1.Berman AT, Litzky L, Livolsi V, et al. Adjuvant radiotherapy for completely resected stage 2 thymoma. Cancer. 2011;117:3502–3508. doi: 10.1002/cncr.25851. [DOI] [PubMed] [Google Scholar]
- 2.Chen YD, Feng QF, Lu HZ, et al. Role of adjuvant radiotherapy for stage II thymoma after complete tumor resection. International Journal of Radiation Oncology Biology Physics. 2010;78:1400–1406. doi: 10.1016/j.ijrobp.2009.09.066. [DOI] [PubMed] [Google Scholar]
- 3.Utsumi T, Shiono H, Kadota Y, et al. Postoperative radiation therapy after complete resection of thymoma has little impact on survival. Cancer. 2009;115:5413–5420. doi: 10.1002/cncr.24618. [DOI] [PubMed] [Google Scholar]
- 4.Curran WJ, Jr, Kornstein MJ, Brooks JJ, et al. Invasive thymoma: The role of mediastinal irradiation following complete or incomplete surgical resection. Journal of Clinical Oncology. 1988;6:1722–1727. doi: 10.1200/JCO.1988.6.11.1722. [DOI] [PubMed] [Google Scholar]
- 5.Ogawa K, Uno T, Toita T, et al. Postoperative radiotherapy for patients with completely resected thymoma: A multi-institutional, retrospective review of 103 patients. Cancer. 2002;94:1405–1413. doi: 10.1002/cncr.10373. [DOI] [PubMed] [Google Scholar]
- 6.Weksler B, Shende M, Nason KS, et al. The role of adjuvant radiation therapy for resected stage III thymoma: A population-based study. Annals of Thoracic Surgery. 2012;93:1822–1829. doi: 10.1016/j.athoracsur.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Fernandes AT, Shinohara ET, Guo M, et al. The role of radiation therapy in malignant thymoma: A surveillance, epidemiology, and end results database analysis. Journal of Thoracic Oncology. 2010;5:1454–1460. doi: 10.1097/JTO.0b013e3181e8f345. [DOI] [PubMed] [Google Scholar]
- 8.Patel S, MacDonald OK, Nagda S, et al. Evaluation of the role of radiation therapy in the management of malignant thymoma. International Journal of Radiation Oncology Biology Physics. 2012;82:1797–1801. doi: 10.1016/j.ijrobp.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Forquer JA, Rong N, Fakiris AJ, et al. Postoperative Radiotherapy After Surgical Resection of Thymoma: Differing Roles in Localized and Regional Disease. International Journal of Radiation Oncology*Biology*Physics. 2010;76:440–445. doi: 10.1016/j.ijrobp.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 10.Huang J, Ahmad U, Antonicelli A, et al. Development of the International Thymic Malignancy Interest Group International Database: An Unprecedented Resource for the Study of a Rare Group of Tumors. J Thorac Oncol. 2014;9:1573–1578. doi: 10.1097/JTO.0000000000000269. [DOI] [PubMed] [Google Scholar]
- 11.Masaoka A, Monden Y, Nakahara K, et al. Follow-up study of thymomas with special reference to their clinical stages. Cancer. 1981;48:2485–2492. doi: 10.1002/1097-0142(19811201)48:11<2485::aid-cncr2820481123>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 12.Tsuchiya R, Koga K, Matsuno Y, et al. Thymic carcinoma: proposal for pathological TNM and staging. Pathol Int. 1994;44:505–512. doi: 10.1111/j.1440-1827.1994.tb02600.x. [DOI] [PubMed] [Google Scholar]
- 13.Detterbeck FC, Nicholson AG, Kondo K, et al. The Masaoka-Koga stage classification for thymic malignancies: clarification and definition of terms. J Thorac Oncol. 2011;6:S1710–S1716. doi: 10.1097/JTO.0b013e31821e8cff. [DOI] [PubMed] [Google Scholar]
- 14.Lim YJ, Kim HJ, Wu HG. Role of Postoperative Radiotherapy in Nonlocalized Thymoma: Propensity-Matched Analysis of Surveillance, Epidemiology, and End Results Database. J Thorac Oncol. 2015;10:1357–1363. doi: 10.1097/JTO.0000000000000619. [DOI] [PubMed] [Google Scholar]
- 15.Ahmad U, Yao X, Detterbeck F, et al. Thymic carcinoma outcomes and prognosis: results of an international analysis. J Thorac Cardiovasc Surg. 2015;149:95–100. 101.e1–101.e2. doi: 10.1016/j.jtcvs.2014.09.124. [DOI] [PubMed] [Google Scholar]
- 16.Ruffini E, Detterbeck F, Van Raemdonck D, et al. Thymic carcinoma: a cohort study of patients from the European society of thoracic surgeons database. J Thorac Oncol. 2014;9:541–548. doi: 10.1097/JTO.0000000000000128. [DOI] [PubMed] [Google Scholar]
- 17.Filosso PL, Yao X, Ahmad U, et al. Outcome of primary neuroendocrine tumors of the thymus: a joint analysis of the International Thymic Malignancy Interest Group and the European Society of Thoracic Surgeons databases. J Thorac Cardiovasc Surg. 2015;149:103–109. e2. doi: 10.1016/j.jtcvs.2014.08.061. [DOI] [PubMed] [Google Scholar]
- 18.Omasa M, Date H, Sozu T, et al. Postoperative radiotherapy is effective for thymic carcinoma but not for thymoma in stage II and III thymic epithelial tumors: the Japanese Association for Research on the Thymus Database Study. Cancer. 2015;121:1008–1016. doi: 10.1002/cncr.29166. [DOI] [PubMed] [Google Scholar]
- 19.Rimner A. Postoperative radiotherapy: not all thymic malignancies are created equal. Cancer. 2015;121:972–974. doi: 10.1002/cncr.29164. [DOI] [PubMed] [Google Scholar]
- 20.Wu KL, Mao JF, Chen GY, et al. Prognostic predictors and long-term outcome of postoperative irradiation in thymoma: a study of 241 patients. Cancer Invest. 2009;27:1008–1015. doi: 10.3109/07357900802563002. [DOI] [PubMed] [Google Scholar]
- 21.Chang JH, Kim HJ, Wu HG, et al. Postoperative radiotherapy for completely resected stage II or III thymoma. Journal of Thoracic Oncology. 2011;6:1282–1286. doi: 10.1097/JTO.0b013e31821f9662. [DOI] [PubMed] [Google Scholar]
- 22.Singhal S, Shrager JB, Rosenthal DI, et al. Comparison of stages I–II thymoma treated by complete resection with or without adjuvant radiation. Ann Thorac Surg. 2003;76:1635–1641. doi: 10.1016/s0003-4975(03)00819-1. discussion 1641-2. [DOI] [PubMed] [Google Scholar]
- 23.Rena O, Papalia E, Oliaro A, et al. Does adjuvant radiation therapy improve disease-free survival in completely resected Masaoka stage II thymoma? Eur J Cardiothorac Surg. 2007;31:109–113. doi: 10.1016/j.ejcts.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 24.Mangi AA, Wain JC, Donahue DM, et al. Adjuvant radiation of stage III thymoma: Is it necessary? Annals of Thoracic Surgery. 2005;79:1834–1839. doi: 10.1016/j.athoracsur.2004.12.051. [DOI] [PubMed] [Google Scholar]
- 25.Korst RJ, Kansler AL, Christos PJ, et al. Adjuvant radiotherapy for thymic epithelial tumors: a systematic review and meta-analysis. Ann Thorac Surg. 2009;87:1641–1647. doi: 10.1016/j.athoracsur.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 26.Fan C, Feng Q, Chen Y, et al. Postoperative radiotherapy for completely resected Masaoka stage III thymoma: a retrospective study of 65 cases from a single institution. Radiat Oncol. 2013;8:199. doi: 10.1186/1748-717X-8-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rimner A, Gomez DR, Wu AJ, et al. Failure patterns relative to radiation treatment fields for stage II–IV thymoma. J Thorac Oncol. 2014;9:403–409. doi: 10.1097/JTO.0000000000000099. [DOI] [PubMed] [Google Scholar]
- 28.Vogel J, Berman AT, Lin L, et al. Prospective study of proton beam radiation therapy for adjuvant and definitive treatment of thymoma and thymic carcinoma: Early response and toxicity assessment. Radiother Oncol. 2016;118:504–509. doi: 10.1016/j.radonc.2016.02.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of contributing institutions