Abstract
The forms of inorganic mercury (HgII) taken up and methylated by bacteria in sediments still remain largely unknown. From pure cultures studies, it has been suggested that dissolved organic matter (DOM) may facilitate the uptake either by acting as a shuttle molecule, transporting the HgII atom to divalent metal transporters, or by binding HgII and then being transported into the cell as a carbon source. Enhanced availability of Hg complexed to DOM has however not yet been demonstrated in natural systems. Here, we show that HgII complexed with DOM of marine origin was up to 2.7 times more available for methylation in sediments than HgII added as a dissolved inorganic complex (HgII(aq)). We argue that the DOM used to complex HgII directly facilitated the bacterial uptake of HgII whereas the inorganic dissolved HgII-complex adsorbed to the sediment matrix before forming bioavailable dissolved HgII complexes. We further demonstrate that differences in net methylation in sediments with high and low organic carbon content may be explained by differences in the availability of carbon to stimulate the activity of Hg methylating bacteria rather than, as previously proposed, be due to differences in HgII binding capacities between sediments.
1. Introduction
Methylmercury (MeHg) is a neurotoxic form of Mercury (Hg) that is produced under anoxic conditions in sediments, soils and aquatic waters from inorganic divalent mercury (HgII) mainly by sulfur and iron reducing bacteria (Compeau and Bartha, 1985; Benoit, et al., 2003). A fraction of the MeHg formed bioaccumulates in aquatic food webs to concentrations of concern for human and wildlife health (Mergler, et al., 2007). Though anthropogenic emissions of Hg have decreased substantially in the US, predicting future concentrations of MeHg amidst changes in global Hg emissions remains a challenge (Mason, et al., 2012; Driscoll, et al., 2013). To address this challenge, a better understanding of the factors that control net methylation in aquatic systems is warranted (Benoit, et al., 1999). While the ability to methylate HgII is restricted to specific strains of bacteria carrying the hgcA and hgcB genes, methylation is known to depend both on the activity and composition of the bacterial community as well as the pool of HgII available to HgII methylating bacteria (Jonsson, et al., 2012; King, et al., 2000; Parks, et al., 2013).
The HgII methylation potential has been widely studied across systems, using isotopically enriched HgII tracers in intact sediment cores or sediment slurries (Hammerschmidt, et al., 2008; Jonsson, et al., 2012; Benoit, et al., 1999; Hollweg, et al., 2010). The HgII methylated is assumed to be taken up from the dissolved pool which, in comparison to the amount of HgII methylated within a day at typically reported potential methylation rate constants (km) (0.01-0.12 d-1) (Hammerschmidt, et al., 2008; Hollweg, et al., 2010; Schartup, et al., 2013; Jonsson, et al., 2012), is at least ten times smaller (a typical distribution coefficient between the solid and aqueous phase, KD, of 103-105) (Schartup, et al., 2013; Hollweg, et al., 2010; Hammerschmidt, et al., 2008). Therefore, desorption and dissolution of HgII from the much more abundant pool of HgII present in the sediment occurs to sustain the typically observed methylation rates (Jonsson, et al., 2012). Hence, the speciation of HgII in both the dissolved and solid phase will influence the pool of HgII available to methylating bacteria. Previous work has shown that the availability for methylation of the adsorbed and solid forms of HgII found in sediments, differs up to two orders of magnitude, and that the rate of methylation was controlled by both the thermodynamic stability of the solid phase as well as the kinetics of HgII desorption/dissolution (Jonsson, et al., 2012). Here, we present an examination of the methylation rates of isotopically enriched HgII tracers added as different solid, adsorbed and dissolved forms to four different sediments. Although the availability of both the adsorbed and solid forms will be discussed in this paper, the focus is primarily on the availability of dissolved HgII added to the sediment as HgII complexed with dissolved organic matter (DOM) extracted from coastal waters, or with inorganic ligands.
The dissolved forms of HgII complexes first proposed to be available for methylation included neutrally charged sulfide complexes, which have been assumed to passively diffuse into the cell of the bacteria (Benoit, et al., 1999). This hypothesis was based on field data and pure culture experiments where the concentration of MeHg was related to the modelled concentration of neutrally charged HgII-S species (Benoit, et al., 1999; Hollweg, et al., 2010; Hammerschmidt and Fitzgerald, 2004). It should be noted that the stability constants used in the speciation models are highly uncertain (Skyllberg, 2011). More recent work done in pure bacterial cultures (Schaefer and Morel, 2009; Schaefer, et al., 2014) has suggested that low-molecular weight thiol complexes facilitate the uptake of HgII by methylating bacteria, by serving as a transporting shuttle for HgII to the cell wall, where HgII is then taken up by a divalent metal ion transporter in place of ZnII (an essential element) (Schaefer, et al., 2014). The methylation rate was found to differ between different Hg-thiol complexes with the highest rate observed for Hg bound to cysteine (Schaefer, et al., 2014). It has also been suggested that HgII-DOM complexes are more available because the DOM is taken up as a source of energy by the bacteria, resulting in the unintentional uptake of HgII (Chiasson-Gould, et al., 2014; Schaefer, et al., 2014) or alternatively that DOM may indirectly enhance the availability of HgII under sulfidic conditions by hindering the formation of β-HgS(s) particles large enough to reduce HgII availability (Graham, et al., 2012; Graham, et al., 2013). Although the different theories have been argued for in various pure culture studies, direct experimental support, except for DOM acting as a shuttle molecule for HgII to the divalent metal ion transporters, is missing (Schaefer, et al., 2014).
One of the major challenges in studying bioavailable forms of HgII in natural samples comes from the multiple effects complexing agents (sulfide, thiols etc.) may have on HgII speciation and availability for methylation as well as their effects on bacterial activity. Here, we have compared the methylation rate constant (km, d-1) determined from HgII added as chloride complexes, hereon referred to as HgII (aq) and HgII complexed to DOM (HgII-DOM) in four different estuarine sediments. To distinguish the effect that the added DOM may have on HgII availability and bacterial activity, we also examined the km of HgII (aq) in presence of an equal amount of simultaneously added DOM (as used to produce the HgII-DOM tracer). We also determined the km of HgII adsorbed onto particulate organic matter of marine origin (HgII-POM), and HgII precipitated with sulfide as micro or nanoparticles of metacinnabar (respectively, β-HgS(s)micro and β-HgS(s)nano), as well as HgII equilibrated with two previously collected sediments.
2. Material and Methods
2.1. Preparation of Hg tracers
Methylation assay sets were used containing a 200Hg and a 199Hg enriched species specific HgII tracer, individually frozen in 15 ml falcon tubes. The isotopically enriched HgII tracers were prepared from 200HgCl2 and 199HgCl2 (Oak Ridge National Laboratory, TN, USA) dissolved in 0.1 M HCl (diluted and pH neutralized before use). HgII tracers complexed to dissolved or particulate organic matter (DOM and POM) were prepared by pre-equilibrating 200HgII with DOM or POM for 24 h. The organic matter was extracted from water sampled at Eastern Long Island Sound (ELIS) using Bond Elute PPL cartridges for DOM (as described in the Electronic annex), and by filtering the water through a 1.0 μm plankton net, after which the collected particles were rinsed, freeze-dried and re-suspended in purified water for POM. Our experiments aimed at studying differences in the availability between different dissolved, adsorbed and solid HgII tracers for methylation. To complex HgII, it was thus desired to have a HgII:ligand ratio low enough to ensure that the binding sites to which HgII would complex under natural concentrations, are not saturated. At the same time, higher amounts of DOM and POM could alter the activity of Hg methylating bacteria and were thus avoided. For DOM, we used a HgII:ligand ratio of 1 μg HgII mg-1 DOC. A high and constant binding coefficient of HgII to DOM has been previously demonstrated at ratios equal to or less than the ratio we used (Haitzer, et al., 2002). For POM, we used a concentration ratio of 2.4 μmol HgII g-1 POM. This is comparable to the HgII:POM ratio used in previous work by Jonsson et al. (2012). For all sets containing DOM and POM, controls (n=2) were prepared where a 200HgII(aq) tracer and a DOM or POM slurry were individually frozen in the assay tubes. These controls were used to test if added amounts of DOM or POM increased HgII methylation when not complexed to added HgII tracer by e.g. altering the activity of HgII methylating bacteria.
Synthesis of β-200HgS(s)micro and β-199HgS(s)nano tracers was done under low O2 conditions (using a N2 flushed glove bag) by adding 1.3 μmoles of dissolved sulfide to equimolar concentrations of 200HgII and 199HgII dissolved in purified water or in purified water with 1.3 mg of DOC extracted from ELIS, respectively. All solutions were prepared in purified water degassed by boiling while purging with N2 for 20 minutes. The Na2S stock solution was prepared by dissolving 10 g of washed and dried sodium sulfide crystals in 5 ml of degassed water and standardized using an ion-selective electrode (Orion) and titrating with 0.1M Pb(NO3)2. The particles were aged for 3 days before the slurries were diluted and individually frozen in the assay tubes. Controls for β-199HgS(s)nano assays were prepared containing the β-199HgS(s)nano and the 200HgII(aq) tracers, also individually frozen. Formation of nanoparticles was confirmed by transmission electron microscopy (TEM, Fig. EA1, Electronic annex). Details for the preparation and analysis of TEM samples are given in the Electronic annex.
Additional sets of tracers, (HgII-LOCsediment and HgII-HOCsediment), were prepared where 200HgII was pre-equilibrated in the dark for 24 h with freeze-dried sediments, of low and high organic carbon content, previously collected from two of the sites (Barn Island LOC and HOC) in 2013. The characteristics of the freeze-dried sediments are presented in Table EA1. The ratio of HgII to sediment in all the tracers was 7 and 35 nmol HgII g-1 d.w Barn Island LOC and HOC sediment, respectively. All the tracers were frozen individually. A conceptual diagram with the study design is presented in Fig. 1.
Fig. 1. Illustration of the experimental design.
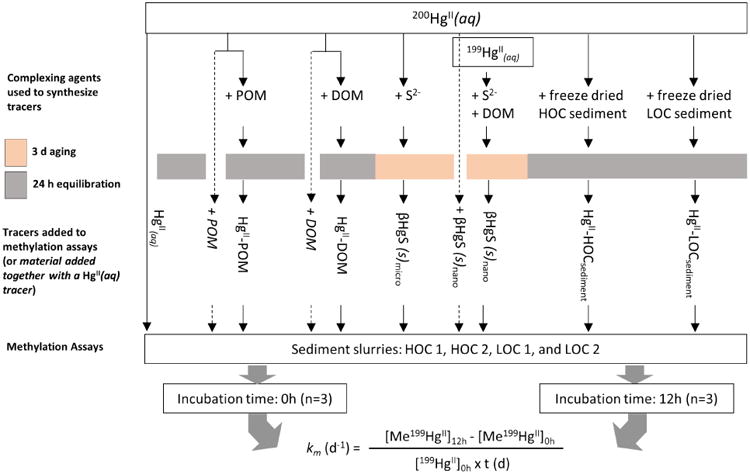
Starting from the top, the illustration shows the complexing agents used to synthesize the different HgII tracers utilized in this study. To prepare HgII-POM, HgII-DOM, HgII-HOCsediment and HgII-LOCsediment, the isotopically enriched HgII(aq) tracer was equilibrated for 24 h (gray field) with the complexing agent. For the case of HgS(s) particles, β-HgS(s)micro was formed when S2- was added to the HgII(aq) tracer while β-HgS(s)nano was formed when DOM followed by S2- were added to the HgII (aq) tracer. Both particles were aged for 3 days (pink field). The synthesized tracers were then added to four different sediment slurries and incubated for 0 h and 12 h. Finally, the methylation rate constant (km) was calculated using the formula shown in the bottom of the illustration. Dashed lines represent control experiments where km of HgII(aq) was quantified in presence of POM, DOM or β-HgS(s)nano (by the simultaneous addition of HgII(aq) with POM, DOM or β-HgS(s)nano to sediment slurries). If methylation was not different from the km of HgII(aq) when added without DOM, POM or β-HgS(s)nano, we conclude that the complexing agent did not impact the rate of methylation by e.g. altering the activity of HgII methylating bacteria.
2.2. Sediment sampling and methylation assays
Sediments from Barn Island (site 1) and Goshen Cove (site 2), Connecticut, USA, were manually collected using acid cleaned polycarbonate core samplers, (diameter of 4.8 cm) from two subsites at each location during low tide in August 2015 (Fig. EA2, Electronic annex). The top 4 cm of multiple sediment cores was pooled and manually homogenized under low oxygen conditions using a N2 flushed glove bag. Tubes with prepared HgII tracers (n=3 per set) were brought to room temperature and while thawing, 10 g of sediment slurry was added and mixed with the tracer using a vortex. The concentration of HgII added to the sediment with the tracer, and the tracer to ambient Hg ratio is given in Table EA2. Samples were incubated for 0 and 12 h (t=0 and t=12, respectively) in a water bath at ambient temperature (±2°C) before the incubation was terminated by flash freezing on dry ice. To verify that steady state in methylation and demethylation was not reached during the course of the 12 h incubations, a subset of experiments with the HgII(aq) tracer were also incubated for 4 h and 24 h. All methylation assays were conducted on the day of sampling. The ambient temperature of the overlying water at the time of sampling is presented in the Electronic annex, Table EA3. All samples were then stored frozen before being freeze-dried. Me201Hg(aq) was added as an internal standard to 0.5-2 g of the sediment and equilibrated for 1 h (in dark) before MeHg was double extracted into purified water using CuSO4/KBr/H2SO4 followed by CH2Cl2 (Lambertsson, et al., 2001). The isotopic composition of extracted MeHg was analyzed by direct ethylation with NaB(C2H5)4, purged and trapped on Carbotrap columns followed by thermal desorption and gas chromatograpy inductively coupled plasma mass spectrometry (GC-ICPMS) as described elsewhere (Hollweg, et al., 2009). Signal deconvolution was performed on mass bias corrected signals (Hintelmann, et al., 2000; Heyes, et al., 2006) and the methylation rate constant (km, d-1,) was calculated in t=12 h samples after correcting for the MeHg concentration detected in t=0 h samples (km (d-1) = ∆[MeHg] · ([HgII]t0 · t(d))-1). The concentration of 200HgII(aq) and 199HgII(aq) stock solutions used to prepare the different tracers as well as the Me201Hg(aq) used as an internal standard, were determined by reversed isotope dilution using solutions of ambient HgII(aq) and MeHg(aq) with known concentrations (diluted from 1000 ppm stock solutions, Alfa Aeser). Methodology for calculating the limit of detection (LOD, Table EA4) is described in the Electronic annex. Statistical data treatment was conducted on log transformed km values using SPSS software (IBM® SPSS). Differences in km values was tested using two-way ANOVA and if the null-hypothesis was rejected (p<0.05) groups statistically differing were identified using Tukey's post hoc analysis. Normal distribution of the data was verified using the Shapiro-Wilk test.
Ancillary parameters (concentration of total Hg and loss on ignition in sediments collected, and the concentration of Hg, MeHg, sulfide and organic carbon (DOC) and fluorescence excitation and emission matrices in sediment pore water) were determined as described in the Electronic annex. The chemical speciation of HgII in the pore water was also calculated as described in the Electronic annex.
3. Results
The concentrations of total Hg (HgT) and MeHg in sediment and sediment pore water (Table 1, Table EA5) collected in the four estuarine locations are within the range typically reported from sites without local point source Hg pollution (Balcom, et al., 2015). Loss on ignition (LOI, Table 1), here used as a proxy for organic matter content (Schartup, et al., 2013), was 15-17 % for two of the sediments (“HOC 1” and “HOC 2”) and 2% for the two more sandy sediments (“LOC 1” and “LOC 2”).
Table 1.
Average (±SD) concentration of total Hg (HgT), MeHg and organic carbon content (measured as the loss on ignition, %) and % MeHg of HgT, in sediments with high (HOC 1 and HOC 2) and low organic content (LOC 1 and LOC 2).
| Sites | HgT (nmol/g) | MeHg (pmol/g) | LOI (%) | % MeHg |
|---|---|---|---|---|
| HOC 1 | 0.3 | 0.4 (0.13) | 17 (0.075) | 0.132 |
| HOC 2 | 0.87 (0.0061) | 0.36 (0.12) | 15 (0.44) | 0.041 (0.008) |
| LOC 1 | 0.051 (0.0097) | 0.73 (0.16) | 1.6 (0.15) | 1.435 (0.243) |
| LOC 2 | 0.042 (0.0012) | 0.34 (0.074) | 2.3 (0.001) | 0.813 (0.101) |
The potential methylation rate constant, km, was calculated assuming pseudo-first-order reaction kinetics (Hintelmann, et al., 2000). To correctly determine km, a linear increase in the amount of MeHg formed from the added HgII tracer is required during the incubation period. Incubation experiments conducted for up to 24 h showed this was true within the first 12 h of the incubations for all sediments, except for HOC 2 (Fig. EA3, Electronic annex). Though the lack of linearity suggests that the system was approaching steady state, the km and the fraction methylated after steady state has been reached (i.e. stable MeHg/HgII) have both been shown useful for comparing differences in availability of HgII(aq) and solid or adsorbed HgII tracers (Jonsson, et al., 2012). In this paper, we use km calculated from 12 h long incubation experiments, even if we recognize that the calculated value may slightly underestimate the true km of added HgII tracers in HOC 2 sediment.
The km of HgII pre-equilibrated for 24 h with POM of marine origin (HgII-POM) was not different than the km of the HgII(aq) tracer in all sediments (Fig. 2a and b, ANOVA, p>0.05). Methylation of HgII(aq) tracer with an equal amount of POM added, as used to synthesize the HgII-POM tracer, (HgII (aq) + POM), was also not different from the km of HgII(aq) tracer in samples with no additional POM added (ANOVA, p>0.05).
Fig 2. Methylation rate constant determined using different HgII tracers in sediments with a) high organic carbon content (HOC 1 and HOC 2) and b) low organic carbon content (LOC 1 and LOC 2).
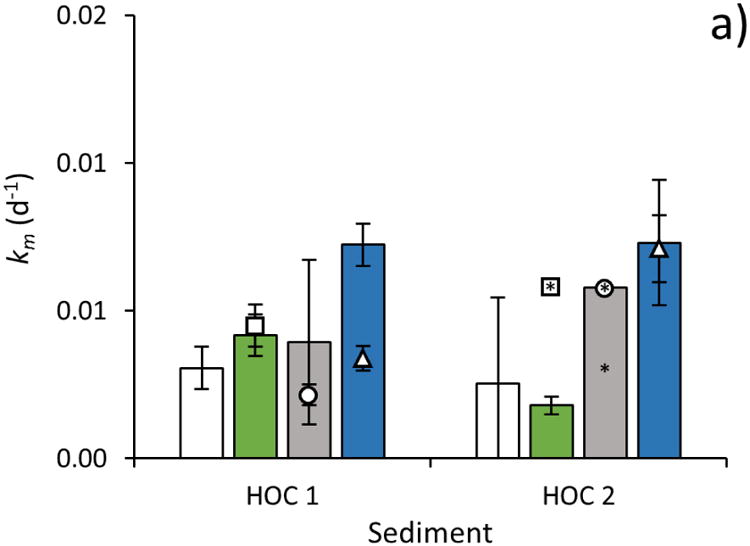
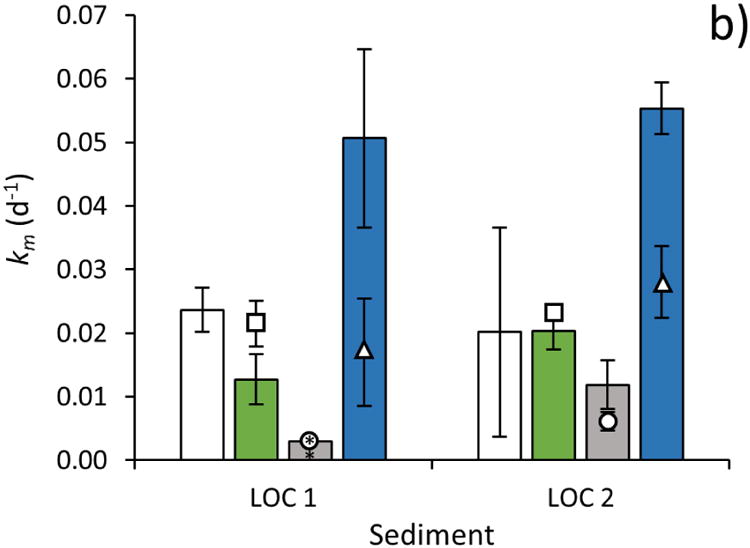
The methylation rate constant (km, d-1; ±SD, n=3) was determined by adding HgII to sediment slurries as an inorganic aqueous tracer (HgII(aq), white bars), as HgII bound to particulate organic matter (HgII-POM, green bars), as β-HgS(s) nanoparticles (β-HgS(s)nano, gray bars), and as HgII bound to dissolved organic matter (HgII-DOM, blue bars). To test how POM and DOM added with the HgII-POM, β-HgS(s)nano, and HgII-DOM tracers affected HgII methylation rates when not complexed to the HgII substrate (by e.g. altering the activity of HgII methylating bacteria), control experiments were conducted. In these control experiments, the HgII(aq) tracer was added to the sediment slurries simultaneously with POM (squares), β-HgS(s)nano (circles) or DOM (triangles). For the controls, error bars show ±SD calculated from n=2. An * associated with any bar or symbol indicates the data < Limit of Detection (LOD) and this value is shown.
Metacinnabar (β-HgS(s)) tracers were synthesized by precipitation of equimolar amounts of isotopically labelled HgII and S2- with and without the presence of DOM. In the absence of DOM, β-HgS(s) precipitated instantly and was assumed, based on previous studies (Jonsson, et al., 2012), to consist of smaller particles (ca 100-200 nm in diameter) aggregated into micrometer sized clusters (β-HgS(s)micro). In the presence of DOM, monodispersed β-HgS(s) particles with a diameter of 4.9 ± 1.2 nm were formed (β-HgS(s)nano) (Fig. EA1, Electronic annex). The methylation of HgII tracer added as β-HgS(s)micro was below the LOD in all the sediments tested and lower than the km of all other tracers tested (ANOVA following Tukey's post hoc analysis, p<0.05), and at least 5-23 times lower than the methylation of HgII added as β-HgS(s)nano.
The km of all tracers added was 2-8 times higher in the LOC sediments than in HOC sediments (Figs. 2-4, ANOVA p<0.05). This is consistent with the higher net methylation observed for ambient HgII in the LOC sediment as evident from the higher fraction (%) of Hg occurring as MeHg (Table 1). To examine how binding of HgII to sediments of different organic carbon content affects the availability for methylation, we prepared two tracers, (HgII-LOCsediment and HgII-HOCsediment), by pre-equilibrating HgII(aq) for 24 h with two freeze-dried sediments of low and high organic carbon content previously collected from the same sites as LOC 1 and HOC 1, respectively. No significant methylation of the tracer was observed during the pre-equilibration time. After incubation with fresh sediment we observed similar km values (ANOVA, p>0.05) for the HgII-LOCsediment and HgII-HOCsediment (Fig. 3).
Fig. 4. Conceptual model of the availability of added tracers (HgII(aq), HgII-DOM, HgII-POM, β-HgS(s)) in marine sediments.
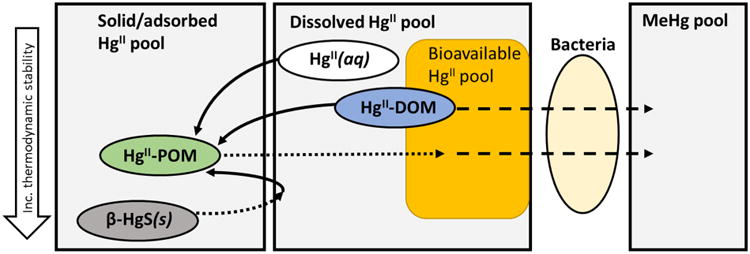
The arrows represent; adsorption (solid lines), dissolution/desorption and formation of bioavailable HgII complexes (dotted lines) and uptake and methylation of HgII (dashed lines).
Fig 3. Methylation rate constant determined using HgII tracers added to sediment slurries (HOC 1, HOC 2, LOC 1 and LOC 2) as HgII bound to freeze dried sediments.
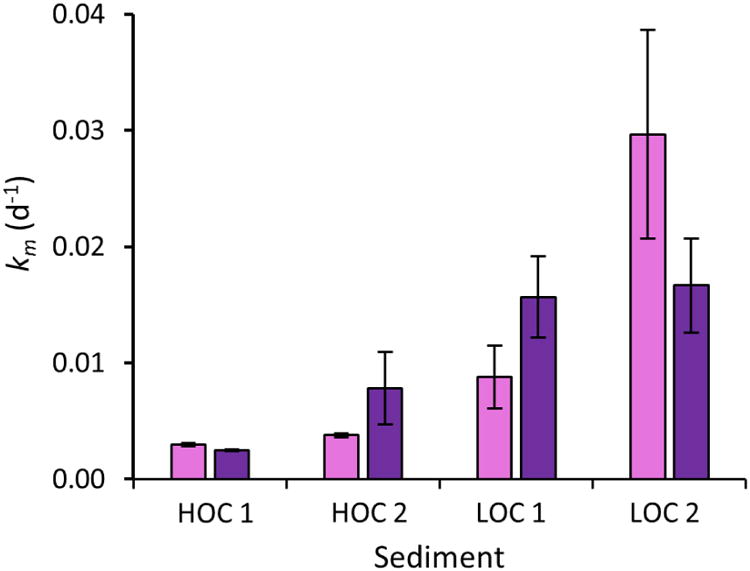
The methylation rate constant (km, d-1; ±SD, n=3) of HgII pre-equilibrated with freeze dried sediment with low organic carbon content (HgII-LOCsediment) is shown in light purple bars and that of HgII pre-equilibrated with freeze dried sediment with high organic carbon content (HgII-HOCsediment) is shown in dark purple bars.
The km of HgII pre-equilibrated for 24 h with DOM of marine origin (HgII-DOM) was 2.1 times and 2.7 times higher (ANOVA following Tukey's post hoc test, p<0.05) than the average km of HgII(aq) in LOC 1 and LOC 2 sediments, respectively (Fig. 2b). The average methylation of HgII-DOM tracer was also higher than that of HgII(aq) in HOC sediments, however, the differences were not statistically significant. In the LOC sediments, the methylation rate of HgII(aq) added to the sediment with the same amount of DOM as used to complex the HgII-DOM tracer, (HgII (aq) + DOM) was similar to observed km of HgII(aq) when no DOM was added (ANOVA following Tukey's post hoc test, p>0.05).
4. Discussion
4.1. Methylation of Hg from the solid/adsorbed Hg pool
The similar km values observed for HgII-POM and HgII(aq) suggests similar availability for methylation of HgII added as complexed to marine POM or as dissolved chloride complexes (Fig. 2a and b). The latter tracer can be assumed to rapidly bind (within seconds) (Hintelmann and Harris, 2004; Jiang, et al., 2015) to easily available adsorption sites (O and N) in the sediment POM matrix, which are much more abundant than any pore water DOM ligands, and with time migrate to less available, but more thermodynamically stable, POM binding sites (reduced sulfur sites) (Jiang, et al., 2015). In contrast to our results, adsorption of HgII complexed to organic matter (OM) derived from a peat soil has previously been demonstrated to reduce the availability of HgII for methylation in a brackish water sediment (Jonsson, et al., 2012). For the POM used by Jonsson et al. (2012), HgII was shown to bind to the OM via a linear coordination to two thiol groups (Skyllberg, et al., 2006). We have no such information available for the specific binding of HgII to the marine POM used in this study, however a growing number of studies suggest that HgII bound to OM of marine origin is more labile than HgII bound to terrestrial OM (Hammerschmidt, et al., 2008). That we, in contrast to Jonsson et al. (2012), did not observe a lower methylation of HgII when added to the sediment as HgII adsorbed onto POM despite using a similar Hg to POM ratio (mol mass-1), may be explained by the differences in the bioavailability (i.e. desorption kinetics) between HgII bound to terrestrial and marine POM. This demonstrates that the type of OM present will determine if, and to what degree, adsorption of HgII to OM affects its availability for methylation.
The lower availability of HgII for methylation when added to the sediment as β-HgS(s)micro has previously been shown (Jonsson, et al., 2012; Zhang, et al., 2014) and is expected due to the higher thermodynamic stability of β-HgS(s) in comparison to e.g. HgII bound to POM or other complexes. The similar availability of β-HgS(s)nano and HgII(aq) tracers (ANOVA, p>0.05) in three of the four sediments (Fig. 2a and b) is in agreement with previous studies (Zhang, et al., 2014). As the nanoparticles are challenging to separate from the surrounding media, it is difficult to evaluate if all HgII has been precipitated and it is possible that the nanoparticle slurry still contained dissolved or clusters of HgII and sulfide. For HgS(s) precipitated in the absence of DOM, previous experiments have confirmed that the HgII methylated originated from the particles themselves and not from HgII remaining in solution (Jonsson, et al., 2012). The higher availability of β-HgS(s)nano relative to β-HgS(s)micro may also be caused by a higher surface area of the nanoparticles which would increase the rate at which the particles dissolve to establish equilibrium, or by a lower presumed thermodynamic stability caused by the DOM preventing aggregation and continuous growth of the particles (Deonarine and Hsu-Kim, 2009). Though the thermodynamic stability of lab synthesized β-HgS(s) nanoparticles and the stability of them in sediment systems has not been well studied, recent work has shown that β-HgS(s) particles forming in presence of terrestrial DOM (Suwanee River and Pony Lake fulvic acid) are more structurally disordered than metacinnabar (Slowey, 2010). It also remains to be demonstrated if β-HgS(s) nanoparticles in sediments constitute a quantitatively important pool of the HgII from which MeHg is formed. Our results and previous work are however of interest since they demonstrate that β-HgS(s), depending on the particle size and stability, differs in its availability for methylation. It is reasonable to assume that the stability of β-HgS(s) precipitated under natural conditions in sediment pore water may be reduced due to the presence of a higher degree of impurities either incorporated or adsorbed onto the solid surface in comparison to β-HgS(s) precipitated in purified water.
4.2. Methylation of tracer and ambient Hg in LOC vs. HOC sediments
Past studies have demonstrated a negative correlation between the km (or fraction of Hg occurring as MeHg) and the content of OM present across different estuarine sediments, and it was suggested that binding to sediment lowers the availability of HgII for methylation (Hammerschmidt and Fitzgerald, 2004; Hollweg, et al., 2010). A negative correlation between km (or % MeHg) and KD was also found in our sediment systems (Fig. EA4, Electronic annex). However, the activity of HgII methylating bacteria has also been coupled to the type of organic carbon present, where autochthonous carbon has been suggested to be a strong driver for HgII methylation than allochthonous carbon (Kim, et al., 2011). The similar availability of the two tracers, HgII-LOCsediment and HgII-HOCsediment, for methylation (Fig. 3, no significant difference between observed km values, ANOVA, p>0.05) in each of the sediment slurries suggest that the binding of Hg to LOC relative to HOC sediment does not limit its availability for methylation. Thus, the higher methylation of HgII in LOC 1 & LOC 2 relative to HOC 1 & HOC 2 (as seen from the added tracers and the higher % MeHg of ambient HgII), is explained by the type of organic matter present for fueling the activity of HgII methylating bacteria (Fig. EA5, Electronic annex), rather than by differences in the binding strength of the sediments. The two HOC sediment samples were collected more inland (Fig. EA2, Electronic annex), and as seen from the fluorescence excitation and emission matrices of pore water collected from these sediments (Fig. EA5, Electronic annex), LOC sediments show a more intense proteinaceous fluorescence (maximum emission at a lower wavelength) and a lower humic-like emission (maximum emission at a higher wavelength) in comparison to the HOC sediments. Previous fluorescence studies in the marine environment have shown that regions of high biological activity have high protein concentrations and that protein-like fluorescence is a proxy for labile DOM (Yamashita and Tanoue, 2003; Mayer, et al., 1999; Para, et al., 2010; Mopper and Schultz, 1993).
4.3. Methylation of Hg complexed to DOM
In the two LOC sediments, the average km of HgII-DOM was 2.1-2.7 times higher in comparison to the average km of the HgII(aq) tracer (Fig. 2b). The methylation rate of HgII(aq) added to the sediment with the same amount of DOM as used to complex the HgII-DOM tracer, (HgII (aq) + DOM) was however not higher, showing that the increased DOC concentration of 8-15% from the added tracer in these sediments (Fig. EA6, Electronic annex) did not have an apparent effect on HgII methylation (by e.g. altering the activity of HgII methylating bacteria). This is further supported by the lack of proteinaceous fluorescence, suggested to be a proxy for labile DOM (Yamashita and Tanoue, 2003; Mopper and Schultz, 1993; Para, et al., 2010), as seen in the EEM recorded for the extracted DOM used in our experiments (Fig. EA7, Electronic annex). The similar methylation rates observed for the HgII(aq) tracer when added to the sediment with and without the simultaneous addition of DOM, also demonstrates that the DOM added did not increase the availability of HgII by increasing the fraction of HgII occurring in the dissolved phase (Waples, et al., 2005; Miller, et al., 2007; Slowey, 2010; Deonarine and Hsu-Kim, 2009). The higher observed km of HgII-DOM in comparison to HgII(aq), thus indicates a higher availability of added HgII-DOM complexes for methylation. This enhanced availability is not apparent in HOC 1 and HOC 2 possibly because here, mercury methylation is limited by bacterial uptake rather than the bioavailability of Hg complexes. Since the HOC sediment slurries have lower bacterial activity as implied by the lower fraction of autochthonous organic matter present in pore water samples (Fig. EA5), mercury uptake by methylating bacteria likely occurs much slower than the combined process of Hg desorption from the solid phase and conversion to a bioavailable complex, (as described in Fig. 4). As such, we find no statistical difference between the availability of HgII(aq) and HgII-DOM in the HOC sediment slurries. Zhang and coworkers observed a similar phenomenon for tracers added to sediments with high and low bacterial activity (Zhang, et al., 2014). Although the higher methylation rate of HgII complexed to low molecular weight thiols and natural DOM has been previously demonstrated in pure bacteria cultures, our study is the first demonstrating this in natural samples.
Below we discuss the enhanced availability of our HgII-DOM tracer based on the assumption that the adsorption of HgII(aq) to the sediment occurs faster than the formation and uptake of bioavailable complexes (presumably dissolved) into HgII methylating bacteria. In more traditional methylation assays, where HgII(aq) is used as the methylation tracer, the HgII(aq) added is assumed to quickly partition to easily available binding sites in the sediment (Hintelmann, et al., 2000; Hintelmann and Harris, 2004; Jiang, et al., 2015) and with time migrate to less available and more stable binding sites thereby adopting an overall binding strength resembling that of ambient Hg (Hintelmann, et al., 2000; Jonsson, et al., 2012). If our assumption was incorrect, a rapid increase in the concentration of MeHg formed from the HgII(aq) tracer would be expected within the first minutes of such incubation experiments. However, the concentration of the methylated tracer in “t=0” samples (which, in reality represents a time of incubation of up to a few minutes, depending on the method of termination used), is typically very low when compared to the concentration of MeHg formed in samples incubated for hours (Jonsson, et al., 2012). This was also true in our LOC sediments (Fig. EA3, Electronic annex), thus justifying our assumption that the adsorption of HgII(aq) to binding sites in the sediment (predominantly surface sites on the POM present) is faster than the formation and uptake of bioavailable HgII complexes. We thus argue that the binding sites to which HgII(aq) first adsorbs to, will also rapidly bind any HgII released by another tracer (e.g. HgII-DOM), before the released HgII is transformed to a bioavailable form and taken up by the bacteria. The readily available and abundant binding sites on POM in the sediment thus act as a buffer, regulating the dissolved concentration of HgII that can be transformed to a bioavailable form.
To explain the observed enhanced availability of HgII complexed with DOM for bacterial uptake and HgII methylation, we consider two main theories that have been suggested from experiments done in pure bacteria cultures: i) the complexation of HgII to DOM favors the formation of smaller β-HgS(s) particles whereas Hg(aq) precipitates as larger, and less available β-HgS(s) particles and ii) HgII-DOM complexes are directly available for uptake by HgII methylating bacteria. We argue that the latter is the more likely explanation of the enhanced availability of added HgII-DOM tracer in our sediment systems.
The formation of smaller, less stable, β-HgS(s) particles from HgII complexed to DOM has previously been used to explain the enhanced methylation of HgII(aq) in pure bacterial cultures under low sulfide conditions (≤ 30 μM) in the presence of various DOM isolates obtained from fresh water and marine environments (Graham, et al., 2012; Graham, et al., 2013). Indeed, we observed a higher availability for methylation of the nano-sized β-HgS(s) particles (β-HgS(s)nano) compared to the larger ones (β-HgS(s)micro) (Table EA6, ANOVA following Tukey's post hoc test, p<0.05). However, as binding to POM would also be expected to partly prevent the precipitation of β-HgS(s), it can be argued that the km obtained for added HgII(aq) should have been lower than the km of β-HgS(s)nano and HgII-POM. As previously discussed, this was not the case in our experiments. Additionally, our speciation calculations, described in the Electronic annex, do not predict the precipitation of β-HgS(s) at the sulfide, DOM and HgII levels present in our sediment pore waters. We thus argue that the enhanced availability of HgII-DOM was not due to formation of smaller sized and less stable β-HgS(s) particles.
Instead, we posit that a fraction of the HgII added as HgII-DOM complexes, was directly available for uptake by HgII methylating bacteria (Fig. 4). The DOM could either be acting as a shuttle for the HgII to divalent metal ion transporters within the cell wall of the bacteria, or be taken up into the cell as a HgII-DOM complex. Both these processes have previously been suggested from studies done in pure bacterial cultures (Schaefer, et al., 2014; Chiasson-Gould, et al., 2014). Chiasson-Gould et al. (2014) further found that the equilibration time of HgII with riverine humic and fulvic acid isolates changed the availability of HgII for uptake into an E. coli strain. The difference observed was suggested to be from the transfer of HgII during equilibration, from labile, bio accessible and/or weaker sites on the DOM to stronger sites on refractory and inaccessible macromolecules. In a similar way, Schartup et al. (2015) found enhanced availability of HgII complexed to marine DOM (extracted from New England shelf waters and similar to the ELIS DOM used in our experiments) in comparison to HgII complexed to, presumably less bioavailable, riverine DOM. The mechanism involving DOM as a shuttle molecule, has been proposed by Schaefer et al. (2014) who found that the methylation of HgII by Geobacter sulfurreducens was fifty times higher in the presence of cysteine, or when sulfide was added with/without cysteine (Schaefer and Morel, 2009). The molecular configuration of the Hg(Cysteine)2 complex was proposed to allow for ligand exchange with metal transporting sites on the cell wall of the bacteria (Schaefer and Morel, 2009; Schaefer, et al., 2014). This mechanism was supported by the addition of ZnII and CdII inhibiting uptake and methylation of HgII (Schaefer, et al., 2014). Though the concentration of cysteine was not determined in our DOM samples, nanomolar levels of low molecular weight thiols have been detected in Long Island Sound waters and other coastal systems (Hu, et al., 2006; Ndu, 2011). Whether the HgII-DOM complexes were taken up by the bacteria or DOM acted as a transporter for HgII to the cell wall of the bacteria cannot be elucidated from this study. It is possible that these two processes are occurring simultaneously.
5. Conclusions and Environmental implications
Although several forms of HgII have been suggested to be available to HgII methylating bacteria, based primarily on pure bacterial culture studies, the form of HgII taken up (i.e. either as the free ion or a complex) and methylated in natural systems remains unknown. Such information is needed in order to fully evaluate the biogeochemical cycle of Hg in sediments and to identify factors limiting the net methylation of HgII. Our results, showing enhanced availability of HgII added as complexed to DOM of marine origin, suggest some HgII-DOM complexes are part of the dissolved HgII pool taken up and methylated by bacteria in sediments. As previously demonstrated (Jonsson, et al., 2012) and as shown here, the speciation of HgII in the solid phase is also an important factor controlling the net methylation of HgII present. Indeed, the fraction of HgII(aq) methylated in our sediments was 60-100 times higher than the pool of added tracer expected to be present in the sediment pore water at equilibrium (based on the measured partition coefficients), demonstrating that the dissolved bioavailable pool of HgII was readily resupplied from the adsorbed/solid phase during the course of the 12 h experiments. This may also suggest that the pool of ligands available to form bioavailable HgII complexes exceeds the dissolved pool of HgII in the pore water, assuming the ligands are also taken up by the bacteria. Whether the size of the pool of these ligands could be a potential factor limiting net methylation of HgII remains unclear as the specific forms bioavailable are still largely unknown. The relatively low concentration of individual HgII-DOM complexes makes them analytically challenging to determine, however emerging analytical techniques (Liem-Nguyen, et al., 2015), and a better understanding of the DOM pools available and HgII-DOM complexes being formed in natural environments could provide further insights in the near future. Our study emphasizes the power of using species/chemical specific forms of isotopically enriched HgII tracers to study which DOM complexes of HgII, are directly bioavailable to HgII methylating bacteria in natural systems.
In our work, we show that HgII added to sediment slurries as HgII equilibrated with freeze-dried sediments of different organic matter content, total sulfur and % autochthonous carbon (HgII-LOCsediment and HgII-HOCsediment) were similar in their availability to the methylating bacteria in each of our four systems. Our results question the hypothesis that OM controls the methylation by controlling the amount of HgII in the dissolved phase. As allochthonous carbon is both suggested to bind HgII more strongly and be less available as a carbon source to HgII methylating bacteria, the KD and bacterial activity can be expected to co-vary, and thus differences in bacterial activity could possibly also explain differences in methylation rates observed among sites where a correlation has been found between KD and km. In line with the earlier proposed hypothesis, that organic matter would control km by controlling the dissolved pool, eutrophication in estuaries has been suggested to limit the net production of MeHg. The opposite would however be expected if a surge in nutrient loading to estuaries would increase the bacterial activity via an increase in deposits of autochthonous carbon to the sediment (following a rise in primary production). It is evident from our study, as well as previous work (Jonsson, et al., 2012; Zhang, et al., 2014; King, et al., 2000), that both bacterial activity and speciation of HgII in the adsorbed/solid phase influences the net methylation of Hg in sediments. It has been suggested that there may be a threshold of bacterial activity, beyond which HgII methylation is controlled mostly by the bioavailability of the HgII species (Kucharzyk, et al., 2015). Our work indicates that HgII bound to OM would not result in conditions where the methylation is entirely controlled by the speciation. Our results also suggest that the higher methylation rates recoded in off shore sediments relative to estuarine sediments (Hollweg, et al., 2010) may be due to a higher proportion of in situ derived organic matter load to the sediment in these locations. It is clear from this research that predicting future changes in MeHg concentrations across estuaries with climate change and changing system eutrophication, requires a holistic approach aimed at examining the factors that affect both HgII bioavailability to methylating bacteria and the bacterial methylating activity.
Acknowledgments
This research was supported by the Swedish Research Council (International Postdoc grant 637-2014-54) to S.J, partial support for N.M.M and R.P.M came from the National Institutes of Health, through collaboration with investigators at Dartmouth College (NIH Grant Number P42 ES007373) and partial supported for S.T was through the FEI Graduate Fellowship award. The TEM studies were performed using the facilities in the UConn/FEI Center for Advanced Microscopy and Materials Analysis (CAMMA). Staff and students from the labs of Robert P. Mason (University of Connecticut) and Celia Chen (Dartmouth College) are acknowledged for their help during field sampling.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balcom PH, Schartup AT, Mason RP, Chen CY. Sources of water column methylmercury across multiple estuaries in the Northeast U.S. Mar Chem. 2015;177:721–730. doi: 10.1016/j.marchem.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit JM, Gilmour CC, Heyes A, Mason RP, Miller CL. Geochemical and Biological Controls over Methylmercury Production and Degradation in Aquatic Ecosystems. In: Cai Y, Braids OC, editors. Biogeochemistry of Environmentally Important Trace Elements. American Chemical Society; 2003. pp. 262–297. [Google Scholar]
- Benoit JM, Gilmour CC, Mason RP, Heyes A. Sulfide controls on mercury speciation and bioavailability to methylating bacteria in sediment pore waters. Environ Sci Technol. 1999;33:951–957. [Google Scholar]
- Chiasson-Gould SA, Blais JM, Poulain AJ. Dissolved organic matter kinetically controls mercury bioavailability to bacteria. Environ Sci Technol. 2014;48:3153–3161. doi: 10.1021/es4038484. [DOI] [PubMed] [Google Scholar]
- Compeau GC, Bartha R. Sulfate-reducing bacteria: Principal methylators of mercury in anoxic estuarine sediment. Appl Environ Microbiol. 1985;50:498–502. doi: 10.1128/aem.50.2.498-502.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deonarine A, Hsu-Kim H. Precipitation of mercuric sulfide nanoparticles in NOM-containing water: Implications for the natural environment. Environ Sci Technol. 2009;43:2368–2373. doi: 10.1021/es803130h. [DOI] [PubMed] [Google Scholar]
- Driscoll CT, Mason RP, Chan HM, Jacob DJ, Pirrone N. Mercury as a global pollutant: Sources, pathways, and effects. Environ Sci Technol. 2013;47:4967–4983. doi: 10.1021/es305071v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham AM, Aiken GR, Gilmour CC. Effect of dissolved organic matter source and character on microbial Hg methylation in Hg-S-DOM solutions. Environ Sci Technol. 2013;47:5746–5754. doi: 10.1021/es400414a. [DOI] [PubMed] [Google Scholar]
- Graham AM, Aiken GR, Gilmour CC. Dissolved organic matter enhances microbial mercury methylation under sulfidic conditions. Environ Sci Technol. 2012;46:2715–2723. doi: 10.1021/es203658f. [DOI] [PubMed] [Google Scholar]
- Haitzer M, Aiken GR, Ryan JN. Binding of mercury(II) to dissolved organic matter: The role of the mercury-to-DOM concentration ratio. Environ Sci Technol. 2002;36:3564–3570. doi: 10.1021/es025699i. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt CR, Fitzgerald WF. Geochemical Controls on the Production and Distribution of Methylmercury in Near-Shore Marine Sediments. Environ Sci Technol. 2004;38:1487–1495. doi: 10.1021/es034528q. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt CR, Fitzgerald WF, Balcom PH, Visscher PT. Organic matter and sulfide inhibit methylmercury production in sediments of New York/New Jersey Harbor. Mar Chem. 2008;109:165–182. [Google Scholar]
- Heyes A, Mason RP, Kim EH, Sunderland E. Mercury methylation in estuaries: Insights from using measuring rates using stable mercury isotopes. Mar Chem. 2006;102:134–147. [Google Scholar]
- Hintelmann H, Harris R. Application of multiple stable mercury isotopes to determine the adsorption and desorption dynamics of Hg(II) and MeHg to sediments. Mar Chem. 2004;90:165–173. [Google Scholar]
- Hintelmann H, Keppel-Jones K, Evans RD. Constants of mercury methylation and demethylation rates in sediments and comparison of tracer and ambient mercury availability. Environ Toxicol Chem. 2000;19:2204–2211. [Google Scholar]
- Hollweg TA, Gilmour CC, Mason RP. Mercury and methylmercury cycling in sediments of the mid-Atlantic continental shelf and slope. Limnol Oceanogr. 2010;55:2703–2722. [Google Scholar]
- Hollweg TA, Gilmour CC, Mason RP. Methylmercury production in sediments of Chesapeake Bay and the mid-Atlantic continental margin. Mar Chem. 2009;114:86–101. [Google Scholar]
- Hu H, Mylon SE, Benoit G. Distribution of the thiols glutathione and 3-mercaptopropionic acid in Connecticut lakes. Limnol Oceanogr. 2006;51:2763–2774. [Google Scholar]
- Jiang T, Skyllberg U, Wei S, Wang D, Lu S, Jiang Z, Flanagan DC. Modeling of the structure-specific kinetics of abiotic, dark reduction of Hg(II) complexed by O/N and S functional groups in humic acids while accounting for time-dependent structural rearrangement. Geochim Cosmochim Acta. 2015;154:151–167. [Google Scholar]
- Jonsson S, Skyllberg U, Nilsson MB, Westlund PO, Shchukarev A, Lundberg E, Björn E. Mercury methylation rates for geochemically relevant Hg II species in sediments. Environ Sci Technol. 2012;46:11653–11659. doi: 10.1021/es3015327. [DOI] [PubMed] [Google Scholar]
- Kim M, Han S, Gieskes J, Deheyn DD. Importance of organic matter lability for monomethylmercury production in sulfate-rich marine sediments. Sci Total Environ. 2011;409:778–784. doi: 10.1016/j.scitotenv.2010.10.050. [DOI] [PubMed] [Google Scholar]
- King JK, Kostka JE, Frischer ME, Saunders FM. Sulfate-reducing bacteria methylate mercury at variable rates in pure culture and in marine sediments. Appl Environ Microbiol. 2000;66:2430–2437. doi: 10.1128/aem.66.6.2430-2437.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharzyk KH, Deshusses MA, Porter KA, Hsu-Kim H. Relative contributions of mercury bioavailability and microbial growth rate on net methylmercury production by anaerobic mixed cultures. Environ Sci Process Impacts. 2015;17:1568–1577. doi: 10.1039/c5em00174a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambertsson L, Lundberg E, Nilsson M, Frech W. Applications of enriched stable isotope tracers in combination with isotope dilution GC-ICP-MS to study mercury species transformation in sea sediments during in situ ethylation and determination. J Anal At Spectrom. 2001;16:1296–1301. [Google Scholar]
- Liem-Nguyen V, Bouchet S, Björn E. Determination of sub-nanomolar levels of low molecular mass thiols in natural waters by liquid chromatography tandem mass spectrometry after derivatization with p-(hydroxymercuri) benzoate and online preconcentration. Anal Chem. 2015;87:1089–1096. doi: 10.1021/ac503679y. [DOI] [PubMed] [Google Scholar]
- Mason RP, Choi AL, Fitzgerald WF, Hammerschmidt CR, Lamborg CH, Soerensen AL, Sunderland EM. Mercury biogeochemical cycling in the ocean and policy implications. Environ Res. 2012;119:101–117. doi: 10.1016/j.envres.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer LM, Schick LL, Loder TC., III Dissolved protein fluorescence in two maine estuaries. Mar Chem. 1999;64:171–179. [Google Scholar]
- Mergler D, Anderson HA, Chan LHM, Mahaffey KR, Murray M, Sakamoto M, Stern AH. Methylmercury exposure and health effects in humans: A worldwide concern. Ambio. 2007;36:3–11. doi: 10.1579/0044-7447(2007)36[3:meahei]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Miller CL, Mason RP, Gilmour CC, Heyes A. Influence of dissolved organic matter on the complexation of mercury under sulfidic conditions. Environ Toxicol Chem. 2007;26:624–633. doi: 10.1897/06-375r.1. [DOI] [PubMed] [Google Scholar]
- Mopper K, Schultz CA. Fluorescence as a possible tool for studying the nature and water column distribution of DOC components. Mar Chem. 1993;41:229–238. [Google Scholar]
- Ndu UC. Ph D thesis. University of Connecticut; 2011. The Mechanisms and Pathways of the Uptake of Inorganic Mercury and Methylmercury Species in Escherichia coli: Possible Implications for Mercury Cycling in the Marine Environment. [Google Scholar]
- Para J, Coble PG, Charrière B, Tedetti M, Fontana C, Sempéré R. Fluorescence and absorption properties of chromophoric dissolved organic matter (CDOM) in coastal surface waters of the northwestern Mediterranean Sea, influence of the Rhône River. Biogeosciences. 2010;7:4083–4103. [Google Scholar]
- Parks JM, Johs A, Podar M, Bridou R, Hurt RA, Jr, Smith SD, Tomanicek SJ, Qian Y, Brown SD, Brandt CC, Palumbo AV, Smith JC, Wall JD, Elias DA, Liang L. The genetic basis for bacterial mercury methylation. Science. 2013;339:1332–1335. doi: 10.1126/science.1230667. [DOI] [PubMed] [Google Scholar]
- Schaefer JK, Morel FMM. High methylation rates of mercury bound to cysteine by Geobacter sulfurreducens. Nat Geosci. 2009;2:123–126. [Google Scholar]
- Schaefer JK, Szczuka A, Morel FMM. Effect of divalent metals on Hg(II) uptake and methylation by bacteria. Environ Sci Technol. 2014;48:3007–3013. doi: 10.1021/es405215v. [DOI] [PubMed] [Google Scholar]
- Schartup AT, Mason RP, Balcom PH, Hollweg TA, Chen CY. Methylmercury production in estuarine sediments: Role of organic matter. Environ Sci Technol. 2013;47:695–700. doi: 10.1021/es302566w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skyllberg U. Chemical speciation of mercury in soil and sediment. In: Liu G, Cai Y, O'Driscoll N, editors. Environmental Chemistry and Toxicology of Mercury. John Wiley & Sons; New Jersey: 2011. pp. 219–258. [Google Scholar]
- Skyllberg U, Bloom PR, Qian J, Lin CM, Bleam WF. Complexation of mercury(II) in soil organic matter: EXAFS evidence for linear two-coordination with reduced sulfur groups. Environ Sci Technol. 2006;40:4174–4180. doi: 10.1021/es0600577. [DOI] [PubMed] [Google Scholar]
- Slowey AJ. Rate of formation and dissolution of mercury sulfide nanoparticles: The dual role of natural organic matter. Geochim Cosmochim Acta. 2010;74:4693–4708. [Google Scholar]
- Waples JS, Nagy KL, Aiken GR, Ryan JN. Dissolution of cinnabar (HgS) in the presence of natural organic matter. Geochim Cosmochim Acta. 2005;69:1575–1588. [Google Scholar]
- Yamashita Y, Tanoue E. Chemical characterization of protein-like fluorophores in DOM in relation to aromatic amino acids. Mar Chem. 2003;82:255–271. [Google Scholar]
- Zhang T, Kucharzyk KH, Kim B, Deshusses MA, Hsu-Kim H. Net methylation of mercury in estuarine sediment microcosms amended with dissolved, nanoparticulate, and microparticulate mercuric sulfides. Environ Sci Technol. 2014;48:9133–9141. doi: 10.1021/es500336j. [DOI] [PubMed] [Google Scholar]


