Abstract
Background
Type 2 myocardial infarction (MI) is defined as myocardial necrosis (myonecrosis) due to an imbalance in supply and demand with clinical evidence of ischemia. Some clinical scenarios of supply-demand mismatch predispose to myonecrosis but limit the identification of symptoms and ECG changes referable to ischemia; therefore, the MI definition may not be met. Factors that predispose to type 2 MI and myonecrosis without definite MI, approaches to treatment, and outcomes remain poorly characterized.
Methods
Patients admitted to an academic medical center with an ICD-9 diagnosis of secondary myocardial ischemia or non-primary diagnosis of non-ST-elevation MI were retrospectively reviewed. Cases were classified as either MI (n=255) or myonecrosis without definite MI (n=220) based on reported symptoms, ischemic ECG changes, and new wall motion abnormalities.
Results
Conditions associated with type 2 MI or myonecrosis included non-cardiac surgery (38%), anemia or bleeding requiring transfusion (32%), sepsis (31%), tachyarrhythmia (23%), hypotension (22%), respiratory failure (23%), and severe hypertension (8%). Inpatient mortality was 5%, with no difference between patients with MI and those with myonecrosis (6% vs. 5%, p=0.41). At discharge, only 43% of patients received aspirin and statin therapy.
Conclusions
Type 2 MI and myonecrosis occur frequently in the setting of supply-demand mismatch due to non-cardiac surgery, sepsis, or anemia. Myonecrosis without definite MI is associated with similar in-hospital mortality as type 2 MI; both groups warrant further workup for cardiovascular disease. Antiplatelet and statin prescriptions were infrequent at discharge, reflecting physician uncertainty about the role of secondary prevention in these patients.
Keywords: myocardial infarction, myocardial ischemia, secondary prevention, risk factor, myocardial injury, myocardial necrosis
Introduction
The 2007 Task Force for the Universal Definition of Myocardial Infarction (MI) introduced five subtypes of MI requiring a rise and/or fall of cardiac biomarkers consistent with myocardial necrosis (myonecrosis) and clinical evidence of ischemia, including symptoms, electrocardiogram (ECG) changes, or new regional wall motion abnormalities.1,2 Type 1 MI results from plaque rupture, erosion, ulceration, fissuring, or dissection in the setting of unstable atherosclerotic coronary artery disease (CAD).2 In contrast, type 2 MI addresses the clinical scenario of infarction due to an imbalance in oxygen supply and demand that is attributed to a condition other than unstable CAD or recent coronary revascularization.2 This definition includes supply demand mismatch in the setting of fixed, stable atherosclerotic CAD.2 Type 2 MI has been reported in 3% to 25% of acute MI, with variations in incidence based on the population studied.3-6 Clinical evidence of ischemia (e.g. symptoms or ECG changes) can be difficult to obtain in the setting of provoking conditions such as surgery or sepsis and may be underreported. The clinical relevance of distinctions between type 2 MI fulfilling the Universal Definition criteria and myocardial necrosis without definite MI has not been established.6-9 We investigated patient characteristics, provoking conditions, management, and in-hospital outcomes of type 2 MI and myonecrosis without definite MI in a large, retrospective, single-center study of patients admitted to an academic tertiary care institution.
Methods
Patients admitted to New York University Langone Medical Center from January 2013 to December 2013 with ≥1 abnormal laboratory value of cardiac troponin were identified and International Classification of Diseases, Ninth Revision (ICD-9) diagnosis codes were obtained. Medical records with a principal or secondary diagnosis code of secondary myocardial ischemia (ICD-9 411.89) or a secondary diagnosis of non-ST-elevation MI (ICD-9 410.71) were retrospectively reviewed. At our institution, these codes are assigned by clinical documentation specialists using a standardized approach that requires provider documentation of the troponin elevation in addition to a positive laboratory result. Cases with admission for heart failure exacerbation, pulmonary embolism, myocarditis, or aortic dissection were excluded. Cases with only one troponin value were excluded, as were cases without either ≥10% rise and fall of troponin or ≥10% fall in troponin if the first value was the highest value. Cases were also excluded when the documented clinical impression of the cardiology consultant was type 1, type 4 (PCI-related) MI, or type 5 (cardiac surgery-related) MI; or complete data were not available from the electronic medical record. Plasma cardiac troponin I (cTnI) was measured using the VITROS cTnI ES assay (Ortho-Clinical Diagnostics, Rochester, NY) or the ST AIA-PACK 2nd generation cTnI assay (Tosoh Bioscience, Tokyo, Japan). The study was approved by the New York University School of Medicine Institutional Review Board with a waiver of informed consent.
Type 2 MI was defined according to the Universal Definition of MI, with a rise and fall (or a fall alone, when the first measured troponin was also the peak value) of serum troponin above the 99% upper reference limit and clinical evidence of ischemia with at least one of the following: (a) symptoms of ischemia; (b) ECG changes with new significant ST-segment–T wave changes, new left bundle branch block (LBBB), or development of pathological Q waves in the ECG; or (c) imaging evidence of a new regional wall motion abnormality.1,2 Cases with a rise and/or fall in serum troponin but without clinical evidence of ischemia were classified as myonecrosis without definite MI, likely due to supply-demand imbalance.
Patient characteristics were ascertained from hospital administrative and laboratory datasets and retrospective review of the medical record. Conditions associated with and possibly provoking type 2 MI and myonecrosis without definite MI were recorded. Anemia was defined as hemoglobin ≤8 g/dL, GI bleeding, or red blood cell transfusion prior to or within 24 hours following the peak serum troponin. Hypotension was defined as a mean arterial pressure (MAP) <65 mmHg. Severe hypertension was defined as a systolic blood pressure >180mmHg or a diastolic blood pressure >110 mmHg. Respiratory failure was defined as the need for high flow oxygen by facemask, non-invasive positive pressure ventilation, or endotracheal intubation and mechanical ventilation. Sepsis was defined as illness meeting systemic inflammatory response syndrome criteria with an infectious source. Tachycardia and bradycardia were recorded as a provoking condition when the dysrhythmia was suspected as an etiology of myocardial ischemia per the treating physician; threshold heart rates for tachycardia or bradycardia were not specified. All ECGs obtained within 48 hours of the peak troponin were retrospectively reviewed for dynamic changes consistent with ischemia. Any cardiovascular testing (echocardiography, stress testing, coronary angiography, coronary CT or cardiac MRI) or ischemic evaluation (stress testing or coronary angiography only) performed during hospital admission or 180 days following hospital discharge were recorded. Medication use was defined from hospital discharge regimens. Subgroup analyses were performed by age, sex, and quartile of peak serum troponin.
Normally distributed continuous data were displayed as means ± standard deviation (SD) and were compared between type 2 MI and myonecrosis without definite MI groups using the two-sided independent sample t test. Skewed continuous data were presented as median [interquartile range] and compared between groups using the Mann-Whitney test. Categorical variables were displayed as n (proportions) and compared by Chi-square or Fisher exact tests. Statistical analyses were performed using SPSS 20 (IBM SPSS Statistics, Armonk, NY). Statistical significance was defined using a two-sided alpha level of 0.05 for all tests.
Results
Among 34,333 admissions to New York University Langone Medical Center in 2013, 3,053 (8.9%) had ≥1 abnormal troponin I value. Of these, 614 admissions (20.1%) had a diagnosis of secondary myocardial ischemia (ICD-9 411.89) and 75 (2.5%) had a secondary diagnosis of non-ST-elevation MI (ICD-9 410.71). Four hundred and seventy five cases (15.6%) met all study inclusion and exclusion criteria (Supplemental Figure).
Baseline demographic and clinical characteristics of patients with type 2 MI or myonecrosis without definite MI are shown in Table 1. Overall, the mean age of patients was 76.2 ± 12.7 years, 53% were male, and 75% were white. Eighty one percent of all patients were ≥65 years old and 92% had at least one baseline cardiovascular risk factor. ECGs and documentation of symptoms were reviewed for all patients in the cohort. Among patients included for analysis, 255 patients (54%) met Universal Definition criteria for type 2 MI; the remaining 220 patients (46%) were classified as myonecrosis without definite MI. In comparison to patients with myonecrosis, patients with type 2 MI were more likely to have a history of hypertension, diabetes, and a history of percutaneous or surgical coronary revascularization.
Table 1.
Baseline characteristics of patients with type 2 myocardial infarction or necrosis.
| Total Cohort (n=475) | Type 2 MI (n=255) | Myonecrosis without definite MI (n=220) | p-value | |
|---|---|---|---|---|
| Age (years), mean ± SD | 76.2 ± 12.7 | 76.0 ± 13.2 | 76.5 ± 12.1 | 0.70 |
| Age ≥65 | 387 (81%) | 208 (82%) | 178 (81%) | 0.85 |
| Male Sex, n (%) | 254 (53%) | 132 (52%) | 122 (56%) | 0.42 |
| Race | ||||
| White | 354 (75%) | 189 (74%) | 165 (75%) | 0.74 |
| Black | 43 (9%) | 26 (10%) | 17 (8%) | |
| Asian | 31 (7%) | 17 (7%) | 14 (6%) | |
| Other | 47 (10%) | 23 (9%) | 24 (10%) | |
| Hispanic Ethnicity | 39 (8%) | 20 (8%) | 19 (9%) | 0.73 |
| BMI (kg/m2), mean ± SD | 25.7 ± 6.2 | 25.6 ± 6.1 | 25.9 ± 6.4 | 0.62 |
| Hypertension | 379 (80%) | 213 (84%) | 166 (76%) | 0.03 |
| Hyperlipidemia | 235 (49%) | 126 (49%) | 109 (50%) | 0.94 |
| Diabetes Mellitus | 166 (35%) | 101 (40%) | 65 (30%) | 0.02 |
| Kidney Disease (eGFR <60)* | 198 (42%) | 108 (46%) | 75 (38%) | 0.09 |
| ESRD | 54 (11%) | 32 (13%) | 22 (10%) | 0.38 |
| Atrial Fibrillation | 176 (37%) | 98 (38%) | 78 (36%) | 0.52 |
| Coronary Artery Disease | 215 (45%) | 128 (50%) | 87 (40%) | 0.02 |
| Prior Myocardial Infarction | 127 (27%) | 76 (30%) | 51 (23%) | 0.1 |
| Prior Revascularization | 155 (32%) | 98 (38%) | 57 (26%) | 0.004 |
| Prior PCI | 99 (21%) | 61 (24%) | 38 (17%) | 0.08 |
| Prior CABG | 85 (18%) | 53 (21%) | 32 (15%) | 0.08 |
| History of Heart Failure | 97 (20%) | 54 (21%) | 43 (20%) | 0.66 |
| History of Malignancy | 134 (28%) | 57 (22%) | 76 (35%) | 0.003 |
BMI: body mass index; CABG: coronary artery bypass graft; eGFR: estimated glomerular filtration rate; ESRD: end stage renal disease; MI: myocardial infarction;; PCI: percutaneous coronary intervention
Among 255 patients with type 2 MI, 68 (27%) patients experienced chest pain, 189 (74%) had dynamic ECG changes concerning for ischemia, and 31 (16%) had new wall motion abnormalities by echocardiography. Proportion of type 2 MI diagnosis and the clinical diagnostic criteria that fulfills the definition of type 2 MI are shown by provoking condition in Figure 1. Admission characteristics and possible provoking conditions in patients with type 2 MI or myonecrosis without definite MI are shown in Table 2. In 182 patients with type 2 MI or myonecrosis attributed to a non-cardiac procedure, orthopedic (29%), vascular (20%), and general surgery (20%) were the most common procedure types. Among 151 patients with bleeding and/or anemia, a median hemoglobin decrease of 2.6 g/dL (IQR 1.8 – 4.2) was observed prior to or within 24 hours following the peak serum troponin. In these patients the mean hemoglobin nadir was 7.5±1.3 g/dL. Eighty-seven patients (57%) received red blood cell transfusion prior to or within 24 hours of the peak troponin. No significant differences in proportions of provoking conditions were observed between patients with type 2 MI and myonecrosis without definite MI.
Figure 1.
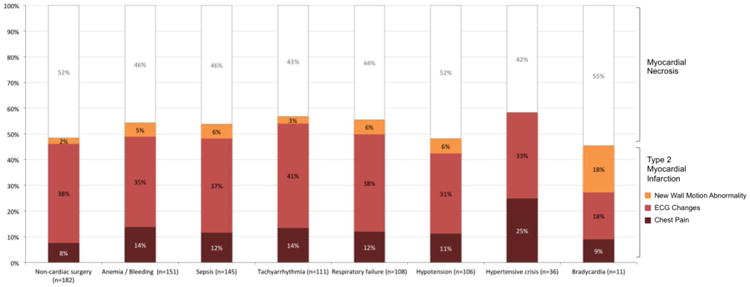
Proportion of Type 2 MI versus myonecrosis without definite MI by provoking condition.
Table 2.
Admission characteristics, associated provoking conditions, and cardiovascular testing in patients with type 2 myocardial infarction or necrosis.
| Total Cohort (n=475) | Type 2 MI (n=255) | Myonecrosis without definite MI (n=220) | p-value | |
|---|---|---|---|---|
| Admission Type | ||||
| Elective | 112 (24%) | 51 (20%) | 61 (28%) | 0.048 |
| Emergency or Urgent | 363 (76%) | 204 (80%) | 159 (72%) | |
| Admission Service | ||||
| Medical | 283 (60%) | 167 (65%) | 116 (53%) | 0.005 |
| Surgical | 192 (40%) | 88 (34%) | 104 (47%) | |
| Provoking Conditions | - | |||
| Non-cardiac surgery | 182 (38%) | 88 (35%) | 94 (43%) | 0.07 |
| Anemia / Bleeding | 151 (32%) | 82 (32%) | 69 (31%) | 0.85 |
| Sepsis | 145 (31%) | 78 (31%) | 67 (31%) | 0.98 |
| Tachyarrhythmia | 111 (23%) | 63 (25%) | 48 (22%) | 0.46 |
| Respiratory failure | 108 (23%) | 60 (24%) | 48 (22%) | 0.66 |
| Hypotension | 106 (22%) | 51 (20%) | 55 (25%) | 0.19 |
| Severe hypertension | 36 (8%) | 21 (8%) | 15 (7%) | 0.56 |
| Bradycardia | 11 (2%) | 5 (2%) | 6 (3%) | 0.58 |
| Other* | 32 (7%) | 20 (8%) | 12 (5%) | 0.30 |
| Multiple Provoking Conditions | 255 (54%) | 129 (51%) | 126 (57%) | 0.15 |
| Peak Troponin (ng/mL) median, IQR | 0.19, 0.09–0.81 | 0.22, 0.09–1.32 | 0.16, 0.09 – 0.50 | 0.003 |
| Transthoracic Echocardiography | 340 (72%) | 189 (74%) | 151 (69%) | 0.19 |
| LV Ejection Fraction, mean ± SD (%) | 58 ± 15% | 56±14 | 61±15 | 0.002 |
| LV Ejection Fraction >55% | 211 (62%) | 105 (56%) | 106 (70%) | 0.008 |
| Left Ventricular Hypertrophy | 118 (32%) | 63 (33%) | 55 (36%) | 0.55 |
| Regional Wall Motion Abnormalities | 135 (40%) | 90 (48%) | 45 (30%) | 0.0008 |
| Stress Testing | 39 (8%) | 25 (10%) | 14 (6%) | 0.17 |
| Abnormal MPI | 26 (67%) | 19 (76%) | 7 (50%) | 0.10 |
| Reversible Perfusion Defect | 18 (46%) | 12 (48%) | 6 (43%) | 0.76 |
| Coronary Angiography | 25 (5%) | 20 (8%) | 5 (2%) | 0.007 |
| No Significant CAD | 2 (8%) | 1 (5%) | 1 (20%) | |
| 1-Vessel CAD | 8 (32%) | 5 (25%) | 3 (60%) | 0.11 |
| Multi-vessel CAD | 15 (60%) | 14 (70%) | 1 (20%) | |
| Percutaneous Coronary Intervention | 9 (36%) | 7 (35%) | 2 (40%) | 0.99 |
CAD: coronary artery disease; LV: left ventricle; MI: myocardial infarction; MPI: myocardial perfusion imaging
Other conditions included: cerebrovascular accident / transient ischemic attack (n=5), metabolic derangements (n=7), syncope (n=5), hemodialysis (n=3), aortic stenosis (n=3), hip fracture without surgery (n=2), coronary vasospasm (n=1), malignancy with brain metastases (n=1), pancreatitis (n=1), palpitations and presumed tachyarrhythmia (n=1), urinary retention and altered mental status (n=1), nausea/vomiting (n=1), hepatic transcatheter arterial chemoembolization (n=1).
Cardiovascular testing was conducted in 352 (74%) patients and an ischemic evaluation was performed in 60 (13%) patients. Proportion of cardiovascular tests performed and the associated results are shown in Table 2. Of the 340 transthoracic echocardiograms performed, 316 (93%) were performed during the index hospitalization. Only coronary angiography was performed more frequently in patients with type 2 MI versus those with myonecrosis without definite MI (9% vs. 2%, p=0.001).
Medical management among 424 patients (89%) surviving to hospital discharge without referral to hospice care is shown in Table 3. Patients with type 2 MI were significantly more likely to receive any antiplatelet therapy at discharge than those with myonecrosis (68% vs. 57%, p=0.02). Only 183 patients (43%) were discharged on a combination of aspirin and statin therapy, and 56 patients (13%) received prescriptions for aspirin, statin, beta-blocker, and ACE inhibitor therapy at discharge. Rates of antiplatelet or anticoagulant prescribing were similar for patients with anemia (71%, p=0.57) and non-cardiac surgery (72%, p=0.75) in comparison to entire cohort (74%).
Table 3.
Medical management among patients with type 2 MI or myonecrosis who survived to hospital discharge without referral to hospice.
| Total Cohort † (n=424) | Type 2 MI † (n=221) | Myonecrosis without definite MI † (n=203) | p-value | |
|---|---|---|---|---|
| Medical Management at Discharge | ||||
| Aspirin | 253 (60%) | 143 (65%) | 110 (54%) | 0.03 |
| Clopidogrel | 82 (19%) | 51 (23%) | 31 (15%) | 0.04 |
| Anticoagulation | 98 (23%) | 47 (21%) | 52 (26%) | 0.29 |
| Any Antiplatelet/Anticoagulant | 312 (74%) | 169 (76%) | 143 (70%) | 0.16 |
| Statin | 256 (60%) | 127 (57%) | 129 (64%) | 0.20 |
| Beta Blocker | 261 (62%) | 142 (64%) | 119 (59%) | 0.23 |
| ACEi or ARB | 138 (32%) | 68 (31%) | 70 (34%) | 0.41 |
| Aspirin + Statin | 183 (43%) | 98 (44%) | 85 (42%) | 0.61 |
| Aspirin + Statin + Beta Blocker | 127 (30%) | 70 (32%) | 57 (28%) | 0.42 |
| Aspirin + Statin + Beta Blocker + ACEi or ARB | 55 (13%) | 29 (13%) | 26 (13%) | 0.92 |
Excludes subjects who died in-hospital or were discharged with a referral to hospice.
ACEi: angiotensin-converting-enzyme inhibitor; ARB: angiotensin receptor blocker; MI: myocardial mnfarction
In hospital outcomes of patients with type 2 MI or myonecrosis without definite MI are shown in Table 4. Overall, in-hospital mortality was 5%, with no significant differences between patients with type 2 MI versus those with myonecrosis (6% vs. 5%, p=0.41). Among the 26 in-hospital deaths, 7 (27%) were due to cardiovascular causes (5 patients with sudden cardiac death due to ventricular arrhythmia and 2 with progressive cardiogenic shock), with no difference between the two groups (31% vs. 20%, p=0.67). Only the composite outcome of all-cause in-hospital death or discharge to hospice was significantly higher in patients with type 2 MI versus those with myonecrosis (13% vs. 8%, p=0.049). Of 146 patients (31%) admitted to an intensive care unit during the hospital stay, in-hospital mortality was 11%.
Table 4. In-Hospital outcomes of subjects with type 2 MI or Myonecrosis.
| Total Cohort (n=475) | Type 2 MI (n=255) | Myonecrosis without definite MI (n=220) | p-value | |
|---|---|---|---|---|
| Outcomes | ||||
| In-Hospital Death | 26 (6%) | 16 (6%) | 10 (5%) | 0.41 |
| Discharge to Hospice | 25 (5%) | 18 (7%) | 7 (3%) | 0.06 |
| Hospice or Death | 51 (11%) | 34 (13%) | 17 (8%) | 0.049 |
| ICU Admission | 146 (31%) | 85 (33%) | 61 (28%) | 0.19 |
| Length of Stay (days), median, IQR | 6 (IQR 3 - 11) | 6 (IQR 4 - 12) | 6 (IQR 3 - 11) | 0.59 |
ICU: Intensive Care Unit; MI: myocardial infarction;
Among 2,122 admissions with an abnormal serum troponin but without an ICD-9 diagnosis code for myocardial ischemia or MI who were not included in the final analysis, the proportion of patients who died in-hospital (5.9% vs. 5.5%, p=0.78) or died or were discharged to hospice (9.5% vs. 10.7%, p=0.45) was similar to the 475 patients included in the final analysis.
Patients age ≤65 years with type 2 MI or myonecrosis (n=89) were less likely to have a prior diagnosis of hyperlipidemia (37% vs. 52%, p=0.01), diabetes (26% vs. 37%, p=0.05), or cardiovascular disease, including atrial fibrillation (16% vs. 42%, p<0.001), heart failure (9% vs. 23%, p=0.003), and CAD (28% vs. 49%, p<0.001), and more likely to have more likely to have end stage renal disease (20% vs. 9%, p=0.004) or present with severe hypertension (13% vs. 6%, p=0.02) in comparison to older individuals. There were no differences in rates of cardiovascular testing by age. In comparison to older patients, those ≤65 years were significantly less likely to receive both aspirin and statin therapy at discharge (29% vs. 47%, p=0.004). In-hospital mortality was not statistically different by age ≤65 or >65 (2% vs. 6%, p=0.14).
Women with type 2 MI or myonecrosis without definite MI (n=222) were less likely to have a history of hyperlipidemia (41% vs. 57%, p<0.001) or CAD (37% vs. 53%, p<0.001) than men. Cardiovascular testing did not differ by sex. Women were significantly less likely than men to be discharged on aspirin (53% vs. 66%, p=0.01), statin (54% vs. 66%, p=0.01), or the combination of aspirin and statin (35% vs. 51%, p=0.001). There was no significant difference in in-hospital mortality by sex.
Patients with the highest quartile of peak troponin elevation (≥0.81 ng/mL, n=120) were more likely to have a history of CAD (60% vs. 40%, p<0.001) in comparison to individuals with lower troponin measurements. Hypotension (33% vs. 18%, p<0.001) and multiple provoking conditions (62% vs. 51%, p=0.04) occurred more frequently in this group. Patients who developed a peak troponin in the top quartile were more likely to receive aspirin (78% vs. 54%, p<0.001) and beta-blocker (74% vs. 58%, p=0.005) at discharge, but not statin (64% vs. 60%, p=0.47) or ACE inhibitor/ARB (39% vs. 30%, p=0.10). Patients with peak serum troponin in the top quartile had significantly higher in-hospital mortality (9% vs. 4%, p=0.04) and composite of death or discharge to hospice (18% vs. 9%, p=0.006) than patients with lower troponin values.
Discussion
In this retrospective, observational cohort study of patients hospitalized with type 2 MI or myonecrosis without definite MI, only a little more than half of patients developed clinical symptoms of ischemia, ECG changes, or new wall motion abnormalities that fulfilled Universal Definition of MI criteria. Although provoking conditions were similar between the two groups, patients meeting criteria for MI had a greater burden of cardiovascular risk factors, CAD, and prior revascularization, and higher peak serum troponin levels than patients with myonecrosis alone. Patients with type 2 MI were also managed more aggressively, with a greater proportion undergoing invasive coronary angiography and antiplatelet treatment than those with myonecrosis without definite MI. Despite these differences in baseline characteristics and management, there were no significant differences in short-term outcomes between patients with type 2 MI and myonecrosis without definite MI, suggesting that troponin signifies increased risk in both groups.9 Consequently, a thorough investigation to determine the presence of CAD and the etiology of the troponin elevation is warranted.
The observed similarity in mortality between patients with myonecrosis attributed to supply-demand mismatch who did or did not meet criteria for MI raises questions about the requirement for symptoms, ECG changes or new wall motion abnormalities in the Universal Definition for this particular MI category. The conditions associated with supply-demand mismatch may limit the ascertainment of clinical manifestations of MI, particularly with regard to communication of symptoms or the documentation of ECG changes. Patients with type 2 MI are less likely to develop chest pain than those with type 1 MI, and other ischemic equivalent symptoms such as dyspnea may be attributed to alternate diagnoses or may have multifactorial etiologies.4 Although clinically silent ST-segment depressions identified by continuous rhythm monitoring are frequently observed during periods of mismatch in myocardial oxygen supply and demand, such changes are rarely identified in standard clinical practice.10 Similarly, echocardiography may not be routinely performed, and the opportunity to detect new wall motion abnormalities may be missed. A diagnosis of type 2 MI according to the Universal Definition necessitates a higher index of suspicion and may require serial ECGs and/or additional cardiac imaging. Thus, revised criteria for the Universal Definition of type 2 MI may be warranted to more accurately convey cardiovascular risks associated with ischemia from myocardial oxygen supply-demand mismatch.
Troponin elevation due to supply-demand mismatch occurred most commonly in the setting of non-cardiac surgery, anemia and/or bleeding, and sepsis; myocardial injury resulting from these clinical scenarios have been well described.11-18 Outcomes were poor overall, consistent with published reports of substantially higher mortality in patients with type 2 MI in comparison to other MI subtypes.6,19 Prior studies report myocardial necrosis occurs in 43% in patients hospitalized with critical illness and is associated with increased hospital length of stay, multi-organ failure, and a 2.5-fold increase in short-term mortality.15,20-25 However, it remains uncertain whether type 2 MI and/or myocardial necrosis contribute to poor outcomes or are instead simply markers of the severity of the underlying illness. It is also uncertain whether any treatment for type 2 MI or myonecrosis may mitigate the risk of cardiovascular mortality.
Patients in this study tended to be older adults with a high burden of cardiovascular risk factors, consistent with similar findings in prior reports.5,9,19 Rates of cardiovascular testing and outpatient antiplatelet and statin prescription were surprisingly low at hospital discharge, suggesting physician uncertainty about the role of secondary risk stratification and prevention.4,6 Perceived contraindications to guideline directed therapy did not appear to affect the low rates of antiplatelet prescribing, as there were no significant differences in discharge medical therapy by the clinical condition associated with Type 2 MI. As clinical practice guidelines do not exist for the management of myocardial necrosis or Type 2 MI, the decision to perform cardiovascular testing or prescribe antiplatelet therapy, statins, and other cardiovascular medications is often made ad-hoc. Once effective treatments have been determined, quality initiatives to ensure proper long-term cardiovascular risk reduction in these patients may be warranted.
There are a number of limitations to this observational study. First, patients with an abnormal serum troponin laboratory value and without an ICD-9 diagnosis code for secondary myocardial ischemia or a secondary diagnosis of non-ST-elevation MI were not included in this analysis. However, the proportion of patients who died in-hospital or were discharged to hospice was similar between those patients included in the final analysis and patients with troponin elevation in the absence of an ICD-9 diagnosis consistent with type 2 MI, who were excluded from analysis. Patients with a diagnosis of Type 1 MI were excluded, given the difficulty establishing a subsequent diagnosis of Type 2 MI within that same admission. Second, data were identified retrospectively from hospital administrative and laboratory datasets, ICD9 coding, and medical record review. Operative or procedural details, including selection of anesthesia, intra-operative hemodynamics, and estimated blood loss were not obtained. Similarly, chest pain, ECG changes, and other clinical criteria necessary for adjudication of MI were assessed retrospectively, and, as such, ascertainment bias may affect study findings. Third, outpatient cardiovascular testing performed at hospitals, clinics, or practices unaffiliated with our institution were not captured. Fourth, classification of in-hospital deaths as cardiovascular or non-cardiovascular in the setting of type 2 MI can be challenging, and misclassification is possible. Finally, long-term mortality data were not available for this cohort. Nonetheless, this study provides in-depth analysis of a large, real-world cohort of patients developing type 2 MI and myonecrosis.
In conclusion, Type 2 MI and myocardial necrosis due to supply-demand mismatch occurs most frequently in the setting of non-cardiac surgery, sepsis, and/or anemia in older patients with established cardiovascular risk factors. Rates of cardiovascular testing and antiplatelet and statin prescription were low at hospital discharge, reflecting physician uncertainty about the role of secondary risk stratification and prevention in this condition. Patients who met the Universal Definition of type 2 MI with symptoms or ECG changes had similar in-hospital mortality compared to those with myocardial necrosis without definite MI, suggesting that elevations in troponin identify patients at increased risk, and that both groups may warrant further evaluation to determine the proximate cause of troponin elevation. Further research into mechanisms and outcomes is needed to inform management of patients with type 2 MI.
Supplementary Material
Acknowledgments
Binita Shah was partially funded by grants from the NIH (UL1 TR000038) and New York State (Empire Clinical Research Investigator Program Fellowship).
Funding Source: Investigator initiated.
Footnotes
Disclosures: The authors report no relationships that could be construed as a conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thygesen K, Mair J, Katus H, et al. Recommendations for the use of cardiac troponin measurement in acute cardiac care. Eur Heart J. 2010 Sep;31(18):2197–2204. doi: 10.1093/eurheartj/ehq251. [DOI] [PubMed] [Google Scholar]
- 2.Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012 Oct 16;60(16):1581–1598. doi: 10.1016/j.jacc.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Morrow DA, Wiviott SD, White HD, et al. Effect of the novel thienopyridine prasugrel compared with clopidogrel on spontaneous and procedural myocardial infarction in the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel-Thrombolysis in Myocardial Infarction 38: an application of the classification system from the universal definition of myocardial infarction. Circulation. 2009 Jun 2;119(21):2758–2764. doi: 10.1161/CIRCULATIONAHA.108.833665. [DOI] [PubMed] [Google Scholar]
- 4.Stein GY, Herscovici G, Korenfeld R, et al. Type-II myocardial infarction--patient characteristics, management and outcomes. PloS one. 2014;9(1):e84285. doi: 10.1371/journal.pone.0084285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Javed U, Aftab W, Ambrose JA, et al. Frequency of elevated troponin I and diagnosis of acute myocardial infarction. Am J Cardiol. 2009 Jul 1;104(1):9–13. doi: 10.1016/j.amjcard.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Saaby L, Poulsen TS, Diederichsen AC, et al. Mortality rate in type 2 myocardial infarction: observations from an unselected hospital cohort. Am J Med. 2014 Apr;127(4):295–302. doi: 10.1016/j.amjmed.2013.12.020. [DOI] [PubMed] [Google Scholar]
- 7.Smilowitz NR, Naoulou B, Sedlis SP. Diagnosis and Management of Type II Myocardial Infarction: Increased Demand for a Limited Supply of Evidence. Current atherosclerosis reports. 2015 Feb;17(2):478. doi: 10.1007/s11883-014-0478-1. [DOI] [PubMed] [Google Scholar]
- 8.Baron T, Hambraeus K, Sundstrom J, et al. Type 2 myocardial infarction in clinical practice. Heart. 2015 Jan;101(2):101–106. doi: 10.1136/heartjnl-2014-306093. [DOI] [PubMed] [Google Scholar]
- 9.Shah AS, McAllister DA, Mills R, et al. Sensitive Troponin Assay and the Classification of Myocardial Infarction. Am J Med. 2015 May;128(5):493–501 e493. doi: 10.1016/j.amjmed.2014.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Landesberg G. Monitoring for myocardial ischemia. Best practice & research Clinical anaesthesiology. 2005 Mar;19(1):77–95. [PubMed] [Google Scholar]
- 11.Oberweis BS, Nukala S, Rosenberg A, et al. Thrombotic and bleeding complications after orthopedic surgery. Am Heart J. 2013 Mar;165(3):427–433 e421. doi: 10.1016/j.ahj.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Landesberg G, Beattie WS, Mosseri M, Jaffe AS, Alpert JS. Perioperative myocardial infarction. Circulation. 2009 Jun 9;119(22):2936–2944. doi: 10.1161/CIRCULATIONAHA.108.828228. [DOI] [PubMed] [Google Scholar]
- 13.Vascular Events In Noncardiac Surgery Patients Cohort Evaluation Study I. Devereaux PJ, Chan MT, et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2012 Jun 6;307(21):2295–2304. doi: 10.1001/jama.2012.5502. [DOI] [PubMed] [Google Scholar]
- 14.van Waes JA, Nathoe HM, de Graaff JC, et al. Myocardial injury after noncardiac surgery and its association with short-term mortality. Circulation. 2013 Jun 11;127(23):2264–2271. doi: 10.1161/CIRCULATIONAHA.113.002128. [DOI] [PubMed] [Google Scholar]
- 15.Lim W, Qushmaq I, Devereaux PJ, et al. Elevated cardiac troponin measurements in critically ill patients. Arch Intern Med. 2006 Dec 11-25;166(22):2446–2454. doi: 10.1001/archinte.166.22.2446. [DOI] [PubMed] [Google Scholar]
- 16.Bessiere F, Khenifer S, Dubourg J, Durieu I, Lega JC. Prognostic value of troponins in sepsis: a meta-analysis. Intensive care medicine. 2013 Jul;39(7):1181–1189. doi: 10.1007/s00134-013-2902-3. [DOI] [PubMed] [Google Scholar]
- 17.Iser DM, Thompson AJ, Sia KK, Yeomans ND, Chen RY. Prospective study of cardiac troponin I release in patients with upper gastrointestinal bleeding. Journal of gastroenterology and hepatology. 2008 Jun;23(6):938–942. doi: 10.1111/j.1440-1746.2007.04940.x. [DOI] [PubMed] [Google Scholar]
- 18.Emenike E, Srivastava S, Amoateng-Adjepong Y, al-Kharrat T, Zarich S, Manthous CA. Myocardial infarction complicating gastrointestinal hemorrhage. Mayo Clinic proceedings Mayo Clinic. 1999 Mar;74(3):235–241. doi: 10.4065/74.3.235. [DOI] [PubMed] [Google Scholar]
- 19.Saaby L, Poulsen TS, Hosbond S, et al. Classification of myocardial infarction: frequency and features of type 2 myocardial infarction. Am J Med. 2013 Sep;126(9):789–797. doi: 10.1016/j.amjmed.2013.02.029. [DOI] [PubMed] [Google Scholar]
- 20.Wu TT, Yuan A, Chen CY, et al. Cardiac troponin I levels are a risk factor for mortality and multiple organ failure in noncardiac critically ill patients and have an additive effect to the APACHE II score in outcome prediction. Shock. 2004 Aug;22(2):95–101. doi: 10.1097/01.shk.0000132484.97424.32. [DOI] [PubMed] [Google Scholar]
- 21.Quenot JP, Le Teuff G, Quantin C, et al. Myocardial injury in critically ill patients: relation to increased cardiac troponin I and hospital mortality. Chest. 2005 Oct;128(4):2758–2764. doi: 10.1378/chest.128.4.2758. [DOI] [PubMed] [Google Scholar]
- 22.Babuin L, Vasile VC, Rio Perez JA, et al. Elevated cardiac troponin is an independent risk factor for short- and long-term mortality in medical intensive care unit patients. Critical care medicine. 2008 Mar;36(3):759–765. doi: 10.1097/CCM.0B013E318164E2E4. [DOI] [PubMed] [Google Scholar]
- 23.Landesberg G, Vesselov Y, Einav S, Goodman S, Sprung CL, Weissman C. Myocardial ischemia, cardiac troponin, and long-term survival of high-cardiac risk critically ill intensive care unit patients. Critical care medicine. 2005 Jun;33(6):1281–1287. doi: 10.1097/01.ccm.0000166607.22550.87. [DOI] [PubMed] [Google Scholar]
- 24.Wright RS, Williams BA, Cramner H, et al. Elevations of cardiac troponin I are associated with increased short-term mortality in noncardiac critically ill emergency department patients. Am J Cardiol. 2002 Sep 15;90(6):634–636. doi: 10.1016/s0002-9149(02)02570-5. [DOI] [PubMed] [Google Scholar]
- 25.Vasile VC, Chai HS, Abdeldayem D, Afessa B, Jaffe AS. Elevated cardiac troponin T levels in critically ill patients with sepsis. Am J Med. 2013 Dec;126(12):1114–1121. doi: 10.1016/j.amjmed.2013.06.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


