Abstract
SCID, a syndrome characterized by the absence of T cells and adaptive immunity, can result from mutations in multiple genes that encode components of the immune system. Three such components are cytokine receptor chains or signaling molecules, five are needed for antigen receptor development, one is adenosine deaminase — a purine salvage pathway enzyme, and the last is a phosphatase, CD45. In this issue of the JCI, a report describes how complete deficiency of the CD3ε chain of the T cell antigen receptor/CD3 complex causes human SCID.
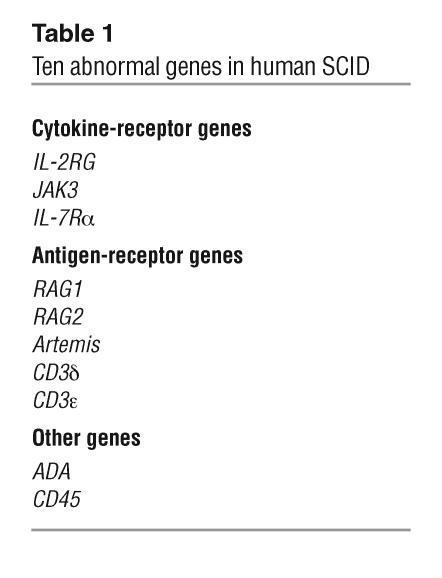
Human SCID was first reported by Glanzmann and Riniker in 1950 (1). Swiss infants with the condition were profoundly lymphopenic and died of infection before their first or second birthdays. In the ensuing years, differences were noted in inheritance patterns for SCID. This indicated that there was more than one cause for this fatal syndrome characterized by an absence of T cells and all adaptive immunity. In many families there was an X-linked recessive mode of inheritance while in others an autosomal recessive mode of inheritance was observed. The first discovered molecular cause of human SCID, adenosine deaminase deficiency, was reported in 1972 (2). However, it was not until 21 years later, in 1993, that a second fundamental cause of the condition was found, i.e., the molecular basis of X-linked human SCID (3, 4). Over the past 11 years, remarkable progress has been made in elucidating several other causes of this syndrome (5). Advances in molecular biology and the Human Genome Project as well as increased knowledge of various components of the immune system through studies of mutant mice and humans with genetically determined immunodeficiencies have all contributed to this understanding. It is now known that SCID can be caused in humans by mutations in at least 10 different genes (Table 1) (6–11), and the likelihood is that there are other causes yet to be discovered. The gene products of 3 of these mutated genes are components of cytokine receptors (the IL-2 receptor γ chain that is also shared with 5 other cytokine receptors [IL-4R, IL-7R, IL-9R, IL-15R, and IL-21R], JAK3, the primary signal transducer from the common γ chain, and the α chain of the IL-7 receptor); the products of 5 more genes (RAG1, RAG2, Artemis, CD3δ, and CD3ε) are necessary for antigen-receptor development; the product of one gene (adenosine deaminase) is necessary for detoxification of metabolic products of the purine salvage pathway that cause lymphocytes to apoptose; and the final gene encodes CD45, a phosphatase that serves as a critical regulator of signaling thresholds in immune cells (Table 1) (12). The most common form of human SCID is the X-linked type, caused by mutations in IL-2RG, which accounts for 46% of cases at the author’s institution, the Duke University Medical Center (Figure 1). This is followed by adenosine deaminase deficiency in 16.1% of cases and IL-7Rα–chain deficiency in 10.3% of cases. The relative frequencies of these different molecular types of human SCID may vary in other geographic areas.
Table 1.
Ten abnormal genes in human SCID
Figure 1.
Relative frequencies of the various molecular defects found in 174 consecutive cases of human SCID evaluated at Duke University Medical Center over the past 3 decades. The most common type is X-linked SCID, due to mutations in the gene encoding the common γ chain for multiple cytokine receptors; the second most common cause is adenosine deaminase deficiency (ADA def.), and the third most common cause is IL-7Rα–chain deficiency. In 25 cases the molecular defect remains unknown (those in the groups labeled autosomal recessive and unknown). No cases of CD45 deficiency have been seen at this institution. Def., deficiency.
A newly discovered cause of human SCID
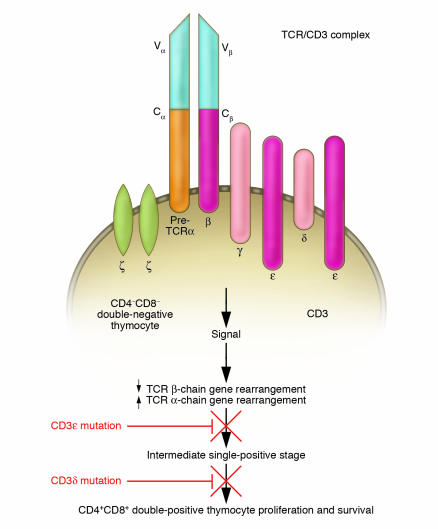
The tenth known cause of human SCID is reported in this issue of the JCI by de Saint Basile et al. (13). The authors identified mutations in the gene encoding the ε component of the T cell receptor/CD3 complex (Figure 2). Building on the discovery of Dadi et al. (11), that mutations in a gene encoding the δ chain of the CD3 complex cause human SCID, de Saint Basile and coworkers used segregation analysis of polymorphic markers for chromosome 11q23 – the location of the CD3 locus — to study 3 families with fetuses or infants who had SCID of unknown molecular type (13). All of the fetuses or infants were characterized by the T–B+NK+ lymphocyte phenotype, i.e., they had no T cells but did have phenotypically normal B cells and NK cells, the same phenotype observed in the SCID infants described by Dadi et al. (11). There was a homozygous haplotype segregation of polymorphic markers for 11q23 in all 3 families studied by de Saint Basile et al. However, mutations in the gene encoding the CD3δ chain were found in only 2 of the families. The investigators then searched for mutations in other components of the CD3 complex and found a homozygous mutation in the gene encoding CD3ε that created a premature stop codon near the start of the extracellular domain, resulting in the absence of CD3 expression in those individuals affected in the 3rd family. In 1993, members of this research group reported a child with low, but detectable, expression levels of CD3ε on circulating T cells and identified a splice site mutation on one allele that did not totally abrogate the normal intron 7 splicing (14). That child did not have impaired T cell development and had only a mild immunodeficiency (14). In the present report (13), the authors show that an absence of CD3ε completely blocks T cell development. In the CD3ε-knockout mouse, this block appears to occur at the pre–T cell receptor α, double-negative stage. Dadi et al. (11) had concluded, based on studies of thymic material from their patients, that an absence of CD3δ also causes a block in T cell development at the pre–T cell receptor α, double-negative stage of intrathymic development. In the present report, de Saint Basile et al. (13) confirm that an absence of CD3δ completely abrogates T cell development but conclude that the block is slightly later — at the intermediate single-positive stage, just before the double-positive (i.e., CD4+CD8+) stage. Thus both CD3δ and CD3ε appear to be essential for intrathymic development of T cells (Figure 2) whereas CD3γ deficiency does not (15). We have also found mutations in these two genes in our human SCID population (J.L. Roberts et al., unpublished results; Figure 1).
Figure 2.
Schematic of the T cell receptor/CD3 (TCR/CD3) complex on the surface of a normal CD4–CD8– double-negative thymocyte. All but one of the normal chains of the CD3 complex, including the β chain, are present, but instead of the CD3α chain, the pre–T cell receptor α gene is expressed at this stage. It is postulated, based on the CD3ε murine knockout studies, that the block in T cell development caused by mutations in CD3ε occurs at or just after this stage. In their study in this issue, de Saint Basile and colleagues (13) report histologic data which suggests that, in the case of mutations in the CD3δ gene, the block in T cell maturation occurs at the next stage, i.e., at the intermediate single-positive stage.
Importance
SCID is a pediatric emergency. If the diagnosis is made at birth or shortly thereafter, definitive therapy in the form of HLA-identical or haploidentical allogeneic bone marrow stem cell transplantation can result in a survival rate as high as 97%, regardless of the molecular type of SCID (5). However, if the diagnosis is made later, serious infections develop for which antibiotics are poorly effective or nonexistent, and the survival rate is significantly less. Allogeneic stem cell transplantation is not a perfect therapy, however, as many recipients experience incomplete B cell and/or NK cell immune reconstitution. The hope is that gene therapy could effect more complete immune reconstitution, and there has already been remarkable success in achieving this in a few patients (16–18). However, gene therapy cannot be performed unless the abnormal gene for a specific patient is known. Knowledge of the specific molecular defect is also essential for genetic counseling and prenatal diagnosis. Of the 174 SCID infants evaluated at the Duke University Medical Center over the past 3 decades, there are still 25 infants for whom the molecular basis of their disease is unknown (Figure 1). It is possible that other genes yet to be discovered can result in human SCID when mutated. Thus knowing all of the abnormal genes that result in human SCID is an important ongoing goal for all who work in this area.
Footnotes
See the related article beginning on page 1512.
Nonstandard abbreviations used: HNF, hepatocyte nuclear factor; IPF-1, insulin promoter factor-1; MIDD, maternally inherited diabetes and deafness; OXPHOS, oxidative phosphorylation; PGC, PPARγ coactivator; T2DM, type 2 diabetes mellitus; VO2max, total body aerobic capacity.
Conflict of interest: Alan R. Shuldiner serves as a consultant for Amgen, Pfizer, Inc., and Serono, Inc. He receives research support from Merck & Co., Inc.
References
- 1.Glanzmann E, Riniker P. Essentielle Lymphocytophtose. Ein neues Krankeitsbild aus der Sauglingspathologie. Ann. Paediat. 1950;174:1–5. [PubMed] [Google Scholar]
- 2.Giblett ER, Anderson JE, Cohen I. Adenosine deaminase deficiency in two patients with severely impaired cellular immunity. Lancet. 1972;2:1067–1070. doi: 10.1016/s0140-6736(72)92345-8. [DOI] [PubMed] [Google Scholar]
- 3.Noguchi M, et al. Interleukin-2 receptor gamma chain mutation results in X-linked severe combined immunodeficiency in humans. Cell. 1993;73:147–157. doi: 10.1016/0092-8674(93)90167-o. [DOI] [PubMed] [Google Scholar]
- 4.Puck JM, et al. The interleukin-2 receptor gamma chain maps to Xq13.1 and is mutated in X-linked severe combined immunodeficiency, SCIDX1. Hum. Mol. Genet. 1993;2:1099–1104. doi: 10.1093/hmg/2.8.1099. [DOI] [PubMed] [Google Scholar]
- 5.Buckley RH. Molecular defects in human severe combined immunodeficiency and approaches to immune reconstitution. Annu. Rev. Immunol. 2004;55:625–656. doi: 10.1146/annurev.immunol.22.012703.104614. [DOI] [PubMed] [Google Scholar]
- 6.Russell SM, et al. Mutation of Jak3 in a patient with SCID: Essential role of Jak3 in lymphoid development. Science. 1995;270:797–800. doi: 10.1126/science.270.5237.797. [DOI] [PubMed] [Google Scholar]
- 7.Puel A, Ziegler SF, Buckley RH, Leonard WJ. Defective IL7R expression in T(-)B(+)NK(+) severe combined immunodeficiency. Nat. Genet. 1998;20:394–397. doi: 10.1038/3877. [DOI] [PubMed] [Google Scholar]
- 8.Schwarz K, et al. RAG mutations in human B cell-negative SCID. Science. 1996;274:97–99. doi: 10.1126/science.274.5284.97. [DOI] [PubMed] [Google Scholar]
- 9.Moshous D, et al. Artemis, a novel DNA double-strand break repair/V(D)J recombination protein, is mutated in human severe combined immune deficiency. Cell. 2001;105:177–186. doi: 10.1016/s0092-8674(01)00309-9. [DOI] [PubMed] [Google Scholar]
- 10.Kung C, et al. Mutations in the tyrosine phosphatase CD45 gene in a child with severe combined immunodeficiency disease. Nat. Med. 2000;6:343–345. doi: 10.1038/73208. [DOI] [PubMed] [Google Scholar]
- 11.Dadi HK, Simon AJ, Roifman CM. Effect of CD3delta deficiency on maturation of alpha/beta and gamma/delta T-cell lineages in severe combined immunodeficiency. N. Engl. J. Med. 2003;349:1821–1828. doi: 10.1056/NEJMoa031178. [DOI] [PubMed] [Google Scholar]
- 12.Hermiston ML, Xu Z, Weiss A. CD45: a critical regulator of signaling thresholds in immune cells. Annu. Rev. Immunol. 2003;21:107–137. doi: 10.1146/annurev.immunol.21.120601.140946. [DOI] [PubMed] [Google Scholar]
- 13.de Saint Basile G, et al. Severe combined immunodeficiency caused by deficiency in either the δ or the ε subunit of CD3. J. Clin. Invest. 2004;114:1512–1517. doi:10.1172/JCI200422588. doi: 10.1172/JCI22588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soudais C, De Villartay JP, Le Deist F, Fischer A, Lisowska-Grospierre B. Independent mutations of the human CD3-epsilon gene resulting in a T cell receptor/CD3 complex immunodeficiency. Nat. Genet. 1993;3:77–81. doi: 10.1038/ng0193-77. [DOI] [PubMed] [Google Scholar]
- 15.Arnaiz-Villena A, et al. T lymphocyte signalling defects and immunodeficiency due to the lack of CD3 gamma. Immunodeficiency. 1993;4:121–129. [PubMed] [Google Scholar]
- 16.Cavazzana-Calvo M, et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288:669–672. doi: 10.1126/science.288.5466.669. [DOI] [PubMed] [Google Scholar]
- 17.Hacein-Bey-Abina S, et al. Sustained correction of X-linked severe combined immunodeficiency by ex vivo gene therapy. N. Engl. J. Med. 2002;346:1185–1193. doi: 10.1056/NEJMoa012616. [DOI] [PubMed] [Google Scholar]
- 18.Aiuti A, et al. Correction of ADA-SCID by stem cell gene therapy combined with nonmyeloablative conditioning. Science. 2002;296:2410–2413. doi: 10.1126/science.1070104. [DOI] [PubMed] [Google Scholar]