Abstract
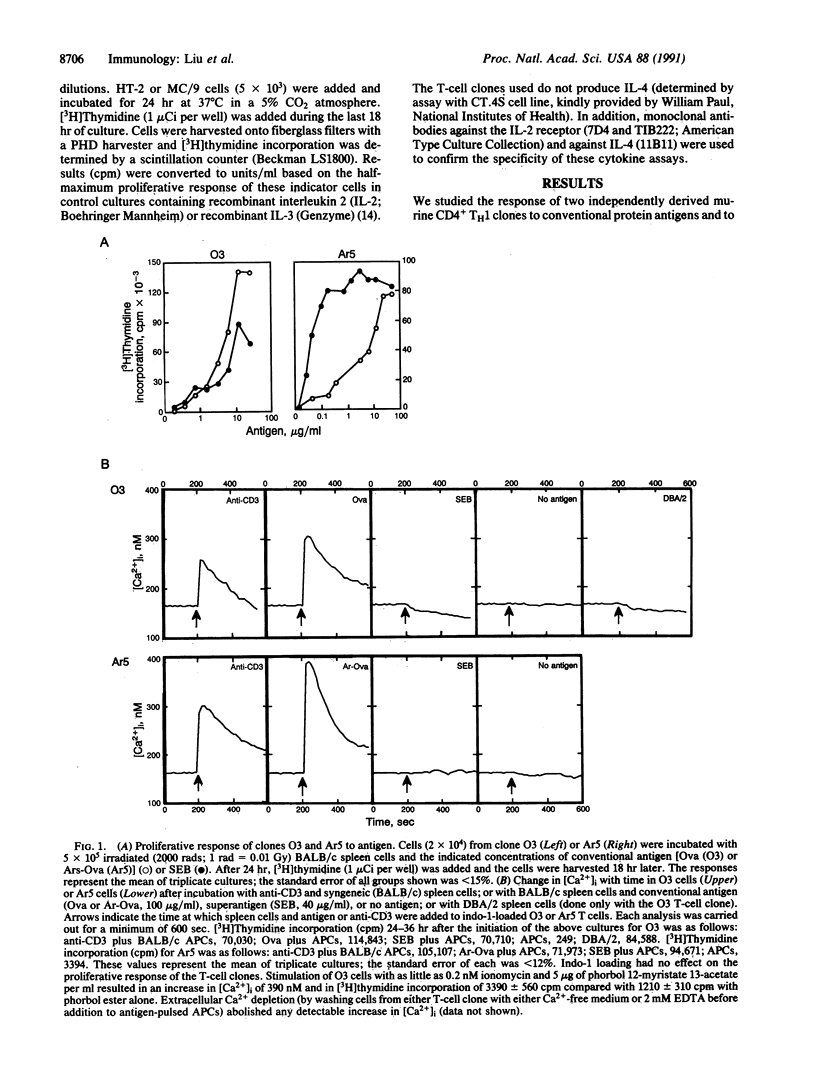
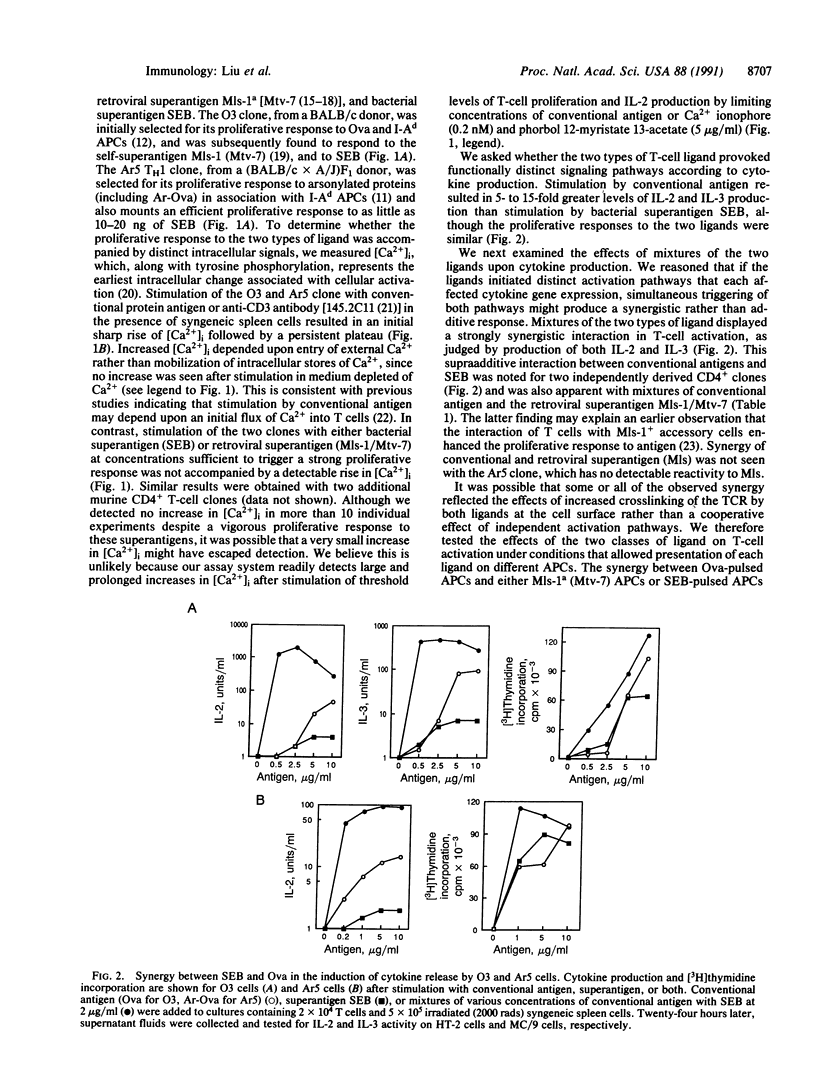
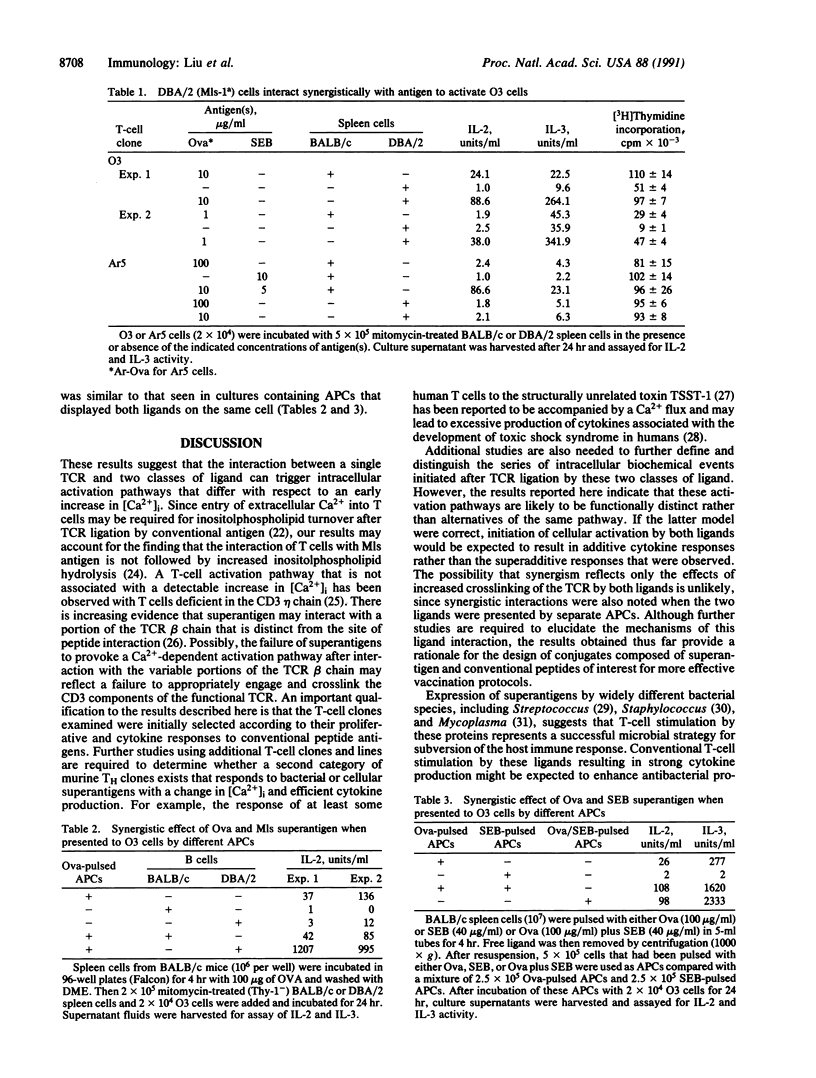
CD4+ T cells are equipped to detect two major classes of ligands. Infectious microbial agents, including bacteria and retroviruses, carry a class of proteins termed superantigens that are recognized by the T-cell receptor in association with class II products of the major histocompatibility complex. Proteins expressed by other cells and organisms are processed by macrophages into peptides that are presented to CD4+ T cells by class II molecules. We have examined CD4+ T-cell clones that proliferate vigorously in response both to conventional peptide antigens and to bacterial or retroviral superantigens. The response to peptide antigen is characterized by a rapid and sustained increase in the levels of intracellular free Ca2+ and a vigorous cytokine response. In contrast, the proliferative response of these clones to bacterial or retroviral superantigen is not accompanied by detectable increases in intracellular Ca2+ or by significant cytokine production. Further analysis of T-cell activation indicates that interaction of a single T-cell receptor with the two types of ligand may be coupled to functionally distinct signaling pathways that interact in a synergistic fashion to achieve T-cell activation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adkins B., Mueller C., Okada C. Y., Reichert R. A., Weissman I. L., Spangrude G. J. Early events in T-cell maturation. Annu Rev Immunol. 1987;5:325–365. doi: 10.1146/annurev.iy.05.040187.001545. [DOI] [PubMed] [Google Scholar]
- Ashwell J. D., Klusner R. D. Genetic and mutational analysis of the T-cell antigen receptor. Annu Rev Immunol. 1990;8:139–167. doi: 10.1146/annurev.iy.08.040190.001035. [DOI] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. The foreign antigen binding site and T cell recognition regions of class I histocompatibility antigens. Nature. 1987 Oct 8;329(6139):512–518. doi: 10.1038/329512a0. [DOI] [PubMed] [Google Scholar]
- Blomster-Hautamaa D. A., Kreiswirth B. N., Kornblum J. S., Novick R. P., Schlievert P. M. The nucleotide and partial amino acid sequence of toxic shock syndrome toxin-1. J Biol Chem. 1986 Nov 25;261(33):15783–15786. [PubMed] [Google Scholar]
- Bonvini E., DeBell K. E., Kolber M. A., Hoffman T., Hodes R. J., Taplits M. S. Hydrolysis of inositol phospholipids induced by stimulation of the T cell antigen receptor complex in antigen-specific, murine helper T cell clones. Requirement for exogenous calcium. J Immunol. 1989 Jul 15;143(2):587–595. [PubMed] [Google Scholar]
- Brown J. H., Jardetzky T., Saper M. A., Samraoui B., Bjorkman P. J., Wiley D. C. A hypothetical model of the foreign antigen binding site of class II histocompatibility molecules. Nature. 1988 Apr 28;332(6167):845–850. doi: 10.1038/332845a0. [DOI] [PubMed] [Google Scholar]
- Cantor H., Boyse E. A. Lymphocytes as models for the study of mammalian cellular differentiation. Immunol Rev. 1977 Jan;33:105–124. doi: 10.1111/j.1600-065x.1977.tb00364.x. [DOI] [PubMed] [Google Scholar]
- Chatila T., Wood N., Parsonnet J., Geha R. S. Toxic shock syndrome toxin-1 induces inositol phospholipid turnover, protein kinase C translocation, and calcium mobilization in human T cells. J Immunol. 1988 Feb 15;140(4):1250–1255. [PubMed] [Google Scholar]
- Choi Y. W., Herman A., DiGiusto D., Wade T., Marrack P., Kappler J. Residues of the variable region of the T-cell-receptor beta-chain that interact with S. aureus toxin superantigens. Nature. 1990 Aug 2;346(6283):471–473. doi: 10.1038/346471a0. [DOI] [PubMed] [Google Scholar]
- Chused T. M., Wilson H. A., Greenblatt D., Ishida Y., Edison L. J., Tsien R. Y., Finkelman F. D. Flow cytometric analysis of murine splenic B lymphocyte cytosolic free calcium response to anti-IgM and anti-IgD. Cytometry. 1987 Jul;8(4):396–404. doi: 10.1002/cyto.990080409. [DOI] [PubMed] [Google Scholar]
- Dellabona P., Peccoud J., Kappler J., Marrack P., Benoist C., Mathis D. Superantigens interact with MHC class II molecules outside of the antigen groove. Cell. 1990 Sep 21;62(6):1115–1121. doi: 10.1016/0092-8674(90)90388-u. [DOI] [PubMed] [Google Scholar]
- Dyson P. J., Knight A. M., Fairchild S., Simpson E., Tomonari K. Genes encoding ligands for deletion of V beta 11 T cells cosegregate with mammary tumour virus genomes. Nature. 1991 Feb 7;349(6309):531–532. doi: 10.1038/349531a0. [DOI] [PubMed] [Google Scholar]
- Frankel W. N., Rudy C., Coffin J. M., Huber B. T. Linkage of Mls genes to endogenous mammary tumour viruses of inbred mice. Nature. 1991 Feb 7;349(6309):526–528. doi: 10.1038/349526a0. [DOI] [PubMed] [Google Scholar]
- Friedman S., Sillcocks D., Cantor H. Alloreactivity of an OVA-specific T-cell clone. I. Stimulation by class II MHC and novel non-MHC B-cell determinants. Immunogenetics. 1987;26(4-5):193–203. doi: 10.1007/BF00346512. [DOI] [PubMed] [Google Scholar]
- Friedman S., Sillcocks D., Rao A., Faas S., Cantor H. A subset of Ly-1 inducer T cell clones activates B cell proliferation but directly inhibits subsequent IgG secretion. J Exp Med. 1985 Apr 1;161(4):785–804. doi: 10.1084/jem.161.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway C. A., Jr, Conrad P. J., Tite J., Jones B., Murphy D. B. Efficiency of antigen presentation differs in mice differing at the Mls locus. Nature. 1983 Nov 3;306(5938):80–82. doi: 10.1038/306080a0. [DOI] [PubMed] [Google Scholar]
- Janeway C. A., Jr, Yagi J., Conrad P. J., Katz M. E., Jones B., Vroegop S., Buxser S. T-cell responses to Mls and to bacterial proteins that mimic its behavior. Immunol Rev. 1989 Feb;107:61–88. doi: 10.1111/j.1600-065x.1989.tb00003.x. [DOI] [PubMed] [Google Scholar]
- Janeway C. Immune recognition. Mls: makes a little sense. Nature. 1991 Feb 7;349(6309):459–461. doi: 10.1038/349459a0. [DOI] [PubMed] [Google Scholar]
- Kotb M., Courtney H. S., Dale J. B., Beachey E. H. Cellular and biochemical responses of human T lymphocytes stimulated with streptococcal M proteins. J Immunol. 1989 Feb 1;142(3):966–970. [PubMed] [Google Scholar]
- Leo O., Foo M., Sachs D. H., Samelson L. E., Bluestone J. A. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1374–1378. doi: 10.1073/pnas.84.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrack P., Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990 May 11;248(4956):705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- Matthes M., Schrezenmeier H., Homfeld J., Fleischer S., Malissen B., Kirchner H., Fleischer B. Clonal analysis of human T cell activation by the Mycoplasma arthritidis mitogen (MAS). Eur J Immunol. 1988 Nov;18(11):1733–1737. doi: 10.1002/eji.1830181112. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Fong T. A. Specific assays for cytokine production by T cells. J Immunol Methods. 1989 Jan 17;116(2):151–158. doi: 10.1016/0022-1759(89)90198-1. [DOI] [PubMed] [Google Scholar]
- O'Rourke A. M., Mescher M. F., Webb S. R. Activation of polyphosphoinositide hydrolysis in T cells by H-2 alloantigen but not MLS determinants. Science. 1990 Jul 13;249(4965):171–174. doi: 10.1126/science.2164711. [DOI] [PubMed] [Google Scholar]
- Patarca R., Wei F. Y., Iregui M. V., Cantor H. Differential induction of interferon gamma gene expression after activation of CD4+ T cells by conventional antigen and Mls superantigen. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2736–2739. doi: 10.1073/pnas.88.7.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullen A. M., Wade T., Marrack P., Kappler J. W. Identification of the region of T cell receptor beta chain that interacts with the self-superantigen MIs-1a. Cell. 1990 Jun 29;61(7):1365–1374. doi: 10.1016/0092-8674(90)90700-o. [DOI] [PubMed] [Google Scholar]
- Sussman J. J., Merćep M., Saito T., Germain R. N., Bonvini E., Ashwell J. D. Dissociation of phosphoinositide hydrolysis and Ca2+ fluxes from the biological responses of a T-cell hybridoma. Nature. 1988 Aug 18;334(6183):625–628. doi: 10.1038/334625a0. [DOI] [PubMed] [Google Scholar]
- Swain S. L. T cell subsets and the recognition of MHC class. Immunol Rev. 1983;74:129–142. doi: 10.1111/j.1600-065x.1983.tb01087.x. [DOI] [PubMed] [Google Scholar]
- White J., Herman A., Pullen A. M., Kubo R., Kappler J. W., Marrack P. The V beta-specific superantigen staphylococcal enterotoxin B: stimulation of mature T cells and clonal deletion in neonatal mice. Cell. 1989 Jan 13;56(1):27–35. doi: 10.1016/0092-8674(89)90980-x. [DOI] [PubMed] [Google Scholar]
- Woodland D. L., Happ M. P., Gollob K. J., Palmer E. An endogenous retrovirus mediating deletion of alpha beta T cells? Nature. 1991 Feb 7;349(6309):529–530. doi: 10.1038/349529a0. [DOI] [PubMed] [Google Scholar]



