Abstract
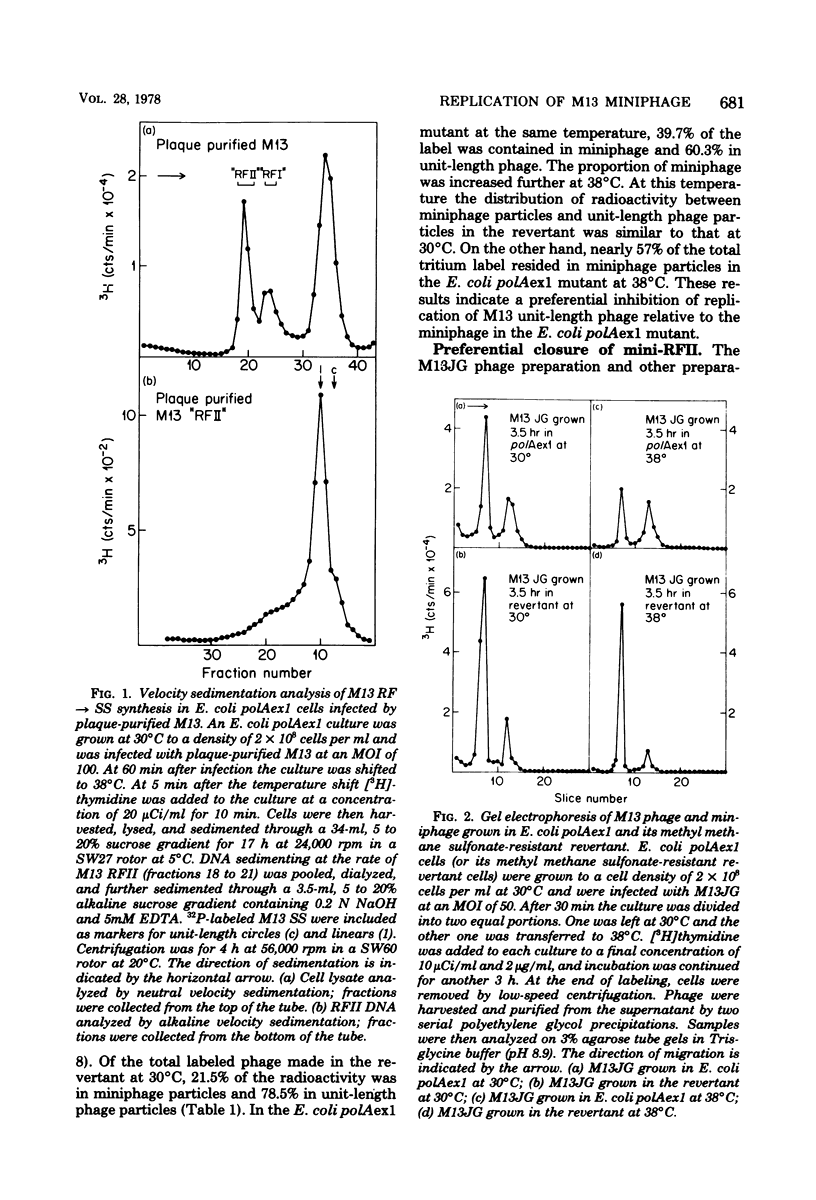
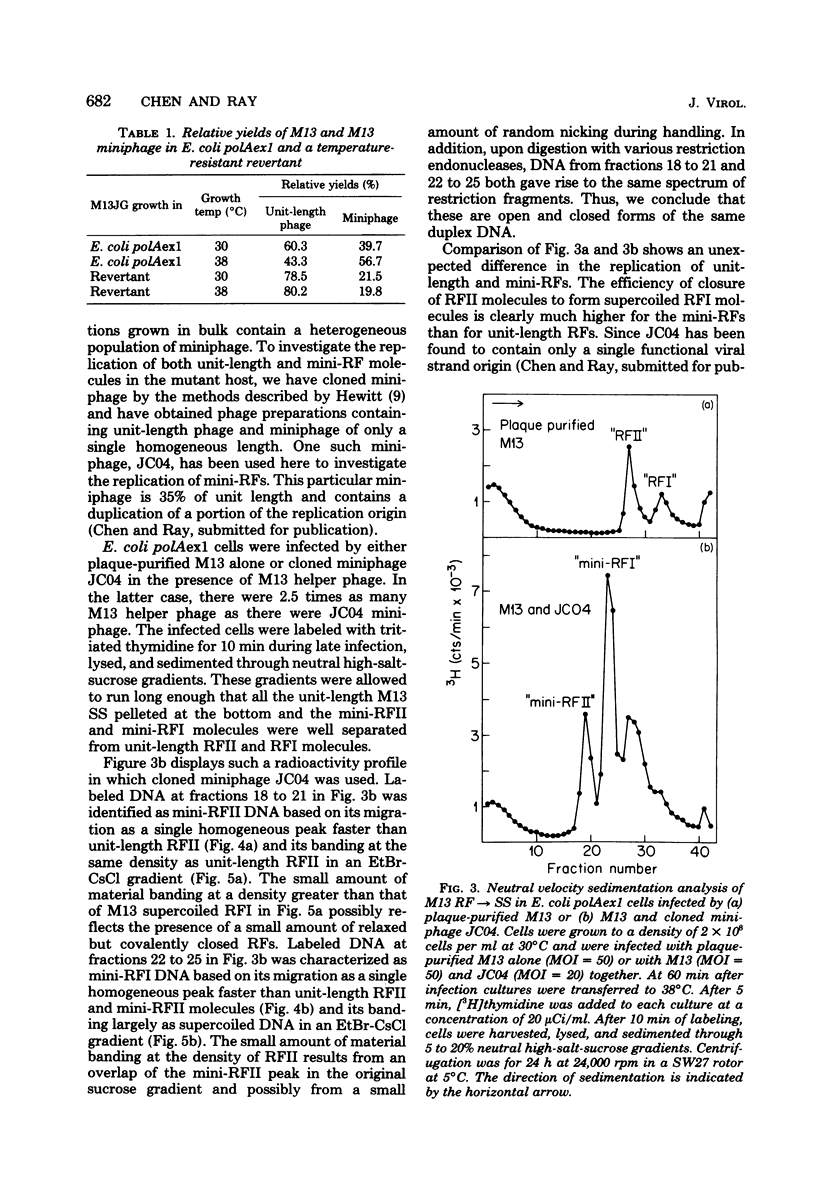
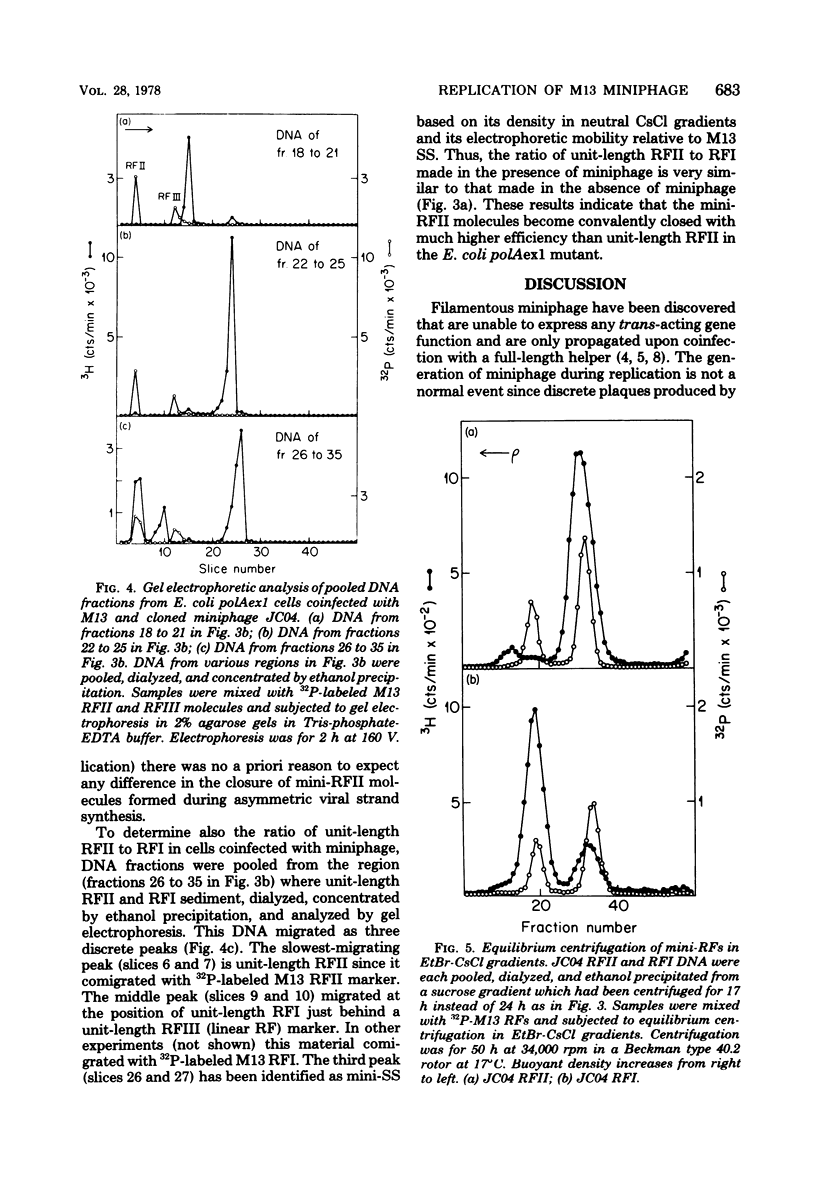
Previous studies have shown that M13 single-strand synthesis is inhibited at nonpermissive temperature in Escherichia coli polAexl, a temperature-sensitive mutant defective in the 5' leads to 3' exonuclease activity of polymerase I (T.-C. Chen and D. S. Ray, J. Mol. Biol. 106:589-604, 1976). Under these conditions the formation of covalently closed replicative form (RF) molecules is greatly reduced, and miniature forms of RF accumulate. We show here that the accumulation of mini-RFs is the consequence of a differential inhibition of the replication of unit-length phage and preexisting miniphage rather than a de novo production of miniphage. Mini-RFs do not accumulate even after as many as nine cycles of growth in the mutant host infected only with unit-length phage. Mixed infections of the mutant host with plaque-purified unit-length phage and a single cloned miniphage show that discontinuities in the mini-RFs are joined with higher efficiency than are those contained in unit-length RFs. After a shift to nonpermissive temperature during single-strand synthesis in cells infected with plaque-purified phage alone, M13 RFs are found largely as RFII molecules (RF form having one or more single-strand discontinuities) containing only a single discontinuity in the viral strand. The inability of the accumulated unit-length RFII molecules to actively replicate may reflect the presence of either a bound protein or RNA primer on the 5' terminus of the viral strand and provides further support for the existence of distinct initiation and termination events in the synthesis of the viral strand.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen T. C., Ray D. S. Replication of bacteriophage M13. X. M13 replication in a mutant of Escherichia coli defective in the 5' leads to 3' exonuclease associated with DNA polymerase I. J Mol Biol. 1976 Sep 25;106(3):589–604. doi: 10.1016/0022-2836(76)90253-9. [DOI] [PubMed] [Google Scholar]
- Dasgupta S., Mitra S. Structure of nascent replicative form DNA of coliphage M13. Proc Natl Acad Sci U S A. 1978 Jan;75(1):153–157. doi: 10.1073/pnas.75.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta S., Mitra S. The role of DNA polymerase I-associated 5'-exonuclease in replication of coliphage M13 replicative-form DNA. Biochem Biophys Res Commun. 1977 Oct 10;78(3):1108–1113. doi: 10.1016/0006-291x(77)90535-6. [DOI] [PubMed] [Google Scholar]
- Enea V., Horiuchi K., Turgeon B. G., Zinder N. D. Physical map of defective interfering particles of bacteriophage f1. J Mol Biol. 1977 Apr 25;111(4):395–414. doi: 10.1016/s0022-2836(77)80061-2. [DOI] [PubMed] [Google Scholar]
- Enea V., Zinder N. D. A delection mutant of bacteriophage f1 containing no intact cistrons. Virology. 1975 Nov;68(1):105–114. doi: 10.1016/0042-6822(75)90152-x. [DOI] [PubMed] [Google Scholar]
- Fidanián H. M., Ray D. S. Replication of bacteriophage M13. VII. Requirement of the gene 2 protein for the accumulation of a specific RFII species. J Mol Biol. 1972 Dec 14;72(1):51–63. doi: 10.1016/0022-2836(72)90067-8. [DOI] [PubMed] [Google Scholar]
- Gilbert W., Dressler D. DNA replication: the rolling circle model. Cold Spring Harb Symp Quant Biol. 1968;33:473–484. doi: 10.1101/sqb.1968.033.01.055. [DOI] [PubMed] [Google Scholar]
- Griffith J., Kornberg A. Mini M13 bacteriophage: circular fragments of M13 DNA are replicated and packaged during normal infections. Virology. 1974 May;59(1):139–152. doi: 10.1016/0042-6822(74)90211-6. [DOI] [PubMed] [Google Scholar]
- Hewitt J. A. Miniphage-a class of satellite phage to M13. J Gen Virol. 1975 Jan;26(1):87–94. doi: 10.1099/0022-1317-26-1-87. [DOI] [PubMed] [Google Scholar]
- Konrad E. B., Lehman I. R. A conditional lethal mutant of Escherichia coli K12 defective in the 5' leads to 3' exonuclease associated with DNA polymerase I. Proc Natl Acad Sci U S A. 1974 May;71(5):2048–2051. doi: 10.1073/pnas.71.5.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lica L., Ray D. S. Replication of bacteriophage M13. XII. In vivo cross-linking of a phage-specific DNA binding protein to the single-stranded DNA of bacteriophage M13 by ultraviolet irradiation. J Mol Biol. 1977 Sep;115(1):45–59. doi: 10.1016/0022-2836(77)90245-5. [DOI] [PubMed] [Google Scholar]
- Loening U. E. The determination of the molecular weight of ribonucleic acid by polyacrylamide-gel electrophresis. The effects of changes in conformation. Biochem J. 1969 Jun;113(1):131–138. doi: 10.1042/bj1130131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suggs S. V., Ray D. S. Replication of bacteriophage M13. XI. Localization of the origin for M13 single-strand synthesis. J Mol Biol. 1977 Feb 15;110(1):147–163. doi: 10.1016/s0022-2836(77)80103-4. [DOI] [PubMed] [Google Scholar]
- Tye B. K., Nyman P. O., Lehman I. R., Hochhauser S., Weiss B. Transient accumulation of Okazaki fragments as a result of uracil incorporation into nascent DNA. Proc Natl Acad Sci U S A. 1977 Jan;74(1):154–157. doi: 10.1073/pnas.74.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyemura D., Eichler D. C., Lehman I. R. Biochemical characterization of mutant forms of DNA polymerase I from Escherichia coli. II. The polAex1 mutation. J Biol Chem. 1976 Jul 10;251(13):4085–4089. [PubMed] [Google Scholar]


