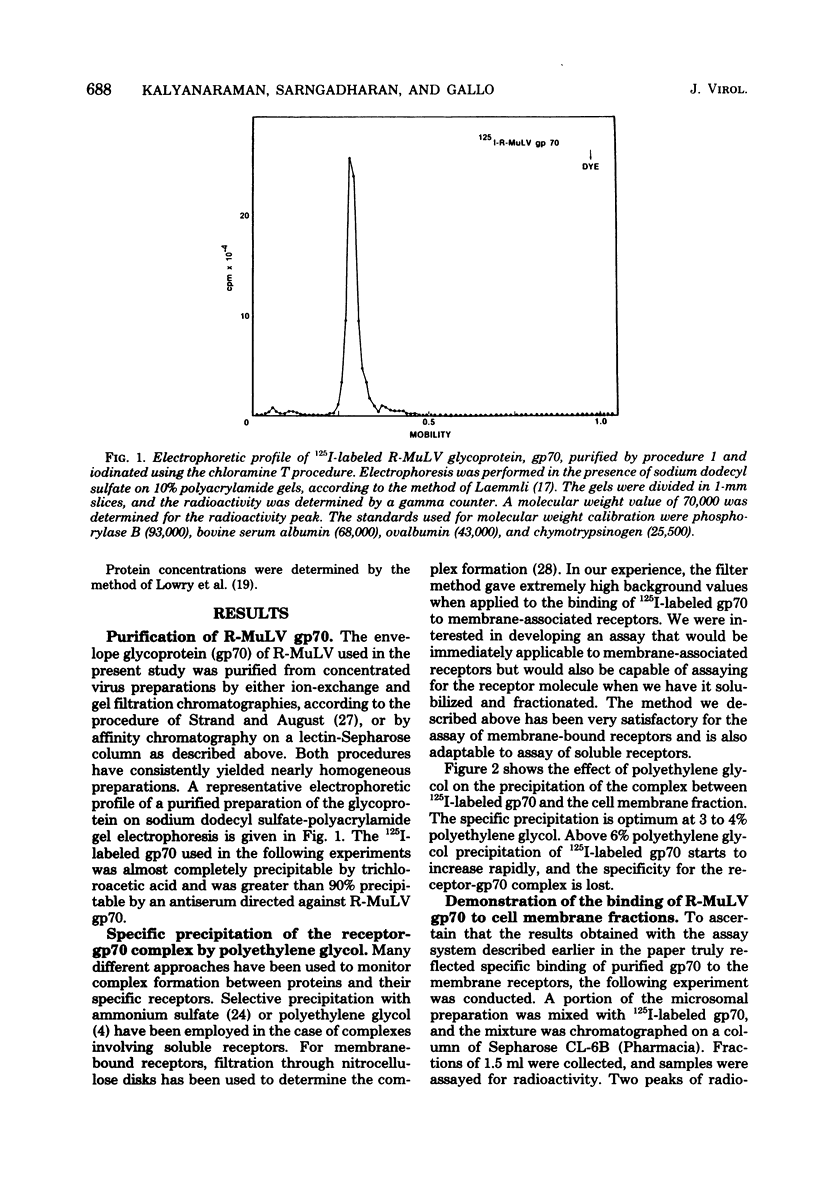
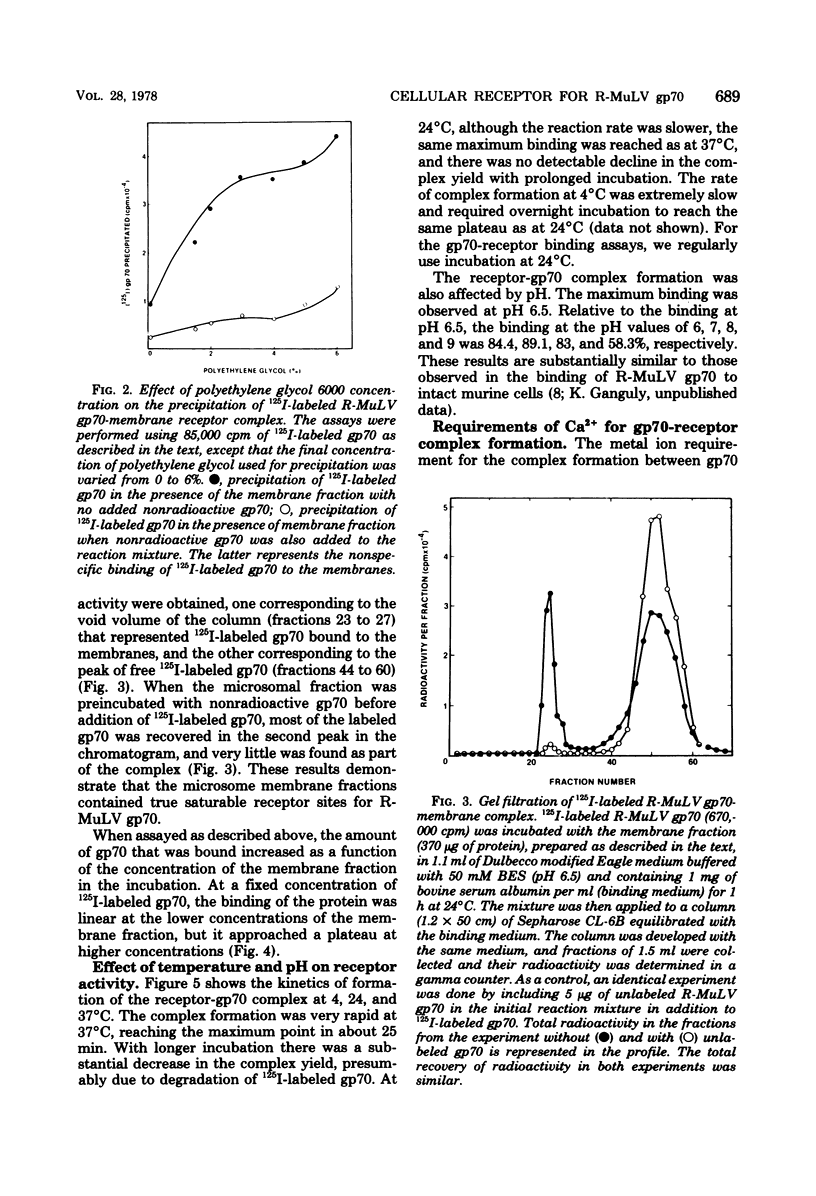
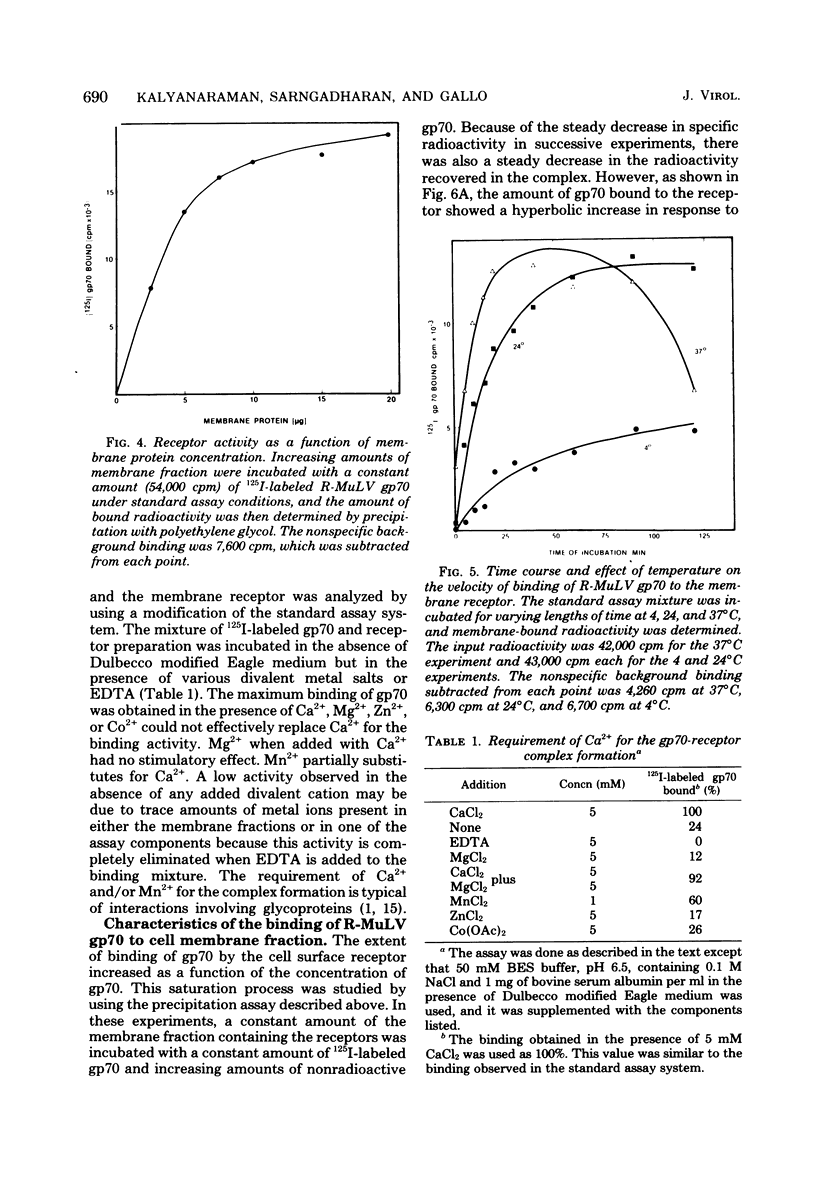
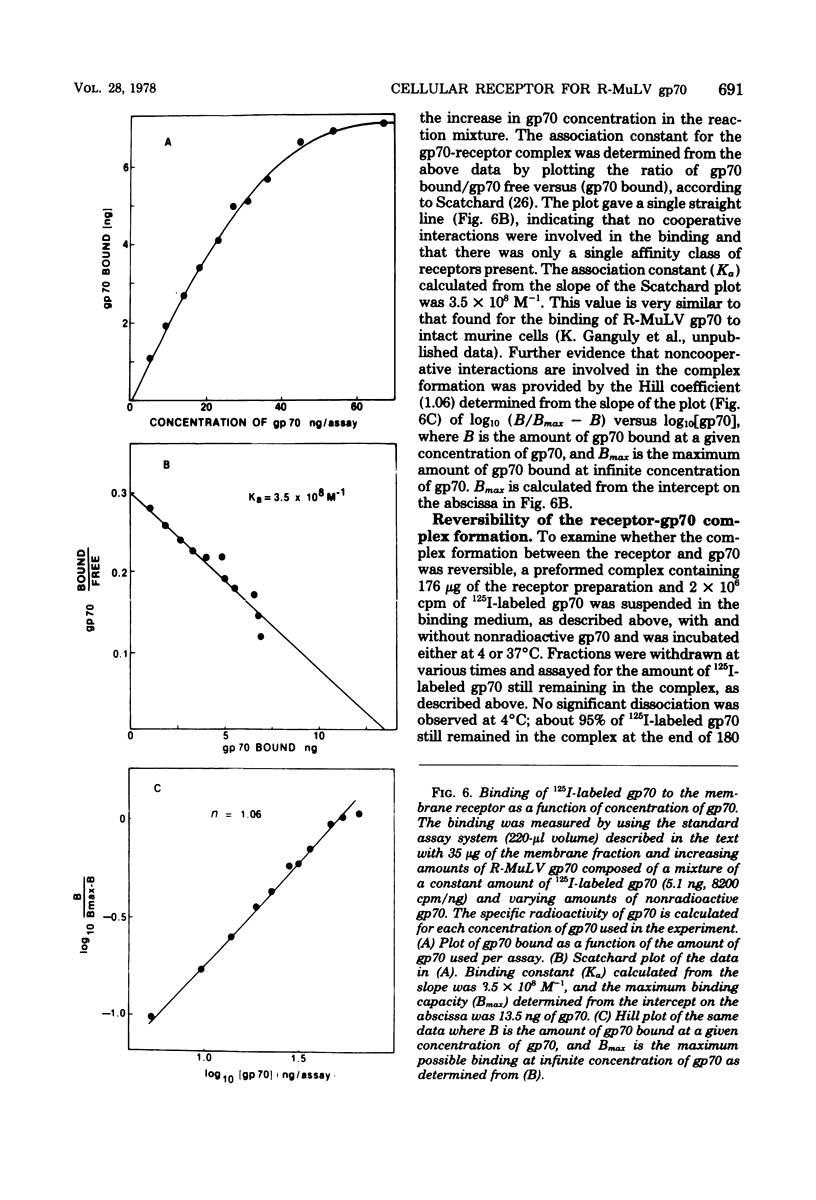
Abstract
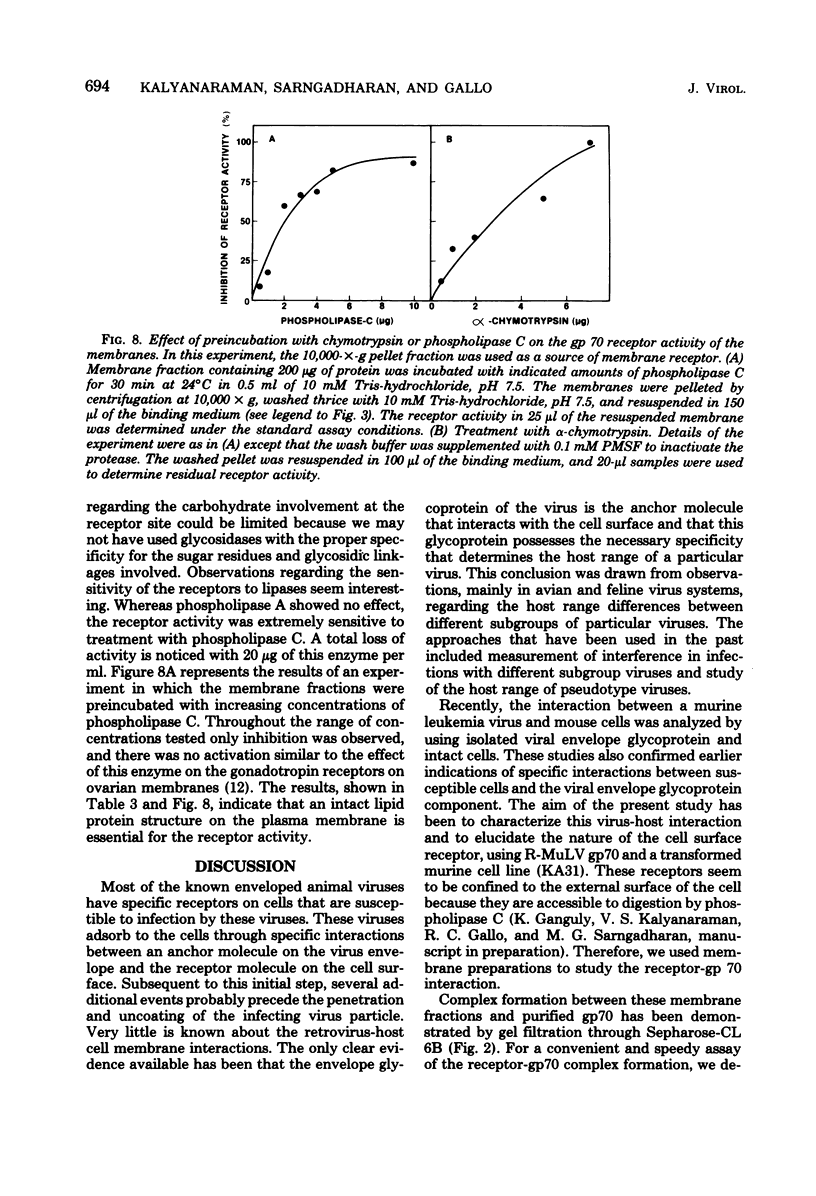
Plasma membrane preparations from KA31 (mouse) cells contained receptors for the binding of Rauscher murine leukemia virus (R-MuLV) envelope glycoprotein, gp70. This binding was demonstrated by gel filtration of a mixture of the microsomal fraction of the cells and 125I-labeled gp70. A rapid and convenient assay was developed to measure the complex formation between the membrane receptors and gp70 involving specific precipitation of the complex by 3 to 4% polyethylene glycol. The complex formation was responsive to the concentrations of both the receptor and gp70 and also to changes in temperature and pH. The gp70 binding was a noncooperative, saturable process, and an association constant of 3.5 X 10(8) M-1 was estimated from the binding data. The complex formation was reversible and a near-total exchange of 125I-labeled gp70 in the complex was achieved by incubation with excess of unlabeled gp70. The complex formation was inhibited by protein denaturing agents, guanidine-hydrochloride and urea. Pretreatment of the membrane fractions with either chymotrypsin or phospholipase C led to a loss of the membrane-associated receptor activity, indicating that a lipoprotein structure was important for the receptor function, consistent with the observation that nonionic detergents strongly inhibited the complex formation.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach M. K., Brashler J. R. On the nature of the presumed receptor for IgE on mast cells. I. The effect of sialidase and phospholipase C treatment on the capacity of rat peritoneal cells to participate in IgE-mediated, antigen-induced histamine release in vitro. J Immunol. 1973 Jun;110(6):1599–1608. [PubMed] [Google Scholar]
- Baird S., Raschke W., Weissman I. L. Evidence that MuLV-induced thymic lymphoma cells possess specific cell membrane binding sites for MuLV. Int J Cancer. 1977 Mar 15;19(3):403–413. doi: 10.1002/ijc.2910190319. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Isolation of the insulin receptor of liver and fat-cell membranes (detergent-solubilized-( 125 I)insulin-polyethylene glycol precipitation-sephadex). Proc Natl Acad Sci U S A. 1972 Feb;69(2):318–322. doi: 10.1073/pnas.69.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dales S. Early events in cell-animal virus interactions. Bacteriol Rev. 1973 Jun;37(2):103–135. doi: 10.1128/br.37.2.103-135.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLarco J., Todaro G. J. Membrane receptors for murine leukemia viruses: characterization using the purified viral envelope glycoprotein, gp71. Cell. 1976 Jul;8(3):365–371. doi: 10.1016/0092-8674(76)90148-3. [DOI] [PubMed] [Google Scholar]
- Duff R. G., Vogt P. K. Characteristics of two new avian tumor virus subgroups. Virology. 1969 Sep;39(1):18–30. doi: 10.1016/0042-6822(69)90344-4. [DOI] [PubMed] [Google Scholar]
- Fowler A. K., Twardzik D. R., Reed C. D., Weislow O. S., Hellman A. Binding characteristics of Rauscher leukemia virus envelope glycoprotein gp71 to murine lymphoid cells. J Virol. 1977 Dec;24(3):729–735. doi: 10.1128/jvi.24.3.729-735.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haour F., Saxena B. B. Characterization and solubilization of gonadotropin receptor of bovine corpus luteum. J Biol Chem. 1974 Apr 10;249(7):2195–2205. [PubMed] [Google Scholar]
- Hayman M. J., Crumpton M. J. Isolation of glycoproteins from pig lymphocyte plasma membrane using Lens culinaris phytohemagglutinin. Biochem Biophys Res Commun. 1972 May 26;47(4):923–930. doi: 10.1016/0006-291x(72)90581-5. [DOI] [PubMed] [Google Scholar]
- Howard I. K., Sage H. J., Stein M. D., Young N. M., Leon M. A., Dyckes D. F. Studies on a phytohemagglutinin from the lentil. II. Multiple forms of Lens culinaris hemagglutinin. J Biol Chem. 1971 Mar 25;246(6):1590–1595. [PubMed] [Google Scholar]
- Kalb A. J., Levitzki A. Metal-binding sites of concanavalin A and their role in the binding of alpha-methyl d-glucopyranoside. Biochem J. 1968 Oct;109(4):669–672. doi: 10.1042/bj1090669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koide N., Nose M., Muramatsu T. Recognition of IgG by Fc receptor and complement: effects of glycosidase digestion. Biochem Biophys Res Commun. 1977 Apr 25;75(4):838–844. doi: 10.1016/0006-291x(77)91458-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lonberg-Holm K., Philipson L. Early interaction between animal viruses and cells. Monogr Virol. 1974;9:1–148. [PubMed] [Google Scholar]
- Miyamoto K., Gilden R. V. Electron microscopic studies of tumor viruses. I. Entry of murine leukemia virus into mouse embryo fibroblasts. J Virol. 1971 Mar;7(3):395–406. doi: 10.1128/jvi.7.3.395-406.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldow C. F., McGrath M., Peterson C. Solubilization of initial attachment site activity for avian tumor viruses with lithium diiodosalicylate. Proc Soc Exp Biol Med. 1977 Feb;154(2):201–205. doi: 10.3181/00379727-154-39637. [DOI] [PubMed] [Google Scholar]
- Perdue J. F. The isolation and characterization of plasma membrane from cultured chicken embryo fibroblasts. Methods Enzymol. 1974;31:162–168. doi: 10.1016/0076-6879(74)31017-8. [DOI] [PubMed] [Google Scholar]
- Rifkin D., Compans R. W. Identification of the spike proteins of Rous sarcoma virus. Virology. 1971 Nov;46(2):485–489. doi: 10.1016/0042-6822(71)90049-3. [DOI] [PubMed] [Google Scholar]
- Rossi G., Newman S. A., Metzger H. Assay and partial characterization of the solubilized cell surface receptor for immunoglobulin E. J Biol Chem. 1977 Jan 25;252(2):704–711. [PubMed] [Google Scholar]
- Strand M., August J. T. Structural proteins of ribonucleic acid tumor viruses. Purification of envelope, core, and internal components. J Biol Chem. 1976 Jan 25;251(2):559–564. [PubMed] [Google Scholar]
- Tate R. L., Schwartz H. I., Holmes J. M., Kohn L. D. Thyrotropin receptors in thyroid plasma membranes. Characteristics of thyrotropin binding and solubilization of thyrotropin receptor activity by tryptic digestion. J Biol Chem. 1975 Aug 25;250(16):6509–6515. [PubMed] [Google Scholar]
- Vogt P. K., Ishizaki R. Patterns of viral interference in the avian leukosis and sarcoma complex. Virology. 1966 Nov;30(3):368–374. doi: 10.1016/0042-6822(66)90115-2. [DOI] [PubMed] [Google Scholar]
- Witte O. N., Tsukamoto-Adey A., Weissman I. L. Cellular maturation of oncornavirus glycoproteins: topological arrangement of precursor and product forms in cellular membranes. Virology. 1977 Feb;76(2):539–553. doi: 10.1016/0042-6822(77)90236-7. [DOI] [PubMed] [Google Scholar]
- de Giuli C., Kawai S., Dales S., Hanafusa H. Absence of surface projections of some noninfectious forms of RSV. Virology. 1975 Jul;66(1):253–260. doi: 10.1016/0042-6822(75)90195-6. [DOI] [PubMed] [Google Scholar]



