Abstract
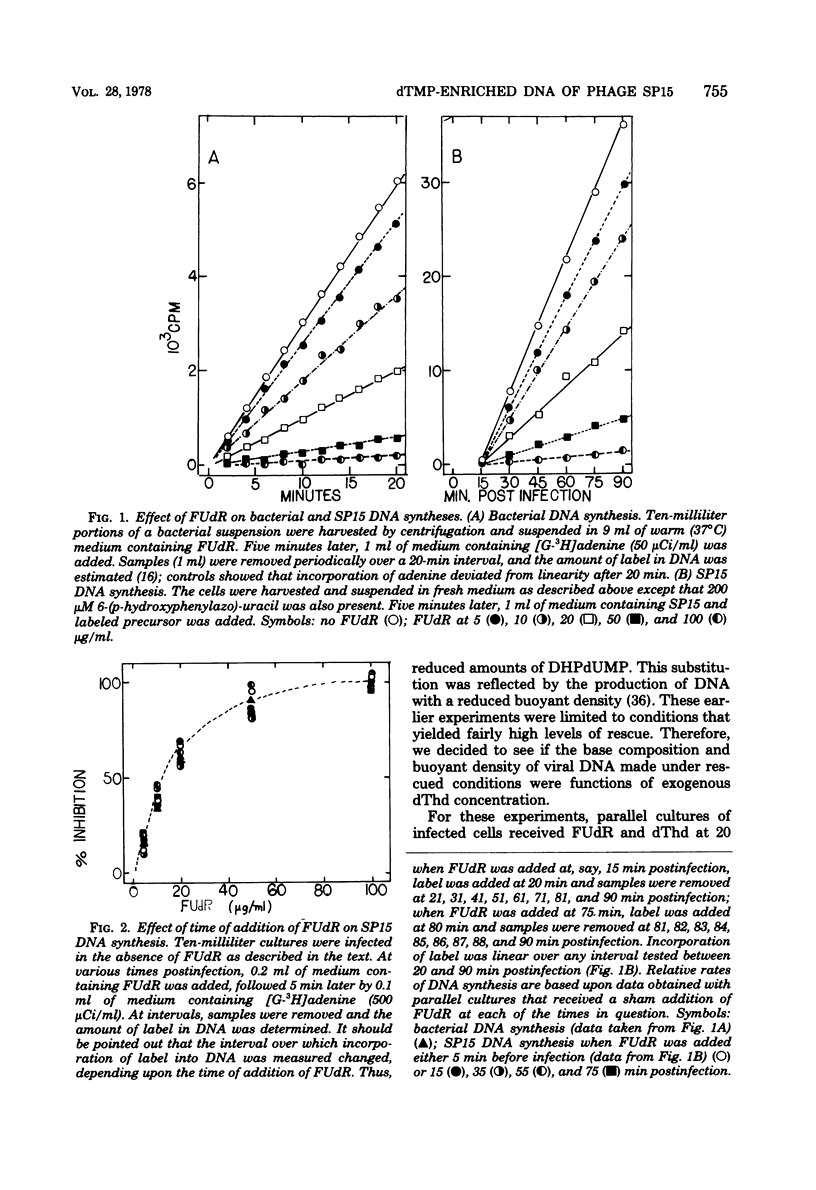
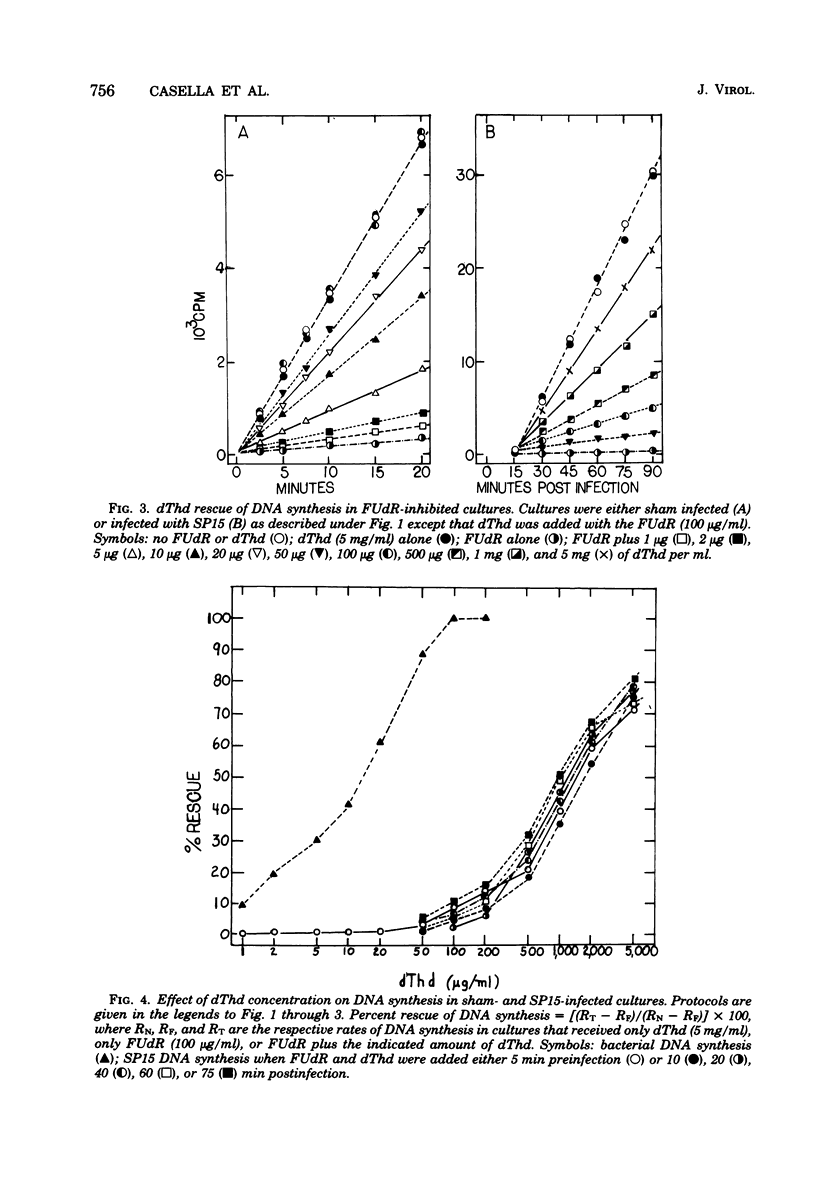
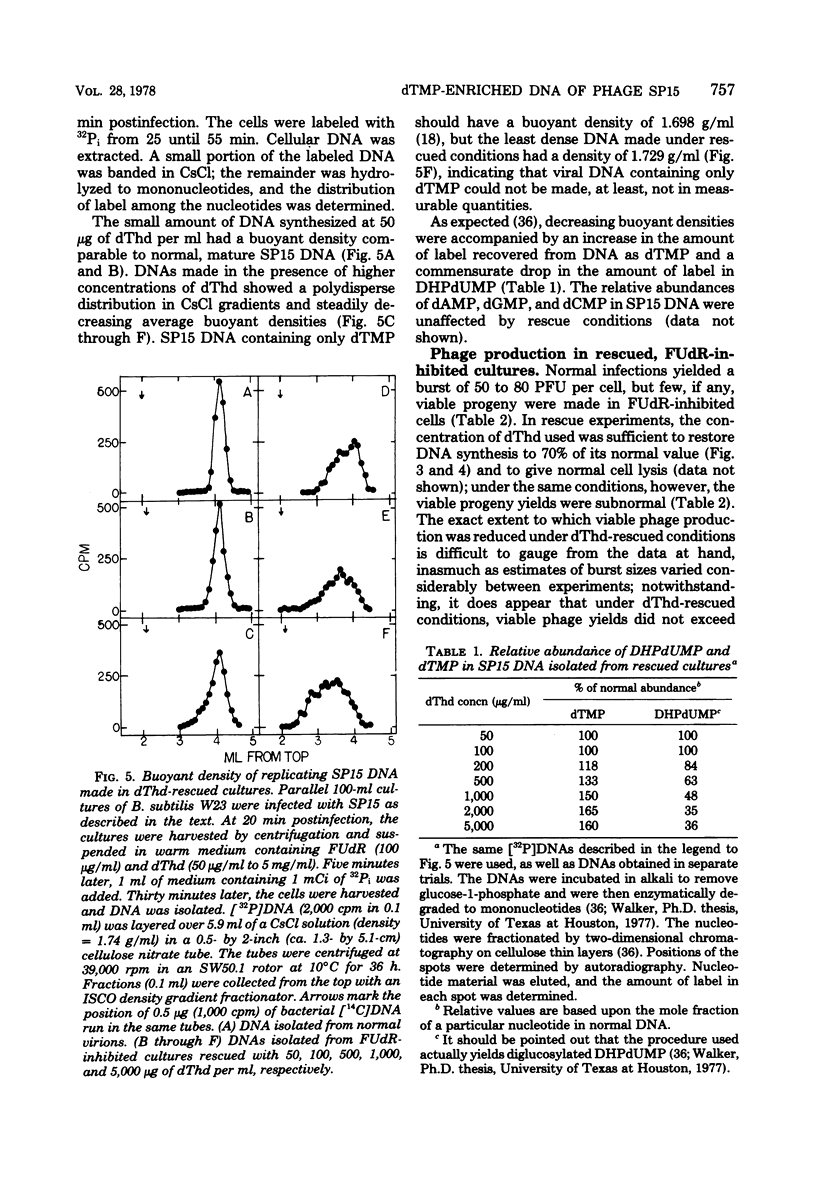
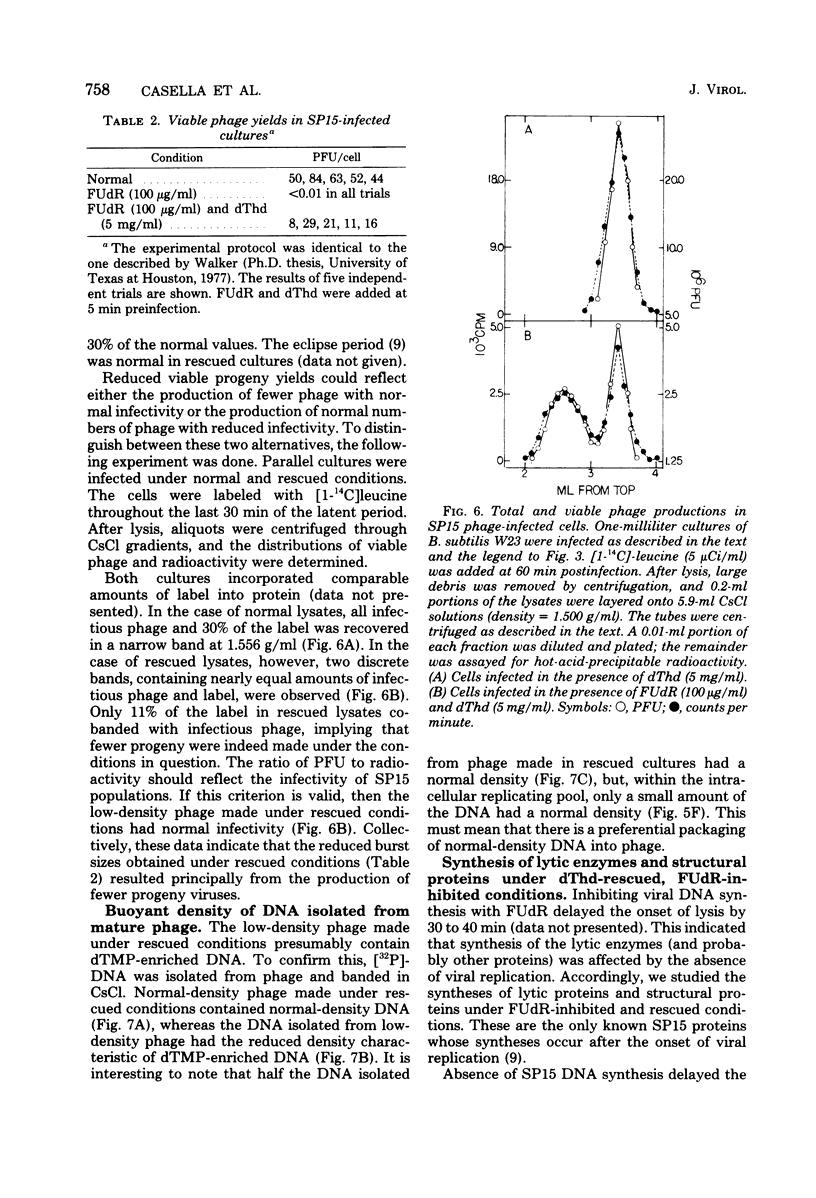
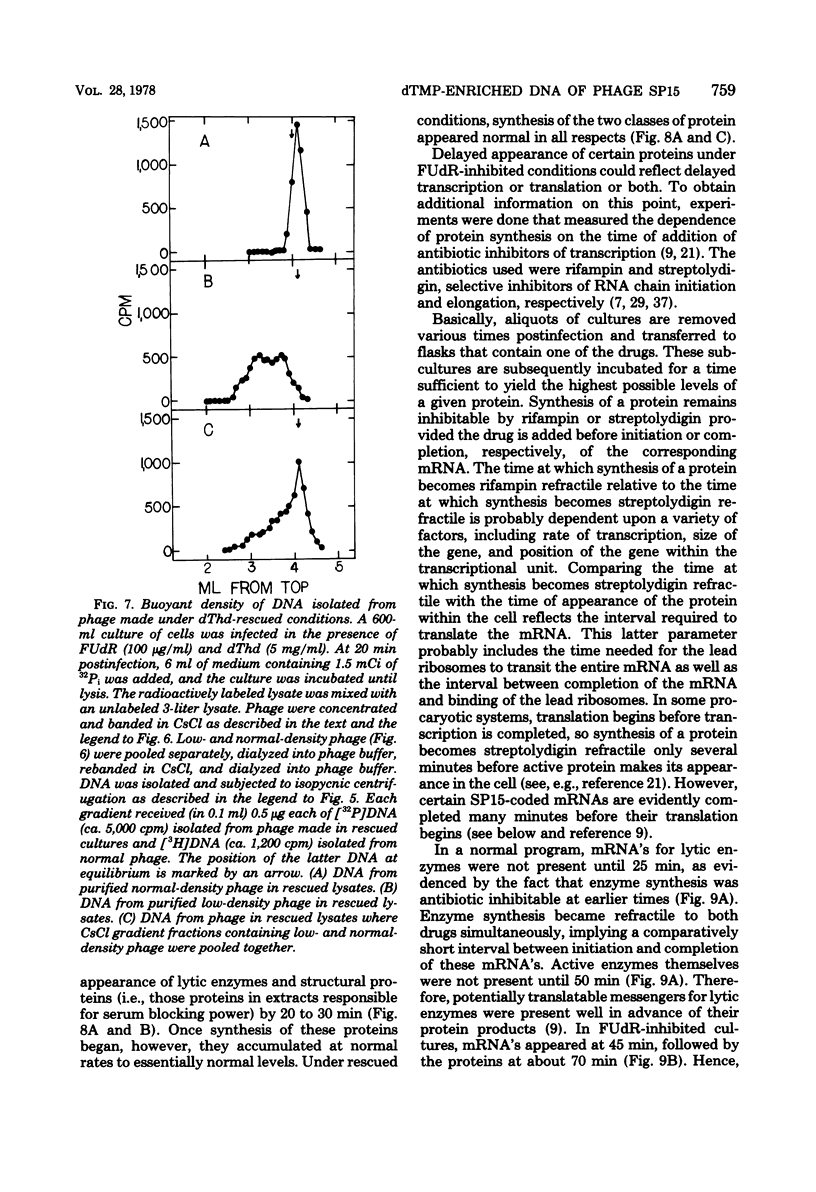
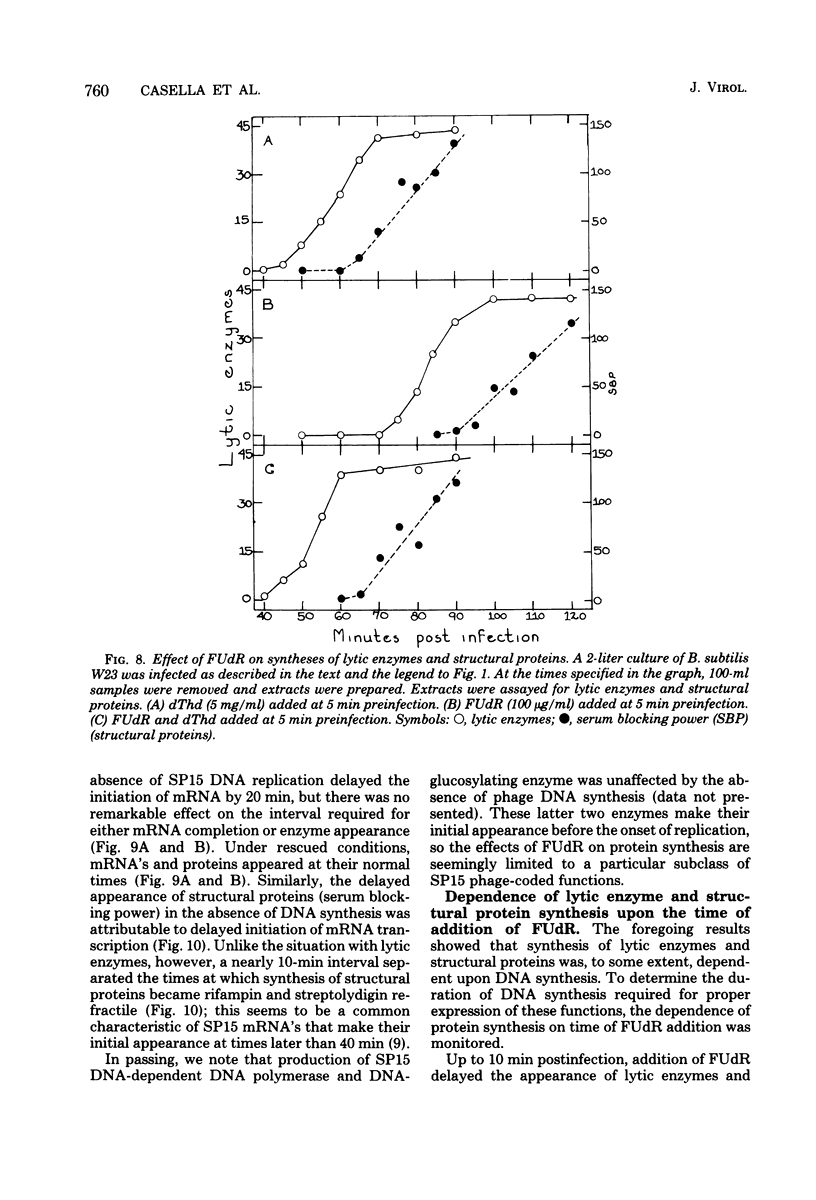
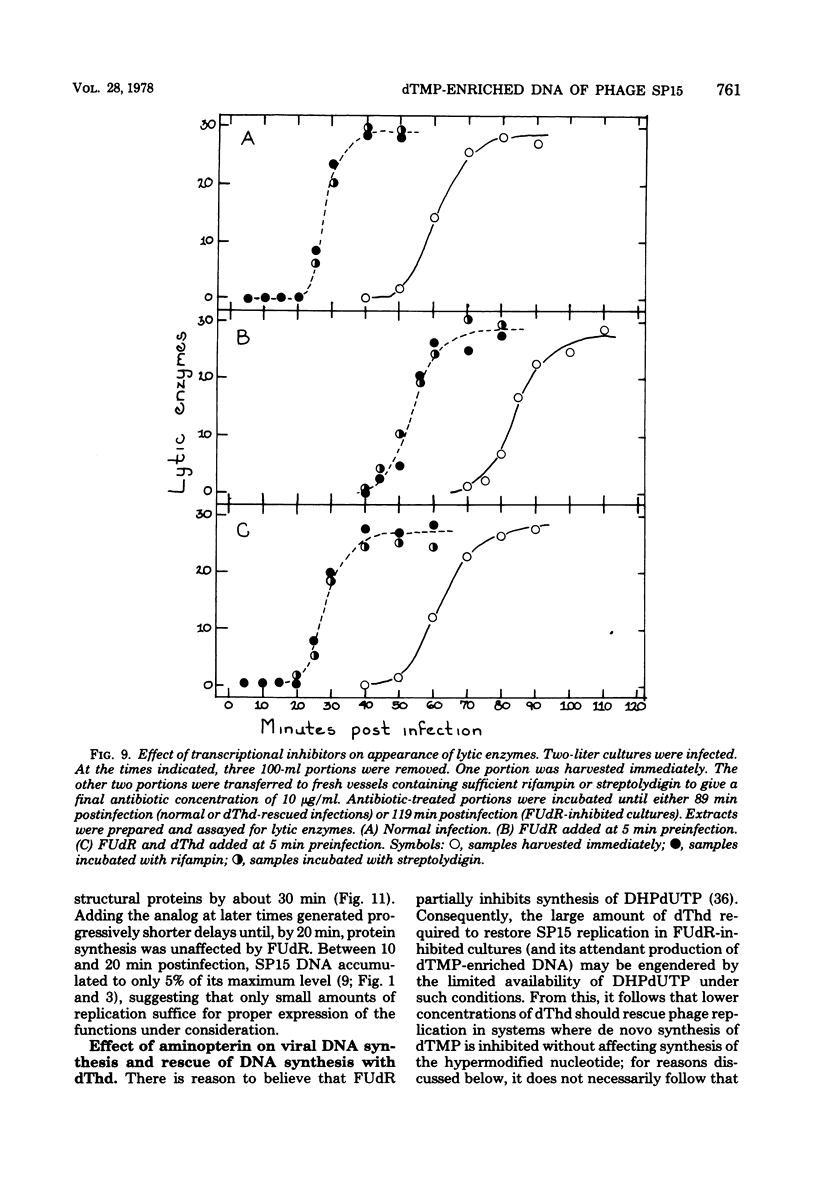
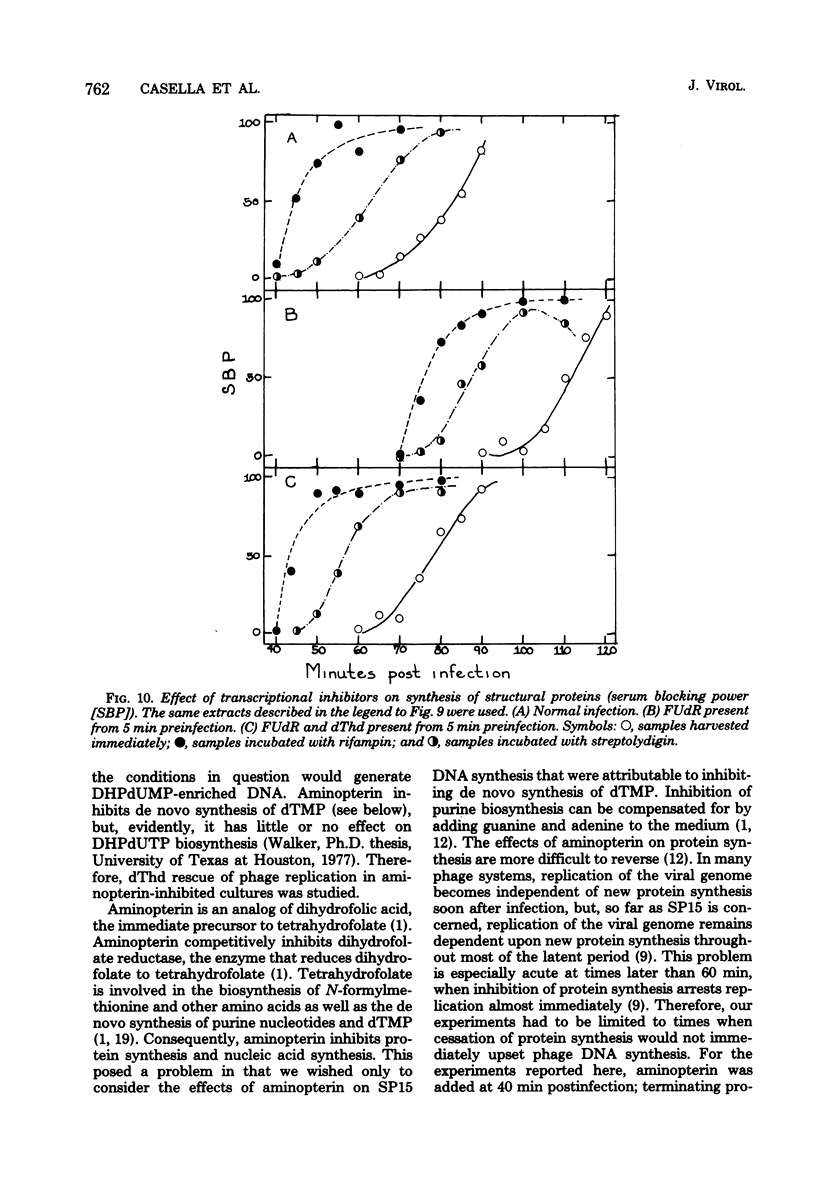
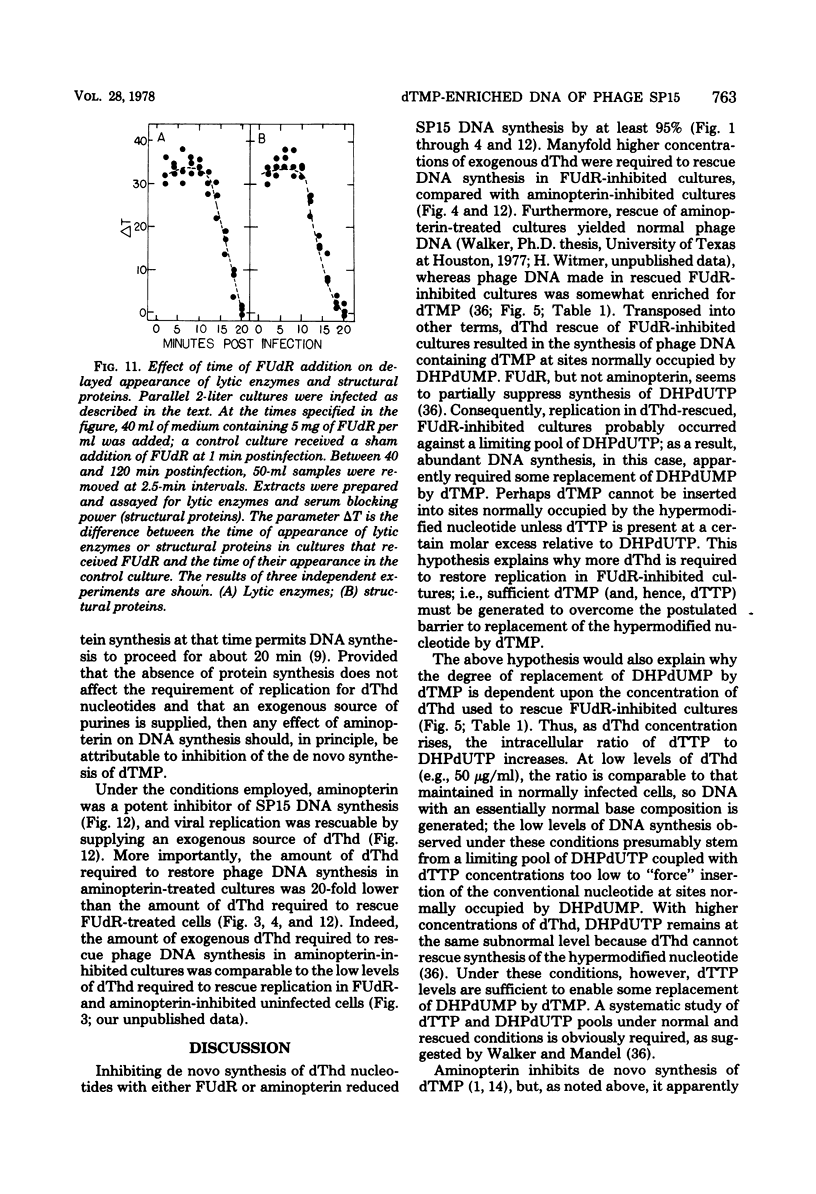
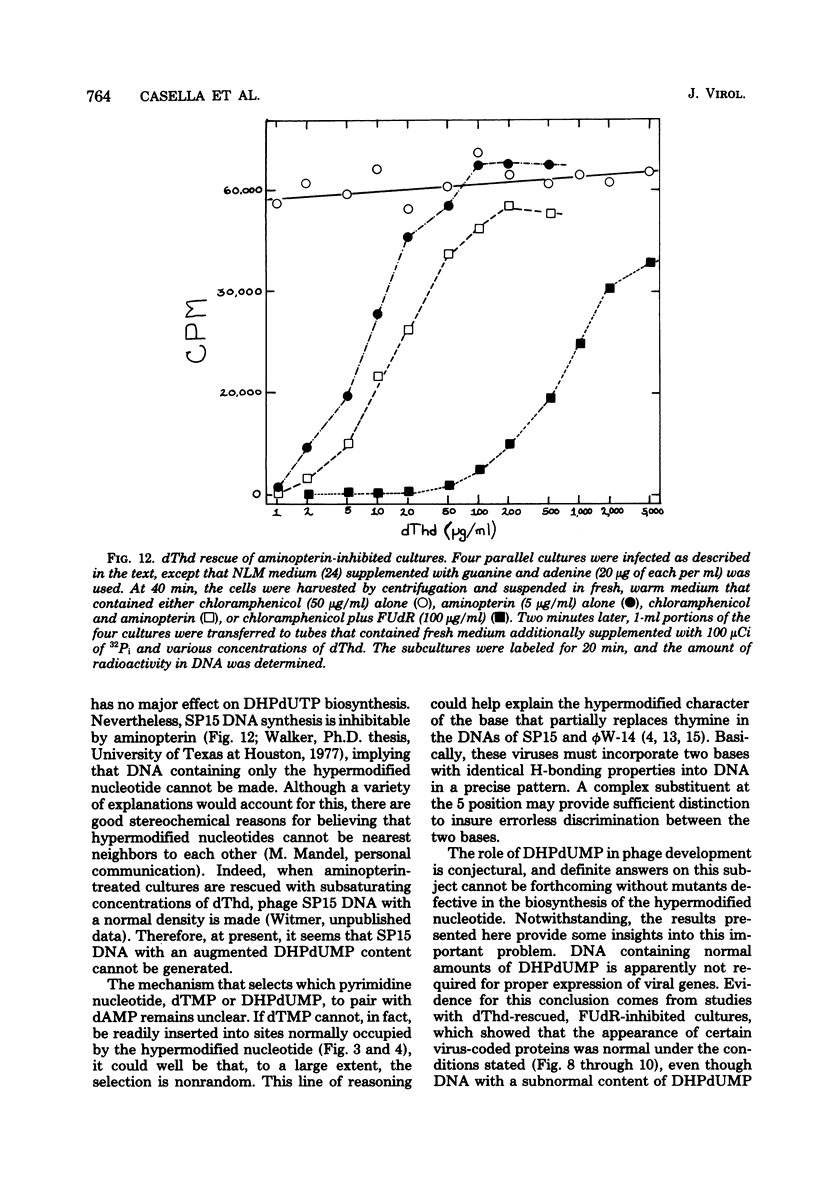
Normal DNA of Bacillus subtilis phage SP15 contains approximately equimolar quantities of dTMP and a hypermodified nucleotide, 5-dihydroxypentyl-dUMP (DHPdUMP). Deoxythymidine (dThd) rescue of phage DNA synthesis in 5-fluorodeoxyuridine (FUdR)-inhibited cultures resulted in the synthesis of SP15 DNA containing enhanced levels of dTMP and correspondingly reduced levels of DHPdUMP. This rescued system was used to probe possible roles of DHPdUMP in phage development. The results suggested that normal levels of DHPdUMP were not required for proper transcription of phage DNA, but normal amounts of DHPdUMP were indispensable for phage assembly and/or DNA maturation. The amount of exogenous dThd required to rescue phage DNA synthesis in FUdR-inhibited cultures was 20-fold higher than the concentration required to rescue cellular replication, whereas the same low concentrations of dThd sufficed to rescue viral and bacterial DNA syntheses in aminopterin-inhibited cultures. Normal SP15 DNA was made in rescued, aminopterin-inhibited cultures. We suggest that FUdR (but not aminopterin) partially suppresses biosynthesis of the hypermodified nucleotide and that there is a barrier to replacement of DHPdUMP by dTMP; therefore, exceptionally large amounts of dThd must be salvaged in FUdR-inhibited cultures to force replacement of the unusual nucleotide by dTMP.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandon C., Gallop P. M., Marmur J., Hayashi H., Nakanishi K. Structure of a new pyrimidine from Bacillus subtilis phage SP-15 nucleic acid. Nat New Biol. 1972 Sep 20;239(90):70–71. doi: 10.1038/newbio239070a0. [DOI] [PubMed] [Google Scholar]
- Brown N. C. 6-(p-hydroxyphenylazo)-uracil: a selective inhibitor of host DNA replication in phage-infected Bacillus subtilis. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1454–1461. doi: 10.1073/pnas.67.3.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE MARS R. I. The production of phage-related materials when bacteriophage development in interrupted by proflavine. Virology. 1955 May;1(1):83–99. doi: 10.1016/0042-6822(55)90007-6. [DOI] [PubMed] [Google Scholar]
- Dosmar M., Markewych O., Witmer H. Effect of antibiotics on certain aspects of bacteriophage SP-15 development in Bacillus subtilis W23. J Virol. 1977 Mar;21(3):924–931. doi: 10.1128/jvi.21.3.924-931.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDLIN G. GENE REGULATION DURING BACTERIOPHAGE T4 DEVLOPMENT. I. PHENOTYPIC REVERSION OF T4 AMBER MUTANTS BY 5-FLUOROURACIL. J Mol Biol. 1965 Jun;12:363–374. doi: 10.1016/s0022-2836(65)80260-1. [DOI] [PubMed] [Google Scholar]
- Glassberg J., Slomiany R. A., Stewart C. R. Selective screening procedure for the isolation of heat- and cold-sensitive, DNA replication-deficient mutants of bacteriophage SPO1 and preliminary characterization of the mutants isolated. J Virol. 1977 Jan;21(1):54–60. doi: 10.1128/jvi.21.1.54-60.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey R. J. Growth and initiation of protein synthesis in Escherichia coli in the presence of trimethoprim. J Bacteriol. 1973 Apr;114(1):309–322. doi: 10.1128/jb.114.1.309-322.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi H., Nakanishi K., Brandon C., Marmur J. Structure and synthesis of dihydroxypentyluracil from bacteriophage SP-15 deoxyribonucleic acid. J Am Chem Soc. 1973 Dec 26;95(26):8749–8757. doi: 10.1021/ja00807a041. [DOI] [PubMed] [Google Scholar]
- Kropinski A. M., Bose R. J., Warren R. A. 5-(4-Aminobutylaminomethyl)uracil, an unusual pyrimidine from the deoxyribonucleic acid of bacteriophage phiW-14. Biochemistry. 1973 Jan 2;12(1):151–157. doi: 10.1021/bi00725a025. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lembach K. J., Buchanan J. M. The relationship of protein synthesis to early transcriptive events in bacteriophage T4-infected Escherichia coli B. J Biol Chem. 1970 Apr 10;245(7):1575–1587. [PubMed] [Google Scholar]
- MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol. 1962 Jul;5:109–118. doi: 10.1016/s0022-2836(62)80066-7. [DOI] [PubMed] [Google Scholar]
- Marcker K. The formation of N-formyl-methionyl-sRNA. J Mol Biol. 1965 Nov;14(1):63–70. doi: 10.1016/s0022-2836(65)80230-3. [DOI] [PubMed] [Google Scholar]
- Marcus M., Newlon M. C. Control of DNA synthesis in Bacillus subtilis by phage phi e. Virology. 1971 Apr;44(1):83–93. doi: 10.1016/0042-6822(71)90155-3. [DOI] [PubMed] [Google Scholar]
- Markewych O., Boghosian A., Dosmar M., Ende D., Witmer H. SP-10 bacteriophage-specific nucleic acid and enzyme synthesis in Bacillus subtilis W23. J Virol. 1977 Jan;21(1):84–95. doi: 10.1128/jvi.21.1.84-95.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmur J., Brandon C., Neubort S., Ehrlich M., Mandel M., Konvicka J. Unique properties of nucleic acid from Bacillus subtilis phage SP-15. Nat New Biol. 1972 Sep 20;239(90):68–70. doi: 10.1038/newbio239068a0. [DOI] [PubMed] [Google Scholar]
- Neubort S., Marmur J. Synthesis of the unusual DNA of Bacillus subtilis bacteriophage SP-15. J Virol. 1973 Nov;12(5):1078–1084. doi: 10.1128/jvi.12.5.1078-1084.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville M. M., Brown N. C. Inhibition of a discrete bacterial DNA polymerase by 6-(p-hydroxyphenylazo)-uracil and 6-(p-hydroxyphenylazo-)-isocytosine. Nat New Biol. 1972 Nov 15;240(98):80–82. doi: 10.1038/newbio240080a0. [DOI] [PubMed] [Google Scholar]
- Price A. R., Frato J. Bacillus subtilis deoxyuridinetriphosphatase and its bacteriophage PBS2-induced inhibitor. J Biol Chem. 1975 Nov 25;250(22):8804–8811. [PubMed] [Google Scholar]
- ROMIG W. R., BRODETSKY A. M. Isolation and preliminary characterization of bacteriophages for Bacillus subtilis. J Bacteriol. 1961 Jul;82:135–141. doi: 10.1128/jb.82.1.135-141.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roscoe D. H. Synthesis of DNA in phage-infected Bacillus subtilis. Virology. 1969 Aug;38(4):527–537. doi: 10.1016/0042-6822(69)90173-1. [DOI] [PubMed] [Google Scholar]
- Siddhikol C., Erbstoeszer J. W., Weisblum B. Mode of action of streptolydigin. J Bacteriol. 1969 Jul;99(1):151–155. doi: 10.1128/jb.99.1.151-155.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR M. J., THORNE C. B. TRANSDUCTION OF BACILLUS LICHENIFORMIS AND BACILLUS SUBTILIS BY EACH OF TWO PHAGES. J Bacteriol. 1963 Sep;86:452–461. doi: 10.1128/jb.86.3.452-461.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M. J., Goldberg I. D. Growth and cultivation of the unusual generalized transducing Bacillus bacteriophage SP-15. Appl Microbiol. 1971 Jul;22(1):113–119. doi: 10.1128/am.22.1.113-119.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyeryar F. J., Jr, Lawton W. D., MacQuillan A. M. Sequential replication of the chromosome of Bacillus licheniformis. J Bacteriol. 1968 Jun;95(6):2062–2069. doi: 10.1128/jb.95.6.2062-2069.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyeryar F. J., Jr, Taylor M. J., Lawton W. D., Goldberg I. D. Cotransduction and cotransformation of genetic markers in Bacillus subtilis and Bacillus licheniformis. J Bacteriol. 1969 Nov;100(2):1027–1036. doi: 10.1128/jb.100.2.1027-1036.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker M. S., Mandel M. Biosynthesis of 5-(4'5'-dihydroxypentyl) uracil as a nucleoside triphosphate in bacteriophage SP15-infected Bacillus subtilis. J Virol. 1978 Feb;25(2):500–509. doi: 10.1128/jvi.25.2.500-509.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrli W., Staehelin M. Actions of the rifamycins. Bacteriol Rev. 1971 Sep;35(3):290–309. doi: 10.1128/br.35.3.290-309.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagil E., Rosner A. Phosphorolysis of 5-fluoro-2'-deoxyuridine in Escherichia coli and its inhibition by nucleosides. J Bacteriol. 1971 Nov;108(2):760–764. doi: 10.1128/jb.108.2.760-764.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]


