Abstract
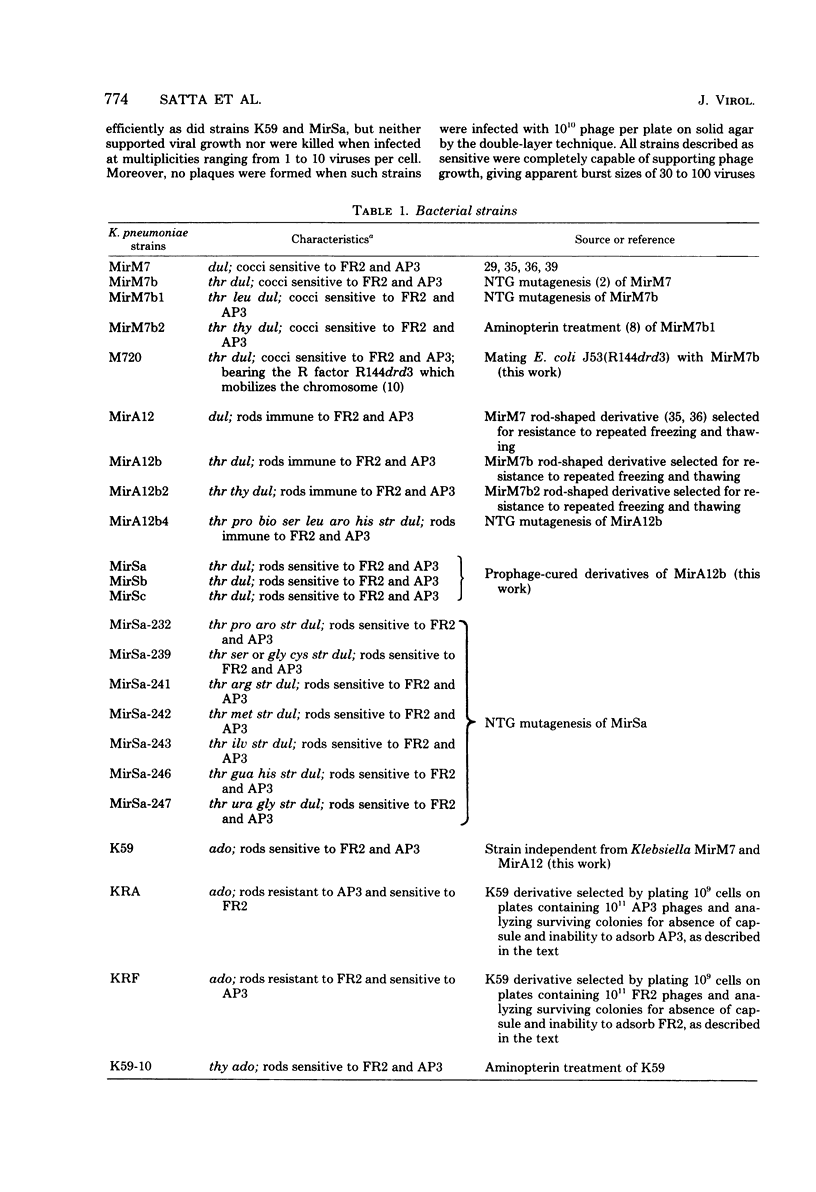
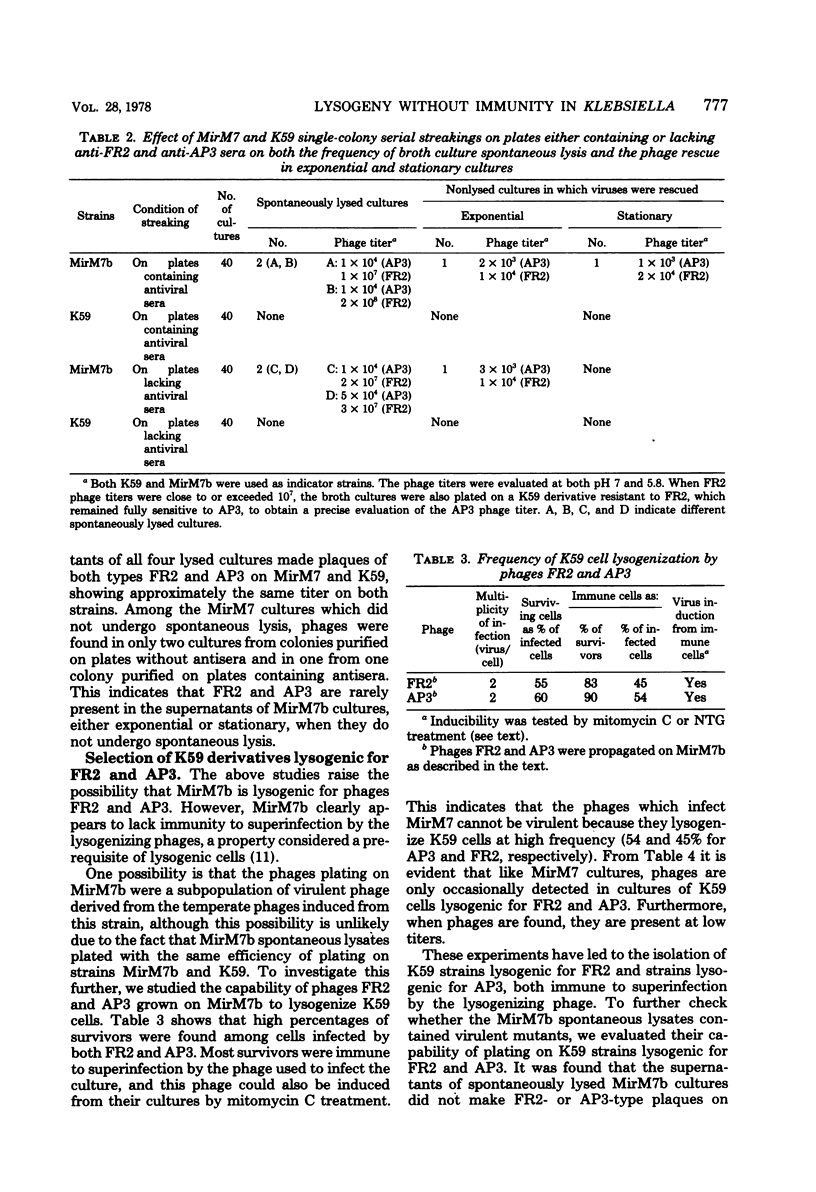
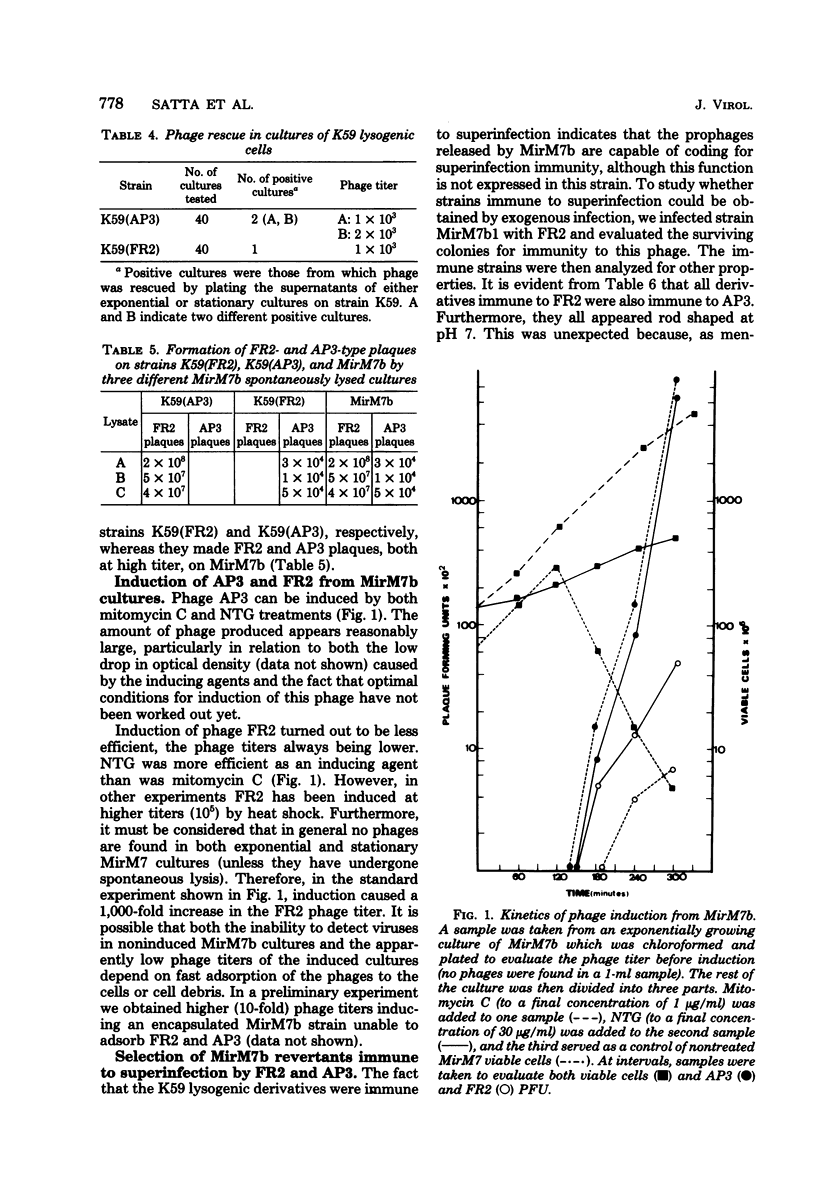
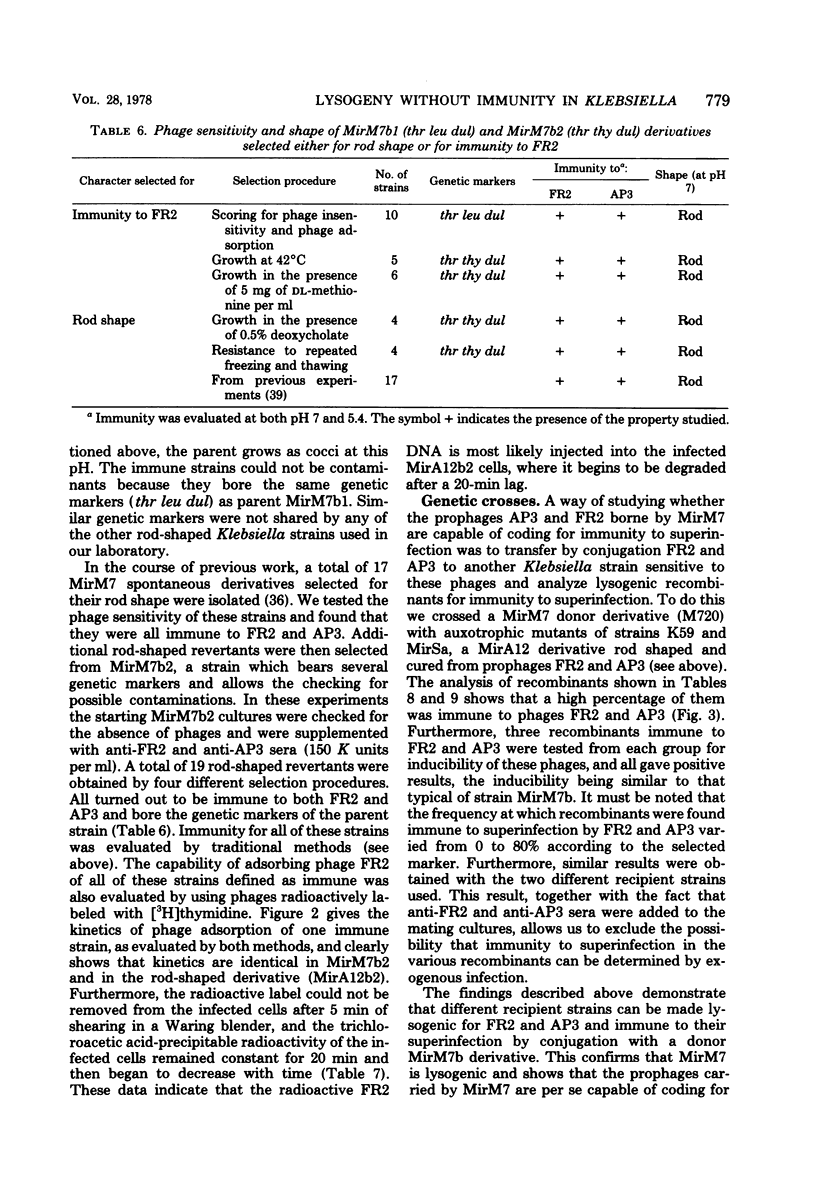
Klebsiella pneumoniae MirM7 is a wild-type strain which grows as cocci at pH 7 and above and as rods at pH 6.5 and below. Cultures of this strain and an auxotrophic derivative, MirM7b, have been found to undergo spontaneous lysis after purification from possible contaminating viruses. Lysates always contained two phages, FR2 and AP3, most often at high titers. FR2 and AP3 plated with the same efficiency on both MirM7b and K59 (another K. pneumoniae strain sensitive to FR2 and AP3) and lysogenized 45 and 54% of the K59-infected cells, respectively. These findings raise the possibility that MirM7b is lysogenic for FR2 and AP3, although nonimmune to their superinfection. The fact that mitomycin C and N-methyl-N'-nitro-N-nitrosoguanidine can induce phages FR2 and AP3 from MirM7b confirmed this possibility. When MirM7b was infected with FR2 several strains immune to FR2 and AP3, which were all rod shaped, were obtained. Furthermore, 19 derivatives, rod shaped at all pH's have been isolated from MirM7b. They were all immune to both FR2 and AP3. From mating experiments between the MirM7b donor derivative, strain M720, and either K59 or MirCV5, a rod-shaped MirM7b derivative cured from the prophages, cysteine recombinants were obtained which were most often (80%) immune to FR2 and AP3. Nonimmune and still lysogenic recombinants were obtained by mating M720 with a rod-shaped immune MirM7b derivative; the majority of the non-immune strains maintained the rod shape. Five coccus-shaped recombinants were also isolated; they were nonimmune to superinfection. Several physiological properties of strain MirM7b and the other nonimmune coccal recombinants have been studied in comparison with those of the rod-shaped immune derivatives. All of the coccal strains have shown several alterations with respect to the rods. The role of possible derepressed prophage genes in the various physiological alterations of MirM7 is discussed, and the analogies between this system and those of vertebrate cells transformed by proviruses are stressed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S., Campbell A. Crypticogenicity of bacteriophage lambda. J Mol Biol. 1970 Jun 14;50(2):481–490. doi: 10.1016/0022-2836(70)90206-8. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barksdale L., Arden S. B. Persisting bacteriophage infections, lysogeny, and phage conversions. Annu Rev Microbiol. 1974;28(0):265–299. doi: 10.1146/annurev.mi.28.100174.001405. [DOI] [PubMed] [Google Scholar]
- Bertani L. E. Split-operon control of a prophage gene. Proc Natl Acad Sci U S A. 1970 Feb;65(2):331–336. doi: 10.1073/pnas.65.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botstein K., Lew K. K., Jarvik V., Swanson C. A. Role of antirepressor in the bipartite control of repression and immunity by bacteriophage P22. J Mol Biol. 1975 Feb 5;91(4):439–462. doi: 10.1016/0022-2836(75)90271-5. [DOI] [PubMed] [Google Scholar]
- Dixon R., Cannon F. C., Postgate J. R. Properties of the R factor R144drd3 in Klebsiella pneumoniae strain M5al. Genet Res. 1975 Jun;25(3):327–338. doi: 10.1017/s0016672300015743. [DOI] [PubMed] [Google Scholar]
- Dulbecco R. Cell transformation by viruses. Science. 1969 Nov 21;166(3908):962–968. doi: 10.1126/science.166.3908.962. [DOI] [PubMed] [Google Scholar]
- Echols H. Developmental pathways for the temperate phage: lysis vs lysogeny,. Annu Rev Genet. 1972;6(0):157–190. doi: 10.1146/annurev.ge.06.120172.001105. [DOI] [PubMed] [Google Scholar]
- Edlin G., Lin L., Bitner R. Reproductive fitness of P1, P2, and Mu lysogens of Escherichia coli. J Virol. 1977 Feb;21(2):560–564. doi: 10.1128/jvi.21.2.560-564.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlin G., Lin L., Kudrna R. Lambda lysogens of E. coli reproduce more rapidly than non-lysogens. Nature. 1975 Jun 26;255(5511):735–737. doi: 10.1038/255735a0. [DOI] [PubMed] [Google Scholar]
- FISCHER-FANTUZZI L., CALEF E. A TYPE OF LAMBDA PROPHAGE UNABLE TO CONFER IMMUNITY. Virology. 1964 Jun;23:209–216. doi: 10.1016/0042-6822(64)90284-3. [DOI] [PubMed] [Google Scholar]
- George J., Castellazzi M., Buttin G. Prophage induction and cell division in E. coli. III. Mutations sfiA and sfiB restore division in tif and lon strains and permit the expression of mutator properties of tif. Mol Gen Genet. 1975 Oct 22;140(4):309–332. [PubMed] [Google Scholar]
- Golub E. I., Zwenigorodsky V. I. Defective thermal induction of a noninducible bacteriophage. Virology. 1969 Dec;39(4):919–921. doi: 10.1016/0042-6822(69)90027-0. [DOI] [PubMed] [Google Scholar]
- Gudas L. J., Pardee A. B. Model for regulation of Escherichia coli DNA repair functions. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2330–2334. doi: 10.1073/pnas.72.6.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliker S., Botstein D. Specificity of genetic elements controlling regulation of early functions in temperate bacteriophages. J Mol Biol. 1976 Sep 25;106(3):537–566. doi: 10.1016/0022-2836(76)90251-5. [DOI] [PubMed] [Google Scholar]
- Huebner R. J., Todaro G. J. Oncogenes of RNA tumor viruses as determinants of cancer. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1087–1094. doi: 10.1073/pnas.64.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James R. Identification of an outer membrane protein of Escherichia coli, with a role in the coordination of deoxyribonucleic acid replication and cell elongation. J Bacteriol. 1975 Nov;124(2):918–929. doi: 10.1128/jb.124.2.918-929.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAISER A. D., JACOB F. Recombination between related temperate bacteriophages and the genetic control of immunity and prophage localization. Virology. 1957 Dec;4(3):509–521. doi: 10.1016/0042-6822(57)90083-1. [DOI] [PubMed] [Google Scholar]
- Katzir N., Oppenheim A., Belfort M., Oppenheim A. B. Activation of the lambda int gene by the cii and ciii gene products. Virology. 1976 Oct 15;74(2):324–331. doi: 10.1016/0042-6822(76)90339-1. [DOI] [PubMed] [Google Scholar]
- Krizsanovich K. Cryptic lysogeny in Proteus mirabilis. J Gen Virol. 1973 Jun;19(3):311–320. doi: 10.1099/0022-1317-19-3-311. [DOI] [PubMed] [Google Scholar]
- Levine M., Truesdell S., Ramakrishnan T., Bronson M. J. Dual control of lysogeny by bacteriophage P22: an antirepressor locus and its controlling elements. J Mol Biol. 1975 Feb 5;91(4):421–438. doi: 10.1016/0022-2836(75)90270-3. [DOI] [PubMed] [Google Scholar]
- Levy J. A. Xenotropic type C viruses. Curr Top Microbiol Immunol. 1978;79:111–213. doi: 10.1007/978-3-642-66853-1_4. [DOI] [PubMed] [Google Scholar]
- Lin L., Bitner R., Edlin G. Increased reproductive fitness of Escherichia coli lambda lysogens. J Virol. 1977 Feb;21(2):554–559. doi: 10.1128/jvi.21.2.554-559.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H., Tazaki T. Genetic mapping of aro, pyr, and pur markers in Klebsiella pneumoniae. Jpn J Microbiol. 1971 Jan;15(1):11–20. [PubMed] [Google Scholar]
- Novick R. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology. 1967 Sep;33(1):155–166. doi: 10.1016/0042-6822(67)90105-5. [DOI] [PubMed] [Google Scholar]
- Reichardt L. F. Control of bacteriophage lambda repressor synthesis: regulation of the maintenance pathway of the cro and cI products. J Mol Biol. 1975 Apr 5;93(2):289–309. doi: 10.1016/0022-2836(75)90133-3. [DOI] [PubMed] [Google Scholar]
- Satta G., Canepari P., Fontana R., Calegari L. Envelope protein alterations in a conditional mutant of Klebsiella pneumoniae with pH dependent morphology and temperature dependent division. Ann Microbiol (Paris) 1974 Sep;125 B(2):259–273. [PubMed] [Google Scholar]
- Satta G., Fontana R. Cell division, macromolecular synthesis and morphology dependent on the state of the envelope in a mutant of Klebsiella pneumoniae. J Gen Microbiol. 1974 Jan;80(1):65–75. doi: 10.1099/00221287-80-1-65. [DOI] [PubMed] [Google Scholar]
- Satta G., Fontana R. Characterization of a conditional mutant with altered envelope showing pH-dependent morphology and temperature-dependent division. J Gen Microbiol. 1974 Jan;80(1):51–63. doi: 10.1099/00221287-80-1-51. [DOI] [PubMed] [Google Scholar]
- Satta G., Pardee A. B. Inhibition of Escherichia coli division by protein X. J Bacteriol. 1978 Mar;133(3):1492–1500. doi: 10.1128/jb.133.3.1492-1500.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satta G., Pruzzo C., Debbia E., Calegari L. Lysogenic conversion in Klebsiella pneumoniae: system which requires active immunity regulation for expression of the conversion phenomenon. J Virol. 1978 Dec;28(3):786–794. doi: 10.1128/jvi.28.3.786-794.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. R. A defective P1 prophage with a chromosomal location. Virology. 1970 Jan;40(1):144–151. doi: 10.1016/0042-6822(70)90386-7. [DOI] [PubMed] [Google Scholar]
- Temin H. M. Mechanism of cell transformation by RNA tumor viruses. Annu Rev Microbiol. 1971;25:609–648. doi: 10.1146/annurev.mi.25.100171.003141. [DOI] [PubMed] [Google Scholar]
- Thomson J. A., Woods D. R. Bacteriophages and cryptic lysogeny in Achromobacter. J Gen Virol. 1974 Jan;22(1):153–157. doi: 10.1099/0022-1317-22-1-153. [DOI] [PubMed] [Google Scholar]
- Truitt C. L., Haldenwang W. G., Walker J. R. Interaction of host and viral regulatory mechanisms: effect of the ion cell division defect on regulation of repression by bacteriophage lambda. J Mol Biol. 1976 Aug 5;105(2):231–244. doi: 10.1016/0022-2836(76)90109-1. [DOI] [PubMed] [Google Scholar]


