Abstract
Objective
In nonpregnant populations the waist to hip ratio (WHR) is a better predictor of obesity related outcomes than BMI. Our objective was to determine, in pregnancy, the relationship between these measures of obesity and LGA and cesarean delivery (CD).
Methods
This is a secondary analysis of data from the Combined Antioxidant and Preeclampsia Prediction Study (CAPPS). Women a WHR of ≥0.85 and 0.80–0.84 at 9–16 weeks gestation, were compared to those with a WHR <0.80. Women with early pregnancy BMI ≥ 30.0 kg/m2 (obese) and 25.0–29.9 kg/m2 (overweight) were compared to those <25.0 kg/m2. LGA was defined as >90% by Alexander nomogram. Univariable analysis, logistic regression and ROC curves were used.
Results
Data from 2,276 women were analyzed. After correcting for potential confounders, only BMI ≥ 30 was significantly associated with LGA (aOR 2.07, 1.35–3.16) while BMI 25.0–29.9 (aOR 1.5, 0.98–2.28), WHR 0.8–0.84 (aOR 1.33, 0.83–2.13) and WHR≥0.85, (aOR 1.05, 0.67–1.65) were not. Risk for CD was increased for women with elevated WHR and with higher BMI compared to normal.
Conclusion
WHR is not associated with LGA. While BMI performed better than WHR, neither was a strong predictor of LGA or need for CD in low risk nulliparous women.
Introduction
Obesity is now considered to have reached epidemic proportions. During pregnancy, obesity has been associated with increased maternal and neonatal morbidity which includes an increased risk for large for gestational age infants (LGA).1 LGA fetuses are more likely to experience stillbirth, shoulder dystocia and admission to the neonatal intensive care unit compared to normal weight fetuses.2,3 Multiple prior studies have linked LGA to increasing maternal body mass index (BMI).4–8 A study of singleton births found the strongest modifiable predictor of LGA to be prepregnancy weight.9 The outcome is highly multifactorial as other studies link parity, gender, race, glucose intolerance, diabetes, and maternal weight gain to LGA.10–12
BMI is an estimate of body fatness used as a screening tool for obesity and predictor of obesity related disorders.13 The correlation of BMI and body fatness varies with age, race, gender and athletic ability and does not have the ability to differentiate fatness as central or visceral. Alternatively, as a screening tool, the waist to hip ratio (WHR) has been shown to be more reflective of visceral fat and central adiposity, as well as a better predictor of obesity related disorders, such as stroke, myocardial infarction, or cardiovascular death, than BMI.14–19 This anthropometric index has not been as widely studied in relation to pregnancy outcomes, but in a prospective cohort of pregnancy, both increased WHR and BMI were associated with an increased risk of preeclampsia.20 Central adiposity and LGA infants are both associated with elevated triglycerides, LDL cholesterol, free fatty acids, fasting glucose and insulin, suggesting fat distribution may affect fetal growth independent of fat mass.21–23 In addition, central adiposity is associated with variations in the concentrations of specific sex hormones which may play a role in the maternal regulation of fetal growth.24 The WHR is largely unaffected until 20 weeks of gestation.21,24 A measurement in early pregnancy would be a reliable indicator of central adiposity.
Our objective was to determine the relationship between WHR in early pregnancy and LGA and CD, as well as compare the predictive value of this measure of obesity to BMI in a population of low risk nulliparous women.
Methods
This is a secondary analysis of the Combined Antioxidant and Preeclampsia Prediction Study (CAPPS), a multicenter randomized control trial of vitamin C and E to prevent preeclampsia which enrolled 9,969 nulliparous women prior to 16 weeks gestation.25 For the secondary analysis inclusion criteria included women with data available to determine WHR and BMI at the time of enrollment. Exclusion criteria included congenital anomalies and intrauterine fetal demise. Intervention and control groups were combined into one cohort for analysis. WHR was evaluated as a categorical variable, comparing women with a ratios of ≥0.85, 0.80–0.84, and <0.80 representing high, moderate and low risk respectively for adverse outcomes.16 Likewise, BMI was also evaluated categorically, comparing ≥ 30 kg/m2 (obese) and 25–29 kg/m2 (overweight) to <25 kg/m2. Outcomes were also measured using both WHR and BMI as continuous variables.
The primary outcome was LGA as defined by birth weight >90% by the Alexander nomogram.26 Secondary outcomes included overall need for cesarean delivery (CD) and need for CD due to cephalopelvic disproportion. Univariable analysis was performed followed by multivariable logistic regression adjusting for maternal age, gestational age at enrollment, years of schooling, race, alcohol, and smoking status. The Breslow-Day test for homogeneity was used to determine if there was a difference in the association of WHR and BMI with LGA and CD by race/ethnicity. Receiver operator curves (ROC) were calculated to determine the relationship between both WHR and BMI with the incidence of LGA and need for CD. Categorical variables were analyzed using the chi square test or Fisher’s exact test. Continuous variables were analyzed using the Wilcoxon rank sum test or the Kruskal-Wallis test. Statistical analysis was conducted with SAS software (SAS Institute, Cary, NC). A nominal two-sided P value less than 0.05 was considered to indicate statistical significance and no adjustments were made for multiple comparisons.
Results
Of the 9,969 women analyzed in the CAPPS trial, 2,276 met inclusion criteria and had data available for calculation of WHR and BMI at < 16 weeks gestation. Group demographic information is displayed in Table 1.
Table 1.
Group demographics by WHR and BMI
| WHR <0.8 n=639 |
WHR 0.8–<0.85 n=588 |
WHR ≥ 0.85 n=1049 |
P value | BMI <25.0 kg/m2 n=1184 |
BMI 25.0 – 29.9 kg/m2 n=597 |
BMI ≥ 30.0 kg/m2 n=495 |
P value | |
|---|---|---|---|---|---|---|---|---|
| Age | 23.5 ± 4.9 | 23.8 ± 4.8 | 23.1 ± 4.7 | 0.01 | 23.5 ± 4.8 | 23.2 ± 4.8 | 23.2 ± 4.6 | 0.17 |
| Gestational age at enrollment | 11.4 ± 1.1 | 11.5 ± 1.1 | 11.4 ± 1.1 | 0.30 | 11.5 ± 1.1 | 11.4 ± 1.1 | 11.3 ± 1.1 | 0.004 |
| Years of schooling | 13.6 ± 2.2 | 13.5 ± 2.3 | 12.3 ± 2.7 | <0.001 | 13.2 ± 2.7 | 12.8 ± 2.7 | 12.7 ± 2.2 | 0.001 |
| Race | <0.001 | <0.001 | ||||||
| Hispanic | 60 (9.4%) | 106 (18.0%) | 416 (39.7%) | 300 (25.3%) | 181 (30.3%) | 101 (20.4%) | ||
| AA | 213 (33.3%) | 125 (21.3%) | 207 (19.7%) | 212 (17.9%) | 138 (23.1%) | 195 (39.4%) | ||
| White | 354 (55.4%) | 342 (58.2%) | 408 (38.9%) | 644 (54.4%) | 268 (44.9%) | 192 (38.8%) | ||
| Other | 12 (1.9%) | 15 (2.6%) | 18 (1.7%) | 28 (2.4%) | 10 (1.7%) | 7 (1.4%) | ||
| Alcohol use | 76 (11.9%) | 66 (11.2%) | 102 (9.7%) | 0.34 | 129 (10.9%) | 54 (9.1%) | 61 (12.3%) | 0.21 |
| Smoking | 90 (14.1%) | 97 (16.5%) | 188 (17.9%) | 0.12 | 170 (14.4%) | 105 (17.6%) | 100 (20.2%) | 0.009 |
On initial univariate analysis, WHR was not significantly associated with LGA. BMI was associated with LGA. Both increased WHR and BMI were associated with an increased need for CD.
Results of the multivariable regression are displayed in Table 2. BMI ≥ 30 remained significantly associated with LGA, but WHR was not. Both categories of elevated WHR and BMI remained predictive of CD. There were no significant interactions between race/ethnicity and WHR or BMI for these outcomes (data not shown).
Table 2.
Rates and Adjusted Odd Ratios (aOR) for LGA and Cesarean Delivery by WHR and BMI Categories
| WHR | No./Total (%) | aOR (95% CI) | BMI (kg/m2) | No./Total (%) | aOR (95% CI) |
|---|---|---|---|---|---|
| LGA | |||||
| <0.80 | 35/639 (5.5) | Referent | <25.0 | 54/1182 (4.6) | Referent |
| 0.80–84 | 42/587 (7.2) | 1.33 (0.83, 2.13) | 25.0–29.9 | 41/597 (6.9) | 1.50 (0.98, 2.28) |
| ≥0.85 | 64/1048 (6.1) | 1.05 (0.67, 1.65) | ≥30.0 | 46/495 (9.3) | 2.07 (1.35, 3.16) |
| CD | |||||
| <0.80 | 116/639 (18.2) | Referent | <25.0 | 225/1184 (19.0) | Referent |
| 0.80–84 | 142/588 (24.1) | 1.43 (1.08, 1.89) | 25.0–29.9 | 153/597 (25.6) | 1.45 (1.14, 1.84) |
| ≥0.85 | 303/1049 (28.9) | 1.74 (1.35, 2.25) | ≥30.0 | 183/495 (37.0) | 2.52 (1.98, 3.21) |
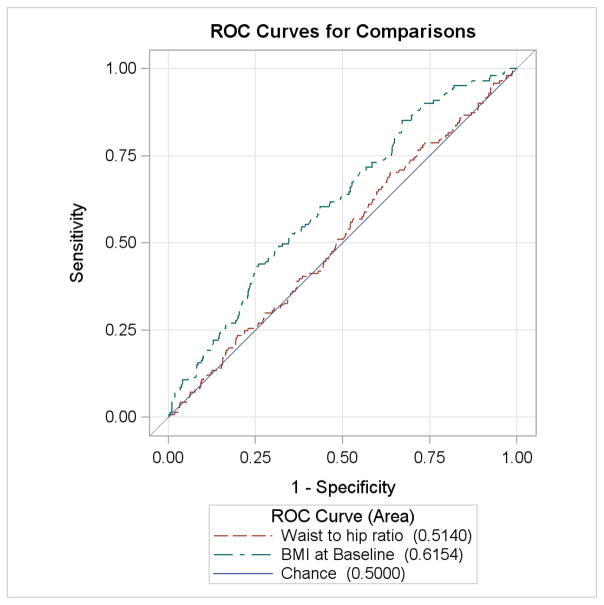
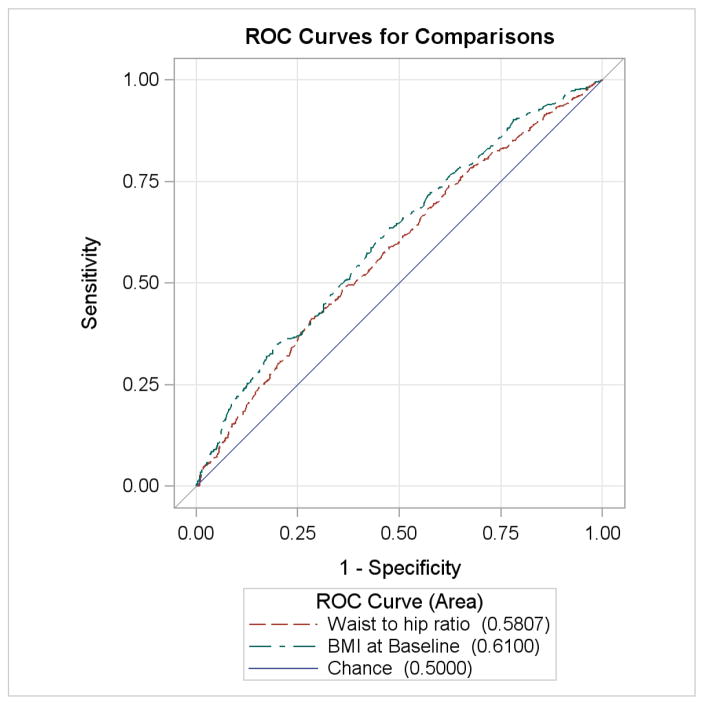
ROC curves for the risk of LGA and CD are displayed in Figures 1 and 2. WHR performed no better than chance alone in prediction of LGA (AUC 0.514, p=0.5724) while increased BMI was slightly more predictive of LGA (AUC 0.615, p<0.001). Both variables were similarly associated with CD and both significantly more predictive than chance alone (WHR AUC 0.58, p<0.0001; BMI AUC 0.61, p<0.0001). These curves were similar when only CD performed for cephalopelvic disproportion was analyzed.
Figure 1.
ROC curve of LGA
Figure 2.
ROC curve of CD
Data were then further analyzed by comparing outcomes from women meeting both criteria of WHR ≥ 0.85 and BMI ≥ 30 kg/m2 to those meeting only one criteria or neither criterion. On univariate analysis, there was a significant difference between groups for both prediction of LGA and CD (Table 3). After multivariable regression, the OR of LGA for women with both WHR ≥ 0.85 and BMI ≥ 30 kg/m2 was no longer significant as compared to nonobese women (OR 1.40, 95% CI 0.86–2.29), but the risk of CD was significantly elevated (OR 2.48, 95% CI 1.88–3.28).
Table 3.
Rates for LGA and Cesarean Delivery by One or Both Obesity Categories
| Not Obese | BMI ≥ 30 kg/m2 | WHR ≥ 0.85 | Both Obesity Categories | P value | |
|---|---|---|---|---|---|
| LGA No./Total (%) | 58/1061 (5.5) | 19/165 (11.5) | 37/718 (5.2) | 27/330 (8.2) | 0.006 |
| CD No./Total (%) | 200/1062 (18.8) | 58/165 (35.2) | 178/719 (24.8) | 125/330 (37.9) | <0.001 |
Discussion
In this cohort of low risk nulliparous women, we found no association between increased WHR and the incidence of LGA, while maternal obesity (BMI ≥ 30) in early pregnancy was predictive of development of LGA. Both measurements were similarly predictive of the need for CD.
Multiple prior studies have found similar results in regards to the relationship between BMI and LGA or macrosomia.4–7 A prospective cohort study in Sweden found women with a BMI of 35.1–40 and a BMI of >40 have an adjusted odds ratio of 3.11 and 3.82 respectively for having an LGA infant.10 These findings are supported by other studies noting increased risks for LGA infants when mothers were obese (OR ranges 2.32–3.1).7,8
Three previous studies have evaluated the relationship between WHR and infant size. Brown et al prospectively followed 521 low risk, primarily Caucasian women with singleton pregnancies who had WHR measured in early pregnancy. They found that the birthweight increased by 120 gm for each 0.1 unit increase in WHR, but did not specifically address birthweight percentiles.24 A separate study of 700 Caucasian women, found central obesity, as defined by WHR of >0.9, to have a 1.81-fold increased risk of LGA.27 Salem et al, in a cohort study of nulliparous women in the United Kingdom, noted a WHR in the third and fourth quartiles of their cohort was associated with macrosomia, as defined by birthweight ≥4000g, or LGA, as defined by birthweight ≥95% of the cohort.21 The median values of the third and fourth quartile for WHR were 0.75 and 0.81 respectively, lower than in our US-based study. A further study evaluating the association of waist circumference with macrosomia in Brazilian women found an AUC for the ROC curve to be 0.645, similar to the predictive value of BMI, which had an AUC of 0.632.28 The variations in study design and differences in patient population may explain these differences. In their study, Brown et al initially controlled for gestational diabetes, but found that the variable did not contribute to outcomes and subsequently dropped it from further analysis.24 Bo et al also controlled for the presence of gestational diabetes in their study and found the increased rate of LGA to be independent of its presence.27 As the predictive value of elevated WHR was being evaluated in our study, the incidence of GDM and gestational weight gain were not included in our multivariable analysis, given these variables lie in the causative pathway for the studied outcomes. In addition, obesity itself has been shown to be independently associated with infant size.5,29
Central adiposity is associated with the presence of visceral adipose tissue, which is associated with multiple abnormal markers of metabolism including insulin resistance, dyslipidemia, and hypertension.17 In turn, these markers have been shown to be predictive of fetal size. While an excess of glucose supply may cause fetal hyperinsulinemia and lead to macrosomia, other abnormal metabolic factors may also contribute to fetal growth.29,30 Studies of women with positive diabetic screens but normal glucose tolerance tests found fasting maternal hypertriglyceridemia in the third trimester to be predictive of LGA infants independent of prepregnancy BMI.23,30 Other studies have found an association between maternal free fatty acid concentrations or amino acids with fetal weight.23,29 These abnormalities are hypothesized to lead to increased fetal growth as the hydrolysis of maternal triglycerides by placental lipoprotein lipase leads to increased free fatty acids crossing the placenta.23 We did not find the hypothesized association between central adiposity and fetal size, but this is possibly due to the low risk nature of the cohort.
Our finding that the rate of CD increases with BMI is consistent with prior studies.4,5,7,8,31 In cohort studies focused on singleton pregnancies, odds ratios for CD of 1.35 for overweight women to 2.92 for morbidly obese women (BMI>40) were found compared to women with normal BMI.7,8,10 This outcome was not reported by Brown, and Salem found no difference in the rate of spontaneous birth between quartiles, but used a lower cutoff value for WHR.21,24 In their cohort, Bo et al found women with a WHR of >0.9 had a 1.51-fold increased risk of CD, similar to our results.27 Although we did not find an increased incidence of LGA associated with increased WHR, the abnormal markers of metabolism associated with both central adiposity and abnormal fetal growth may affect other measures of newborn size which may then contribute to cephalopelvic disproportion. For example, Brown et al found a 0.3 cm increase in head circumference for every 0.1 increase in WHR.24 Further study would be required to determine if other neonatal anthropometric measurements are affected by maternal central adiposity.
Weaknesses of this study include the fact that it is a secondary analysis and therefore data is limited by availability from the primary study. We did not have information available for other risks associated with LGA, such as shoulder dystocia, obstetrical laceration, or neonatal hypoglycemia. Strengths included that the study population is a large, heterogenous group from multiple institutions across the United States, and therefore applicable to wider populations than prior studies. The fact that only nulliparous patients were included eliminates further bias as WHR increases with parity.
In conclusion, in a pregnant heterogenous low risk cohort, BMI but not WHR is predictive of LGA. Both measurements are similarly predictive of the need for CD, and further study is required to determine if this is due to an alteration in neonatal growth or other factors related to obesity.
Acknowledgments
The project described was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) [HD34208, HD27869, HD40485, HD40560, HD40544, HD34116, HD40512, HD21410, HD40545, HD40500, HD27915, HD34136, HD27860, HD53118, HD53097, HD27917, and HD36801]; the National Heart, Lung, and Blood Institute; and the National Center for Research Resources [M01 RR00080, UL1 RR024153, UL1 RR024989] and its contents do not necessarily represent the official views of NICHD, NHLBI, NCRR or NIH.
The authors thank the following Network members who participated in protocol development and coordination between clinical research centers (Sabine Bousleiman, R.N.C., M.S.N. and Margaret Cotroneo, R.N.), protocol/data management and statistical analysis (Elizabeth Thom, Ph.D. and Rebecca Clifton, Ph.D.), and protocol development and oversight (George Saade, M..D., Gail D. Pearson, M.D., Sc.D. and Catherine Y. Spong M.D.).
In addition to the authors, other members of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network are as follows:
University of Texas Medical Branch, Galveston, TX – G. Saade, J. Moss, B. Stratton, G. Hankins, J. Brandon, C. Nelson-Becker, G. Olson, L. Pacheco
University of Pittsburgh, Pittsburgh, PA – J. Roberts, S. Caritis, T. Kamon (deceased), M. Cotroneo, D. Fischer
University of Utah, Salt Lake City, UT – M. Varner, P. Reed, S. Quinn (LDS Hospital), V. Morby (McKay-Dee Hospital), F. Porter (LDS Hospital), R. Silver, J. Miller (Utah Valley Regional Medical Center), K. Hill
University of Alabama at Birmingham, Birmingham, AL – D.J. Rouse, A. Northen, P. Files, J. Grant, M. Wallace, K. Bailey
Columbia University, New York, NY – R. Wapner, S. Bousleiman, R. Alcon, K. Saravia, F. Loffredo, A. Bayless (Christiana), C. Perez (St. Peter’s University Hospital), M. Lake (St. Peter’s University Hospital), M. Talucci
University of North Carolina at Chapel Hill, Chapel Hill, NC – J. Thorp, K. Boggess, K. Dorman, J. Mitchell, K. Clark, S. Timlin
Case Western Reserve University-MetroHealth Medical Center, Cleveland, OH – J. Bailit, C. Milluzzi, W. Dalton, C. Brezine, D. Bazzo
University of Texas Southwestern Medical Center, Dallas, TX – J. Sheffield, L. Moseley, M. Santillan, K. Buentipo, J. Price, L. S. Hermann, C. Melton, Y. Gloria-McCutchen, B. Espino
Northwestern University, Chicago, IL – A. Peaceman, M. Dinsmoor (NorthShore University HealthSystem), T. Matson-Manning, G. Mallett
University of Texas Health Science Center at Houston-Children’s Memorial Hermann Hospital, Houston, TX – S. Ramin, S. Blackwell, K. Cannon, S. Lege-Humbert, Z. Spears
Brown University, Providence, RI – M. Carpenter, J. Tillinghast, M. Seebeck
The Ohio State University, Columbus, OH – P. Samuels, J. Iams, F. Johnson, S. Fyffe, C. Latimer, S. Frantz, S. Wylie
Drexel University, Philadelphia, PA – A. Sciscione, M. Talucci, M. Hoffman (Christiana), J. Benson (Christiana), Z. Reid, C. Tocci
Wake Forest University Health Sciences, Winston-Salem, NC – M. Harper, P. Meis, M. Swain
Oregon Health & Science University, Portland, OR – J. Tolosa, W. Smith, L. Davis, E. Lairson, S. Butcher, S. Maxwell, D. Fisher
Wayne State University, Detroit, MI – Y. Sorokin, G. Norman, S. Blackwell, P. Lockhart, D. Driscoll, M. Dombrowski
The George Washington University Biostatistics Center, Washington, DC – E. Thom, R. Clifton, T. Boekhoudt, L. Leuchtenburg
National Heart, Lung, and Blood Institute, Bethesda, MD – G. Pearson, V. Pemberton, J. Cutler, W. Barouch
Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD – C. Spong, S. Tolivaisa
Footnotes
Presented in Poster Format at The Society of Gynecologic Investigation Annual Meeting, Orlando, FL, March 2013
Literature Cited
- 1.Lu G, Rouse D, DuBard M, Cliver S, Kimberlin D, Hauth J. The effect of the increasing prevalence of maternal obesity on perinatal mortality. Am J Obstet Gynecol. 2001;184:845–9. doi: 10.1067/mob.2001.117351. [DOI] [PubMed] [Google Scholar]
- 2.Ju H, Chadha Y, Donovan T, O’Rourke P. Fetal Macrosomia and pregnancy outcomes. Aust N Z J Obstet Gynaecol. 2009;49:504–9. doi: 10.1111/j.1479-828X.2009.01052.x. [DOI] [PubMed] [Google Scholar]
- 3.Jolly M, Sebire N, Harris J, Regan L, Robinson S. Risk factors for macrosomia and its clinical consequences: a study of 350,311 pregnancies. Eur J Obstet Gynecol Reprod Biol. 2003;111:9–14. doi: 10.1016/s0301-2115(03)00154-4. [DOI] [PubMed] [Google Scholar]
- 4.Marshall N, Guild C, Cheng Y, Caughey A, Halloran D. Maternal superobesity and perinatal outcomes. Am J Obstet Gynecol. 2012;206:417e1–6. doi: 10.1016/j.ajog.2012.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dennedy M, Avalos G, O’Reilly M, O’Sullivan E, Gaffney G, Dunne F. ATLANTIC-DIP: Raised maternal body mass index (BMI) adversely affects maternal and fetal outcomes in glucose tolerant women according to international association of diabetes and pregnancy study groups (IADPSG) criteria. J Clin Endocrinol Metab. 2012 Apr;97(4):E608–12. doi: 10.1210/jc.2011-2674. [DOI] [PubMed] [Google Scholar]
- 6.Bhattacharya S, Campbell E, Liston W, Bhattacharya S. Effect of body mass index on pregnancy outcomes in nulliparous women delivering singleton babies. BMC Public Health. 2007 Jul 24;7:168. doi: 10.1186/1471-2458-7-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abenhaim H, Kinch R, Morin L, Benjamin A, Usher R. Effect of prepregnancy body mass index categories on obstetrical and neonatal outcomes. Arch Gynecol Obstet. 2007 Jan;275(1):39–43. doi: 10.1007/s00404-006-0219-y. [DOI] [PubMed] [Google Scholar]
- 8.Khashan A, Kenny L. The effects of maternal body mass index on pregnancy outcome. Eur J Epidemiol. 2009;24(11):697–705. doi: 10.1007/s10654-009-9375-2. [DOI] [PubMed] [Google Scholar]
- 9.Jaipaul J, Newburn-Cook C, O’Brien B, Demianczuk N. Modifiable risk factors for term large for gestational age births. Health Care Women Int. 2009 Sep;30(9):802–23. doi: 10.1080/07399330903066160. [DOI] [PubMed] [Google Scholar]
- 10.Cedergren M. Maternal morbid obesity and the risk of adverse pregnancy outcome. Obstet Gynecol. 2004;103:219–24. doi: 10.1097/01.AOG.0000107291.46159.00. [DOI] [PubMed] [Google Scholar]
- 11.Ehrenberg H, Mercer B, Catalano P. The influence of obesity and diabetes on the prevalence of macrosomia. Am J Obstet Gynecol. 2004;191:964–8. doi: 10.1016/j.ajog.2004.05.052. [DOI] [PubMed] [Google Scholar]
- 12.Rode L, Hegaard H, Kjoergaard H, Moller L, Tabor A, Ottesen B. Association between maternal weight gain and birth weight. Obstet Gynecol. 2007;109:1309–15. doi: 10.1097/01.AOG.0000266556.69952.de. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevension. Healthy Weight. 2012 Retrieved from http://www.cdc.gov/healthyweight/assessing/bmi/adult_bmi/index.html.
- 14.Qiao Q, Nyamdorj R. Is the association of type II diabetes with waist circumference or waist to hip ratio stronger than that with body mass index? European Journal of Clinical Nutrition. 2010;64:30–34. doi: 10.1038/ejcn.2009.93. [DOI] [PubMed] [Google Scholar]
- 15.Dalton M, Cameron A, Zimmet P, Shaw J, Jolley D, Dunstan D, Welborn T. Waist circumference, waist-hip ratio and body mass index and their correlation with cardiovascular disease risk factors in Australian adults. Journal of Internal Medicine. 2003;254:555–563. doi: 10.1111/j.1365-2796.2003.01229.x. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. Waist Circumference and Waist to Hip Ratio: Report of a WHO Expert Consultation. Geneva: World Health Organization; Dec, 2008. [Google Scholar]
- 17.de Koning L, Merchant A, Pogue J, Anand S. Waist circumference and waist to hip ratio as predictors of cardiovascular events: meta-regression analysis of prospective studies. Eur Heart J. 2007 Apr;28(7):850–6. doi: 10.1093/eurheartj/ehm026. [DOI] [PubMed] [Google Scholar]
- 18.Schneider H, Friedrich N, Klotsche J. The predictive value of different measures of obesity for incident cardiovascular events and mortality. J Clin Endocrinol Metab. 2010 Apr;95(4):1777–85. doi: 10.1210/jc.2009-1584. [DOI] [PubMed] [Google Scholar]
- 19.Welborn T, Dhaliwal S, Bennet S. Waist-hip ratio is the dominant risk factor predicting cardiovascular death in Australia. Med J Aust. 2003 Dec 1–15;179(11–12):580–5. doi: 10.5694/j.1326-5377.2003.tb05704.x. [DOI] [PubMed] [Google Scholar]
- 20.Taebi M, Sadat Z, Saberi F, Kalahroudi M. Early pregnancy waist-to-hip ratio and risk of preeclampsia: a prospective cohort study. Hypertens Res. 2015 Jan;38(1):80–3. doi: 10.1038/hr.2014.133. [DOI] [PubMed] [Google Scholar]
- 21.Salem W, Adler A, Smith G. Maternal waist to hip ratio is a risk factor for macrosomia. BJOG. 2012;119:291–297. doi: 10.1111/j.1471-0528.2011.03167.x. [DOI] [PubMed] [Google Scholar]
- 22.Clausen T, Burski T, Oyen N, Godang K, Bollerslev J, Henriksen T. Maternal anthropometric and metabolic factors in the first half of pregnancy and risk of neonatal macrosomia in term pregnancies. A prospective study. Eur J Endocrinol. 2005;153:887–94. doi: 10.1530/eje.1.02034. [DOI] [PubMed] [Google Scholar]
- 23.Kitajima M, Oka S, Yasuhi I, Fukuda M, Rii Y, Ishimaru T. Maternal serum triglyceride at 24–32 weeks gestation and newborn weight in nondiabetic women with positive diabetic screens. Obstet Gynecol. 2001;97:776–80. doi: 10.1016/s0029-7844(01)01328-x. [DOI] [PubMed] [Google Scholar]
- 24.Brown J, Potter J, Jacobs D, Kopher R, Rourke M, Barosso G, et al. Maternal waist to hip ratio as a predictor of newborn size: results of the Diana project. Epidemiology. 1996;7:62–6. doi: 10.1097/00001648-199601000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Roberts J, Myatt L, Spong C, et al. Vitamins C and E to prevent complications of pregnancy-associated hypertension. N Engl J Med. 2010 Apr 8;362(14):282–91. doi: 10.1056/NEJMoa0908056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87(2):163–8. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 27.Bo S, Menato G, Signorile A, et al. Obesity or diabetes: what is worse for the mother and for the baby? Diabetes Metab. 2003 Apr;29(2 Pt 1):175–8. doi: 10.1016/s1262-3636(07)70026-5. [DOI] [PubMed] [Google Scholar]
- 28.Wendland E, Duncan B, Mengue S, Nucci L, Schmidt M. Waist circumference in the prediction of obesity-related adverse pregnancy outcomes. Cad Saude Publica. 2007 Feb;23(2):391–8. doi: 10.1590/s0102-311x2007000200015. [DOI] [PubMed] [Google Scholar]
- 29.Catalano P, McIntyre H, Cruickshank J. The hyperglycemia and adverse pregnancy outcome study: Associations of GDM and obesity with pregnancy outcomes. Diabetes Care. 2012 Apr;35(4):780–6. doi: 10.2337/dc11-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Cianni G, Miccoli R, Volpe L, et al. Maternal triglyceride levels and newborn weight in pregnant women with normal glucose tolerance. Diabet Med. 2005 Jan;22(1):21–5. doi: 10.1111/j.1464-5491.2004.01336.x. [DOI] [PubMed] [Google Scholar]
- 31.Chung J, Melsop K, Gilbert W, Caughey A, Walker C, Main E. Increasing pre-pregnancy body mass index is predictive of a progressive escalation in adverse pregnancy outcomes. J Matern Fetal Neonatal Med. 2012;25(9):1635–1639. doi: 10.3109/14767058.2011.648970. [DOI] [PubMed] [Google Scholar]