Abstract
Background It has been over 100 years since the initial description of avascular necrosis of the lunate. Over the last two decades, there has been the introduction of advanced information regarding the etiology, natural history, classification, and treatment options for lunate osteonecrosis. There have been new classifications developed based on advanced imaging, perfusion studies of lunate viability, and arthroscopic assessment of the articular cartilage.
Purpose This article brings together a new treatment algorithm, incorporating the traditional osseous classification system (Lichtman) with the perfusion/viability classification (Schmitt) and the articular cartilage classification (Bain).
Methods We have developed a new algorithm to manage Kienböck avascular necrosis of the lunate. This new algorithm incorporates the current concepts of the diseased lunate and its effects on the remainder of the wrist.
Conclusion For patients with a good prognosis and in the earliest stages, the “intact lunate” is initially protected utilizing nonoperative measures. If this fails, then appropriate lunate unloading procedures should be considered. If the lunate is “compromised” then it can be reconstructed with a medial femoral condyle graft or proximal row carpectomy (PRC). With the further collapse of the lunate, the wrist is then also compromised, with the development of secondary degeneration of the central column articulation. The “compromised wrist” will have functional articulations, which allows motion-preserving procedures to be utilized to maintain a functional wrist. With advanced disease (Kienböck disease advanced collapse), the wrist is not reconstructable, so only a salvage procedure can be performed. Other than these objective pathoanatomical factors, the final decision must accommodate the various patient factors (e.g., age, general health, lifestyle, financial constraints, and future demands on the wrist) and surgeon factors (skill set, equipment, and work environment).
Keywords: Kienböck, lunate, osteonecrosis, avascular, classification
The knowledge of Kienböck disease has evolved over more than 100 years since Robert Kienböck published his article on osteomalacia of the lunate in 1910.1 The purpose of this article is to amalgamate much of this information, particularly the newer advances in diagnosis and treatment, into a practical, yet highly granular algorithm for the management of this disorder. The natural history of Kienböck disease has been reported in terms of the progression of clinical findings and serial changes on standard X-rays.2 3 4 5 The Lichtman classification (1977)6 identifies these changes. The clinical presentation of pediatric Kienböck disease is similar to the adults, with dorsal tenderness, swelling, reduced wrist motion, and grip strength.5 7 8 However, the prognosis is better in the pediatric and elderly patients, than the typical 20- to 40-year-old patient.7
Irisarri et al9 divided pediatric Kienböck disease into infantile (12 years and younger) and juvenile (13 years to skeletal maturity). All patients in the infantile group were treated nonoperatively, with excellent outcomes, including lunate revascularization on magnetic resonance imaging (MRI). The juvenile group was also treated with immobilization, however, 30% had progression and required a joint leveling procedure. Irisarri et al recommended immobilization for patients under 15 years, and joint leveling procedures in the older patient, if there was a disease progression despite immobilization.9
In the elderly patient, Kienböck disease behaves differently in comparison to the pediatric and adult cohorts.10 11 12 13 14 In Kienböck patients older than 70 years, there is less negative ulnar variance, a higher prevalence in women, who had more advanced radiological changes.11 At a mean follow-up of 5.6 years, all 15 patients progressed to stage IV disease but had good-to-excellent clinical outcomes without surgery.
Clinical Assessment
Plain Radiographs
Plain radiographs have been used to diagnose and follow the progression of Kienböck disease, using the osseous Lichtman classification for almost 40 years (Fig. 1).6 It has evolved with time, and now includes stages 0 and IIIC.7 15 16
Fig. 1.
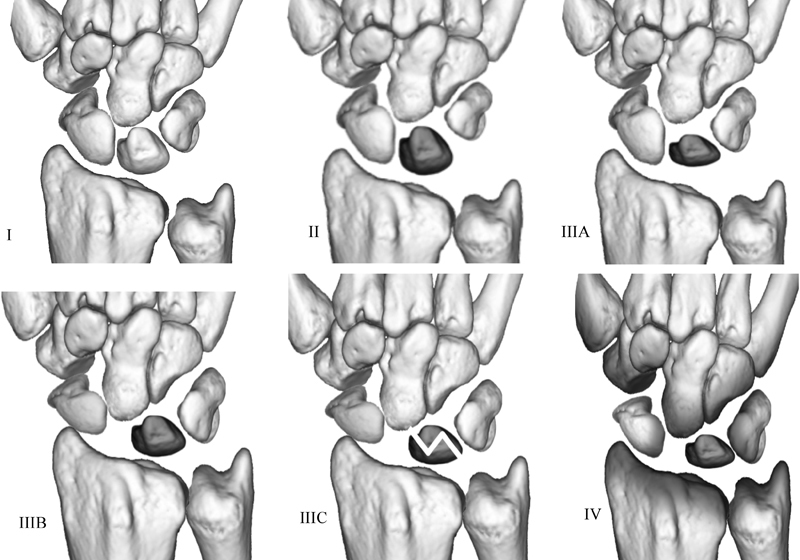
The stages of the Lichtman osseous classification of Kienbock disease.
Magnetic Resonance Imaging
Recently, both T1-weighted fast spin-echo (FSE) and T2-weighted FSE fat-saturated MR images have been used to evaluate viability of bone marrow.17
T1-weighted FSE: Normal marrow will have a high signal with T1 due to the fat in the marrow. The T1 signal will be of low intensity if the fat is replaced with edema, neovascularization, space-occupying lesions, bone necrosis, or sequestrum.
T2-weighted FSE: Normal marrow will have low signal T2, due to its low water content. Ischemic marrow is edematous, so has a high T2 signal. Necrotic marrow has a low signal as it has low water content.
Schmitt et al demonstrated how gadolinium perfusion enhanced T1-weighted FSE fat-saturated sequences so that they are now seen as a high signal.17 This distinguished low-signal edema from the enhanced high signal of neovascular repair tissue. He identified three zones or patterns of enhancement of the necrotic lunate: The proximal necrotic lunate without enhancement, intermediate hypervascular repair zone, and normal distal lunate. The hyperenhancement reparative zone indicates a good-healing prognosis, and low signal indicates a poor prognosis due to the nonviable marrow.
Schmitt et al18 classified the lunate signal changes after administration of intravenous gadolinium contrast (Fig. 2).
Fig. 2.
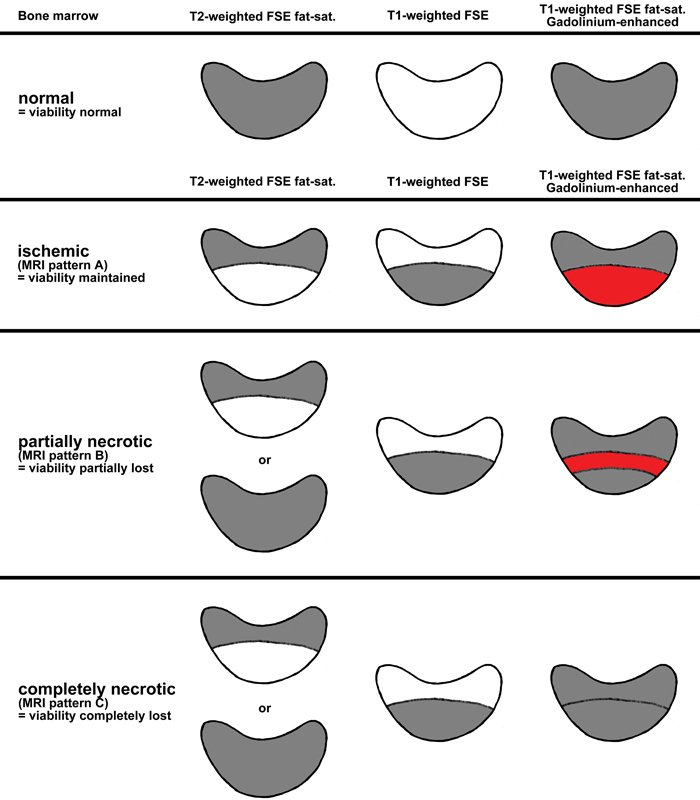
Schmitt schematic diagram of bone-marrow viability in Gadolinium-enhanced MRI normal marrow (viability unaffected). MRI, magnetic resonance imaging.
MRI stage N—Normal: Normal lunate signal with no enhancement.
MRI stage A—Ischemic (viability maintained): Proximal lunate is edematous but well perfused (enhancement with gadolinium).
MRI stage B—Partially necrotic (viability partially lost): Proximal necrotic lunate (no enhancement), with an adjacent reparative zone with enhancement, and viable distal lunate.
MRI stage C—Completely necrotic: Total necrosis, no enhancement corresponding to complete lunate osteonecrosis.
Arthroscopic Assessment of Kienböck Disease
In 2006, Bain et al19 reported an arthroscopic assessment and classification of Kienböck disease, which is based on the number of nonfunctional articular surfaces of the lunate (Fig. 3).19 20 A functional articular surface has a normal smooth arthroscopic appearance and is firm to palpation without significant softening.
Fig. 3.
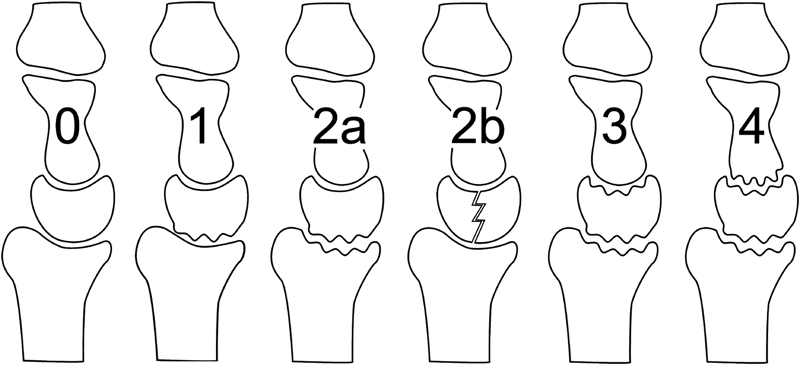
Articular-based approach to Kienböck disease. The Bain/Begg arthroscopic classification system is derived from the number and location of nonfunctional articular-based approach.
A nonfunctional articular surface has at least one of the following: extensive fibrillation, fissuring, localized, or extensive loss, a floating articular surface or fracture. Synovitis was identified in all of our cases; therefore, it was not used for grading.
He reported that plain radiographs often underestimate the severity of articular changes, and arthroscopic findings commonly change the recommended treatment. Also, 82% of cases had at least one nonfunctional articulation, and 61% had at least two nonfunctional articulations.20
From these findings, an articular-based approach to treatment was developed based on the functional articular surfaces.19 20 The nonfunctional (compromised) articulations are identified and either excised, fused, or bypassed. The wrist is then mobilized with the remaining functional articulations.21
A New Treatment Algorithm
Having reviewed these important classifications, we have put together a new treatment algorithm. There are several important concepts that determine the outcome of the patient and need to be considered.
In demonstrating the synergy of the three classifications, we have placed them side-by-side in Table 1.
Table 1. Amalgamation of the osseous, vascular, and cartilage classification systems for the assessment and treatment of Kienböck disease.
| Assessment | Treatment | |||||
|---|---|---|---|---|---|---|
| Osseous (Lichtman) | Vascular (Schmitt) | Cartilage (Bain) | Description | Principle | Procedure | |
| B1: Lunate intact | Lunate protection | |||||
| 0, I, II | A | 0 | Intact lunate | Unload lunate Venous decompression Revascularization |
Immobilize, unloading procedures Lunate decompression Vascularized bone grafta |
|
| B2: Lunate compromised | Lunate reconstruction | |||||
| IIIA | B | 1 | Proximal lunate collapse | Lunate reconstruction | MFTGa, PRC, (RSL fusion, lunate replacementa) | |
| B3: Lunate unreconstructable | Lunate salvage | |||||
| IIIC | C | 2b | Lunate collapse | Lunate excision | Lunate replacementa, capitate lengthening, PRC | |
| C1–3: Wrist compromised | Wrist reconstruction | |||||
| IIIA | B | 2a | RC joint compromised | Fuse or bypass RL joint | RSL fusiona, SC fusion | |
| IIIA or C | B | 3, 4 | RC and MC joint compromised | Bypass central column | SC fusion, hemiarthroplastya | |
| IIB | B | 2–4 | Carpal collapse, (RSA > 60 degrees) | Stabilize radial column | SC fusion | |
| C4: Wrist not reconstructable | Wrist salvage | |||||
| IV, KDAC | C | 4 | Pan–OA | Salvage | Wrist fusion, wrist arthroplastya | |
Abbreviations: KDAC, Kienböck disease advanced collapse; MC, midcarpal; MFTG, medial femoral trochlear graft; Pan–OA, pan carpal osteoarthritis; PRC, proximal row carpectomy; RC, radiocarpal; RL, radiolunate; RSA, radioscaphoid angle; RSL, radioscapholunate; SC, scaphocapitate.
Source: Lichtman et al.43
Notes: Better prognosis: Age < 15 years nonoperative, 16–20 years consider unloading, > 70 years consider synovectomy or unloading procedure. Lunate unloading procedures: Negative ulnar variance: Radial shortening osteotomy; physis intact: Radial epiphysiodesis; neutral or positive ulnar variance: Capitate shortening. Adjunctive procedures: Synovectomy, scaphotrapeziotrapezoid pinning.
Alternative procedures: Used in specialist clinics or in selected cases where the patient has failed less-invasive procedures.
The new classification system extends these concepts further: It respects the importance of the patient's age, the lunate revascularization potential, the pathoanatomical aspects of the lunate disease and the secondary effects that the disorder has had on the wrist. The treatment recommendations are based on these factors.
The five questions underpin the management of Kienböck disease. The first three questions are the essential components of the new combined classification and the treatment recommendations (Fig. 4). The last two concern the capabilities of the surgeon and the needs of the patient and assist in guiding the final decision.
Fig. 4.

The key questions for Kienböck disease: A new algorithm.
Patient's Age
The age at presentation is important, with “Teenbock” and elderly patients having a better prognosis. As these are different prognostic groups, we have separated them from the start. The treatment recommendations for these populations are:
A1 < 15 years: Treat nonoperatively. Consider minimally invasive procedure for symptoms > 6 months.
A2 16–20 years: Trial of nonoperative management. Consider unloading procedure for symptoms > 3 months.
A3 > 70 years: Usually responds to nonoperative management. Consider synovectomy or proceed according to the protocol described below for symptoms > 6 months.
For patients, 21 to 69 years, proceed to sections B or C, as appropriate.
Lunate Stage: How Does the Disease Affect the Lunate?
The lunate consists of osseous, vascular, and cartilaginous components, which are each affected in different ways. (Osseous–Lichtman,6 vascular–Schmitt,18 cartilage–Bain.19)
The lunate is “intact” if it is not fractured and the articular surfaces are “functional.” Lichtman stages 0, I, and II, Schmitt stage A, and Bain grade 0 all represent an intact lunate. A “compromised” lunate has localized areas of collapse or degeneration, but other areas that can be used to reconstruct and maintain a functional lunate. This coincides with the Lichtman stage IIIA, Schmitt stage B, and Bain grade 1. A lunate is “not reconstructable” if it is fragmented, collapsed, or has completely lost its vascular supply. (e.g., Lichtman stage IIIC, Schmitt stage C, and Bain grade 2b).
Lunate Intact—Lunate Protection
By definition, the lunate that has not collapsed (Lichtman stage 0, I, and II), and has functional articular surfaces (Bain 0), with a positive prognosis for revascularization (Schmitt stage A). The three aims of treatment are:
Decrease the patient's pain and improve function.
Protect the vulnerable lunate before collapses.
Set the stage for spontaneous revascularization.
Nonoperative Management
In the earliest stages, patients are managed nonoperatively for at least 3 months (e.g., short arm cast or splint), however, minimally invasive techniques may be appropriate as well. Patients are advised to avoid strenuous activities or lifting over 5 kg (10 lb). Medical causes of osteonecrosis should be treated. If the patient is symptomatic after 3 months, or imaging demonstrates disease progression, a lunate unloading or revascularization procedure is considered.
Lunate Unloading Procedures
Lunate unloading is often used for patients with intact lunates who fail nonoperative treatment. They protect the lunate and set the stage for spontaneous revascularization. It is usually a radial shortening osteotomy, which is effective for the patient with a negative ulnar variance and/or abnormal radial inclination. If the physis is still intact, a radial epiphysiodesis is an alternative.22 If there is a neutral or positive ulnar variance, a capitate shortening osteotomy can be performed.
Lunate Decompression
Lunate forage is another option, which involves drilling the lunate to decompress venous hypertension It can be performed arthroscopically, in conjunction with a synovectomy19 21 23 24 and cancellous bone grafting.23 25 26 27
Revascularization Procedures
Core decompression of the distal radius provides an indirect revascularization of the lunate, due to the increased regional vascularity.28 Direct revascularization of the lunate can be performed, with either a pedicle graft or free vascularized bone graft. Pedicle graft procedures are appropriate for patients with an intact, partially viable lunate (Schmitt grade B). It is also used as an alternative to unloading in patients with a positive ulnar variance.
Lunate Compromised: Lunate Reconstruction (Lichtman Stage IIIA, Schmitt Stage B, and Bain Grade 1)
The lunate has localized disease, with areas of nonfunctioning proximal articular cartilage, but other parts are intact. The ideal treatment would be localized lunate reconstruction.
Vascularized Medial Femoral Trochlea Graft
This is indicated for reconstruction of the proximal lunate, and also to restore carpal height. This is a demanding procedure, so other treatment options are required. These include lunate salvage (PRC or lunate replacement), radioscapholunate (RSL) fusion, or scaphocapitate fusion. Note these alternatives are more destructive than an isolated lunate reconstruction.29 30
Lunate Not Reconstructable: Lunate Salvage (Lichtman Stage IIIC, Schmitt Stage C, and Bain Grade 2b)
The lunate is not reconstructable if the lunate has collapsed, has no revascularization potential or the lunate articular surfaces are nonfunctional. Then a salvage procedure of the lunate is required. Once the lunate has collapsed, it is common for the adjacent articulations to be degenerated and the carpus to collapse. In this case proceed, to the section C of the algorithm.
Lunate Replacement
If the lunate is not reconstructable, then it needs to be excised. The lunate has been replaced with silicone,6 31 autogenous tendon, metallic spheres, and the pisiform,32 but often with poor results.32 If the capitate and lunate fossa articular surfaces are functional, then a lunate excision and capitate lengthening with interposition graft is a possible. Recently, pyrocarbon implant replacement has been advocated. Unfortunately, instability is an issue, and modifications are required to achieve reliable clinical outcomes.33 34
Proximal Row Carpectomy
Proximal row carpectomy is a time tested technique that is indicated for the unreconstructable lunate if the articular surfaces of the lunate facet and the capitate are functional (Bain grade 2b).
Wrist Stage: How Does the Disease Affect the Wrist?
The secondary effects of the collapsing lunate are a “compromised” wrist, which include:
Degeneration of the central column at the radiolunate and midcarpal articulations.
The collapse of the central column.
Proximal row instability and radial column collapse.
Finally, degeneration of the radial column.
Fractures of the lunate produce irregularity of the lunate articular surfaces. If the fracture heals in a stable configuration then the prognosis is good. However, with secondary “kissing lesions” of the lunate facet and capitate, there is likely to be degeneration of the central column will occur.19 20 With fracture propagation, there will be a loss of lunate height, which affects the kinematics of the perilunate ligaments and the central column. Comminution, disruption of the spanning trabeculi, and/or a coronal lunate fracture produces the collapse of the lunate, which allows proximal migration of the capitate, and then the collapse of the entire central column. Scapholunate ligament laxity contributes to proximal migration and collapse of the central column.
With lunate comminution and interposition of the capitate between the lunate fragments, it appears on sagittal X-rays as a widening of the entire lunate. If the major fragments are pushed dorsally, the lunate will appear to be in flexion (volar intercalated segment instability). A dorsal intercalated segment instability deformity occurs if the capitate migrates into a gap due to scapholunate ligament instability or if the main fragments are pushed anteriorly. In each case, the proximal migration of the capitate (and distal row) cause excessive flexion of the scaphoid (Lichtman stage IIIB), as manifested by a radioscaphoid angle > 60 degrees.35 Initially, the scaphoid flexion is correctable. However, after prolonged flexion, it will cause erosive degeneration of radioscaphoid articular cartilage and that joint, too, will become nonfunctional (i.e., Kienböck disease advanced collapse).
Prior to carpal collapse, the wrist with nonfunctional surfaces can be reconstructed with motion preserving procedures by excising, fusing, or bypassing the nonfunctional articulations and maintaining the functional articulations. The recommended procedure will depend upon the extent of osseous collapse, but more importantly the remaining functional articular surfaces. The surgeon matches the remaining functional articular surfaces with the prerequisites.
With carpal collapse, the radial column is initially correctable, so lunate reconstructive procedure can theoretically be effective (e.g., lunate replacement). But if there is a fixed deformity and central column articular degeneration, a limited wrist fusion is required. The scaphocapitate (or scaphotrapeziotrapezoid [STT]) fusion is an effective surgical option, as it bypasses the diseased central column, stabilizes the radial column, and articulates through the intact radio-scaphoid articulation. This is similar to the SLAC wrist procedure for the SLAC wrist.
Once the radioscaphoid articulation is also compromised (nonfunctional), the wrist is unreconstructable and a salvage procedure is required (e.g., very late Kienböck disease or after failed reconstructive surgery).
Central Column Compromised; Central Column Fusion or Bypass (Lichtman Stage IIIA or C, Schmitt Stage B, and Bain Grade 2a, 3, or 4)
-
C.1.a: Radiolunate articulation compromised (Lichtman stage IIIA, Schmitt stage B, and Bain grade 2a) and the midcarpal joint surfaces functional.
A RSL fusion can be performed.
C.1.b: Radiolunate and midcarpal articulations nonfunctional (Lichtman stage IIIA or C, Schmitt stage C, and Bain grade 4).
The central column is not reconstructable and can be bypassed with a scaphocapitate fusion. Occasionally, the capitate articular surface is intact (Lichtman stage IIIA or C, Schmitt stage C, and Bain grade 3), so a hemiarthroplasty can be performed.21 36
Carpal Collapse; Wrist Stabilization (Lichtman Stage IIIB, Schmitt Stage B, and Bain Grade 2–4)
With collapse and/or degeneration of the central column, it is common for the radial column to also collapse (e.g., radioscaphoid angle is greater than 60 degrees).35 37 Fortunately, the radioscaphoid articulation often remains functional, so a scaphocapitate fusion can be performed. A STT fusion is an alternative).
With early carpal collapse, the lunate can be reconstructed, as described in B3. However, this is only if the articulations are intact, and no fixed deformity. We do not recommend a lunate prosthesis in those cases, as it is technically demanding to obtain stability.
Scaphocapitate Fusion
The lunate is excised if the avascular fragments have incited a localized synovitis, or if there is a significant widening of the lunate seen on the sagittal computed tomography scan. The surgeon needs to be cautious to not violating the volar carpal ligaments, which are intimately attached to the volar fragments, as ulnar translocation of the carpus can occur.
Wrist Not Reconstructable; Wrist Salvage (Lichtman Stage 4, Schmitt Stage C, and Bain Grade 4)
Once the radioscaphoid articulation also becomes nonfunctional, the wrist is no longer reconstructable and a salvage procedure is required, such as total wrist fusion or total wrist arthroplasty. The recommendation will depend upon the demands of the patient. Total wrist arthroplasty is only performed in those patients who will not overload the wrist.
Adjuvant Procedures
There are a few surgical procedures that can be considered adjunctive procedures, which can be performed as an independent procedure, or added to other procedures.
Synovitis of the wrist is a common finding in Kienböck disease19 and a frequent source of pain. Synovectomy and joint debridement can be performed as an open or arthroscopic procedure.19 24
Temporary pinning of the STT joint unloads the lunate, and allows time for revascularization.37 38 39 40 41 The STT joint is pinned with the scaphoid in the extension. It is a good option as a primary treatment in the “Teenbock” patient and to protect the lunate in conjunction with a vascularized bone graft.37
Some authors recommend posterior interosseous neurectomy for relief for carpal pathology.42 However, we are concerned that it will negatively affect function and protection of the wrist.36>
What Can the Surgeon Offer?
Surgeons have different abilities, training, and experience. Some surgical options require advanced skills in microsurgery or arthroscopy, which are beyond the capabilities of some surgeons or their facilities. We have identified these procedures within the algorithm. With technical advances, some of these procedures will become more mainstream.
What Does the Patient Want?
The final decision comes down to the needs and desires of the informed, competent patient. The patient's health, lifestyle, and demands on the wrist need careful consideration. Each patient is different, and their personal considerations will affect all levels of the proposed algorithm. Unfortunately, constraints outside of the control of the doctor and patient may affect the final decision (e.g., financial restrictions, the insurer, governing bodies).
No treatment algorithm will cover all contingency, especially when addressing disease condition such as Kienböck disease. However, when armed with this information, we believe the clinician can make an evidence-based decision that is tailored to the unique needs of the patient.
Footnotes
Conflict of Interest None.
References
- 1.Kienböck R. Über traumatische Malazie des Mondbeins und ihre Folgezustände: Entartungsformen und Kompressionsfrakturen. Fortschr Geb Rontgenstr. 1910;XVI(2):77–103. [Google Scholar]
- 2.Lutsky K, Beredjiklian P K. Kienböck disease. J Hand Surg Am. 2012;37(9):1942–1952. doi: 10.1016/j.jhsa.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 3.Keith P P, Nuttall D, Trail I. Long-term outcome of nonsurgically managed Kienböck's disease. J Hand Surg Am. 2004;29(1):63–67. doi: 10.1016/j.jhsa.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 4.Salmon J, Stanley J K, Trail I A. Kienböck's disease: conservative management versus radial shortening. J Bone Joint Surg Br. 2000;82(6):820–823. doi: 10.1302/0301-620x.82b6.10570. [DOI] [PubMed] [Google Scholar]
- 5.Beckenbaugh R D, Shives T C, Dobyns J H, Linscheid R L. Kienböck's disease: the natural history of Kienböck's disease and consideration of lunate fractures. Clin Orthop Relat Res. 1980;1(149):98–106. [PubMed] [Google Scholar]
- 6.Lichtman D M, Mack G R, MacDonald R I, Gunther S F, Wilson J N. Kienböck's disease: the role of silicone replacement arthroplasty. J Bone Joint Surg Am. 1977;59(7):899–908. [PubMed] [Google Scholar]
- 7.Lichtman D M, Degnan G G. Staging and its use in the determination of treatment modalities for Kienböck's disease. Hand Clin. 1993;9(3):409–416. [PubMed] [Google Scholar]
- 8.Fontaine C. Kienböck's disease. Chir Main. 2015;34(1):4–17. doi: 10.1016/j.main.2014.10.149. [DOI] [PubMed] [Google Scholar]
- 9.Irisarri C, Kalb K, Ribak S. Infantile and juvenile lunatomalacia. J Hand Surg Eur Vol. 2010;35(7):544–548. doi: 10.1177/1753193410364913. [DOI] [PubMed] [Google Scholar]
- 10.Geutjens G G. Kienböck's disease in an elderly patient. J Hand Surg Am. 1995;20(1):42–43. doi: 10.1016/S0363-5023(05)80056-5. [DOI] [PubMed] [Google Scholar]
- 11.Taniguchi Y, Yoshida M, Iwasaki H, Otakara H, Iwata S. Kienböck's disease in elderly patients. J Hand Surg Am. 2003;28(5):779–783. doi: 10.1016/s0363-5023(03)00299-5. [DOI] [PubMed] [Google Scholar]
- 12.Yoshida T, Tada K, Yamamoto K, Shibata T, Shimada K, Kawai H. Aged-onset Kienböck's disease. Arch Orthop Trauma Surg. 1990;109(5):241–246. doi: 10.1007/BF00419936. [DOI] [PubMed] [Google Scholar]
- 13.Giunta R, Löwer N, Wilhelm K, Keirse R, Rock C, Müller-Gerbl M. Altered patterns of subchondral bone mineralization in Kienböck's disease. J Hand Surg [Br] 1997;22(1):16–20. doi: 10.1016/s0266-7681(97)80006-x. [DOI] [PubMed] [Google Scholar]
- 14.Thomas A A, Rodriguez E, Segalman K. Kienböck's disease in an elderly patient treated with proximal row carpectomy. J Hand Surg Am. 2004;29(4):685–688. doi: 10.1016/j.jhsa.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 15.Arco News . Association research circulation osseous (ARCO): committee on terminology and classification. ARCO News. 1992;4:41–46. [Google Scholar]
- 16.Lichtman D M, Lesley N E, Simmons S P. The classification and treatment of Kienbock's disease: the state of the art and a look at the future. J Hand Surg Eur Vol. 2010;35(7):549–554. doi: 10.1177/1753193410374690. [DOI] [PubMed] [Google Scholar]
- 17.Schmitt R, Kalb K. Imaging in Kienböck's disease [in German] Handchir Mikrochir Plast Chir. 2010;42(3):162–170. doi: 10.1055/s-0030-1253433. [DOI] [PubMed] [Google Scholar]
- 18.Schmitt R, Heinze A, Fellner F, Obletter N, Strühn R, Bautz W. Imaging and staging of avascular osteonecroses at the wrist and hand. Eur J Radiol. 1997;25(2):92–103. doi: 10.1016/s0720-048x(97)00065-x. [DOI] [PubMed] [Google Scholar]
- 19.Bain G I, Begg M. Arthroscopic assessment and classification of Kienbock's disease. Tech Hand Up Extrem Surg. 2006;10(1):8–13. doi: 10.1097/00130911-200603000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Bain G I, Durrant A. An articular-based approach to Kienbock avascular necrosis of the lunate. Tech Hand Up Extrem Surg. 2011;15(1):41–47. doi: 10.1097/BTH.0b013e31820e82e8. [DOI] [PubMed] [Google Scholar]
- 21.Bain G I, McGuire D T. Decision making for partial carpal fusions. J Wrist Surg. 2012;1(2):103–114. doi: 10.1055/s-0032-1329548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jorge-Mora A, Pretell-Mazzini J, Marti-Ciruelos R, Andres-Esteban E M, Curto de la Mano A. Distal radius definitive epiphysiodesis for management of Kienböcḱs disease in skeletally immature patients. Int Orthop. 2012;36(10):2101–2105. doi: 10.1007/s00264-012-1597-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bain G I, Smith M L, Watts A C. Arthroscopic core decompression of the lunate in early stage Kienbock disease of the lunate. Tech Hand Up Extrem Surg. 2011;15(1):66–69. doi: 10.1097/BTH.0b013e3181e1d2b4. [DOI] [PubMed] [Google Scholar]
- 24.Menth-Chiari W A, Poehling G G, Wiesler E R, Ruch D S. Arthroscopic debridement for the treatment of Kienbock's disease. Arthroscopy. 1999;15(1):12–19. doi: 10.1053/ar.1999.v15.015001. [DOI] [PubMed] [Google Scholar]
- 25.Mehrpour S R, Kamrani R S, Aghamirsalim M R, Sorbi R, Kaya A. Treatment of Kienböck disease by lunate core decompression. J Hand Surg Am. 2011;36(10):1675–1677. doi: 10.1016/j.jhsa.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 26.Leblebicioğlu G, Doral M N, Atay A A, Tetik O, Whipple T L. Open treatment of stage III Kienböck's disease with lunate revascularization compared with arthroscopic treatment without revascularization. Arthroscopy. 2003;19(2):117–130. doi: 10.1053/jars.2003.50009. [DOI] [PubMed] [Google Scholar]
- 27.Pegoli L, Ghezzi A, Cavalli E, Luchetti R, Pajardi G. Arthroscopic assisted bone grafting for early stages of Kienböck's disease. Hand Surg. 2011;16(2):127–131. doi: 10.1142/S0218810411005436. [DOI] [PubMed] [Google Scholar]
- 28.Illarramendi A A, Schulz C, De Carli P. The surgical treatment of Kienböck's disease by radius and ulna metaphyseal core decompression. J Hand Surg Am. 2001;26(2):252–260. doi: 10.1053/jhsu.2001.22928. [DOI] [PubMed] [Google Scholar]
- 29.Higgins J P, Bürger H K. Osteochondral flaps from the distal femur: expanding applications, harvest sites, and indications. J Reconstr Microsurg. 2014;30(7):483–490. doi: 10.1055/s-0034-1372484. [DOI] [PubMed] [Google Scholar]
- 30.Bürger H K, Windhofer C, Gaggl A J, Higgins J P. Vascularized medial femoral trochlea osteocartilaginous flap reconstruction of proximal pole scaphoid nonunions. J Hand Surg Am. 2013;38(4):690–700. doi: 10.1016/j.jhsa.2013.01.036. [DOI] [PubMed] [Google Scholar]
- 31.Roca J, Beltran J E, Fairen M F, Alvarez A. Treatment of Kienböck's disease using a silicone rubber implant. J Bone Joint Surg Am. 1976;58(3):373–376. [PubMed] [Google Scholar]
- 32.Saffar P. Vascularized pisiform transfer in place of lunatum for Kienböck's disease [in French] Chir Main. 2010;29 01:S112–S118. doi: 10.1016/j.main.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 33.Werthel J D, Hoang D V, Boyer P, Dallaudière B, Massin P, Loriaut P. Treatment of Kienböck's disease using a pyrocarbon implant: case report [in French] Chir Main. 2014;33(6):404–409. doi: 10.1016/j.main.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 34.Bellemère P, Maes-Clavier C, Loubersac T, Gaisne E, Kerjean Y, Collon S. Pyrocarbon interposition wrist arthroplasty in the treatment of failed wrist procedures. J Wrist Surg. 2012;1(1):31–38. doi: 10.1055/s-0032-1323641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gelberman R H, Bauman T D, Menon J, Akeson W H. The vascularity of the lunate bone and Kienböck's disease. J Hand Surg Am. 1980;5(3):272–278. doi: 10.1016/s0363-5023(80)80013-x. [DOI] [PubMed] [Google Scholar]
- 36.Boyer J S, Adams B. Distal radius hemiarthroplasty combined with proximal row carpectomy: case report. Iowa Orthop J. 2010;30:168–173. [PMC free article] [PubMed] [Google Scholar]
- 37.Yajima H, Ono H, Tamai S. Temporary internal fixation of the scaphotrapezio-trapezoidal joint for the treatment of Kienböck's disease: a preliminary study. J Hand Surg Am. 1998;23(3):402–410. doi: 10.1016/S0363-5023(05)80457-5. [DOI] [PubMed] [Google Scholar]
- 38.Yasuda M, Okuda H, Egi T, Guidera P M. Temporary scapho-trapezoidal joint fixation for Kienböck's disease in a 12-year-old girl: a case report. J Hand Surg Am. 1998;23(3):411–414. doi: 10.1016/S0363-5023(05)80458-7. [DOI] [PubMed] [Google Scholar]
- 39.Shigematsu K, Yajima H, Kobata Y, Kawamura K, Nakanishi Y, Takakura Y. Treatment of Kienböck disease in an 11-year-old girl with temporary fixation of the scaphotrapeziotrapezoidal joint. Scand J Plast Reconstr Surg Hand Surg. 2005;39(1):60–63. doi: 10.1080/02844310410017988. [DOI] [PubMed] [Google Scholar]
- 40.Kazuki K, Uemura T, Okada M, Egi T. Time course of magnetic resonance images in an adolescent patient with Kienböck's disease treated by temporary scaphotrapezoidal joint fixation: a case report. J Hand Surg Am. 2006;31(1):63–67. doi: 10.1016/j.jhsa.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 41.Ando Y, Yasuda M, Kazuki K, Hidaka N, Yoshinaka Y. Temporary scaphotrapezoidal joint fixation for adolescent Kienböck's disease. J Hand Surg Am. 2009;34(1):14–19. doi: 10.1016/j.jhsa.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 42.Wilhelm A. Partial joint denervation: wrist, shoulder, and elbow. Plast Reconstr Surg. 2010;126(1):345–347. doi: 10.1097/PRS.0b013e3181dab5f3. [DOI] [PubMed] [Google Scholar]
- 43.Lichtman D M, Pientka W F, II, Bain G I. New York: Springer; 2016. The future of Kienbock's disease: a new algorithm; pp. 307–320. [Google Scholar]


