Abstract
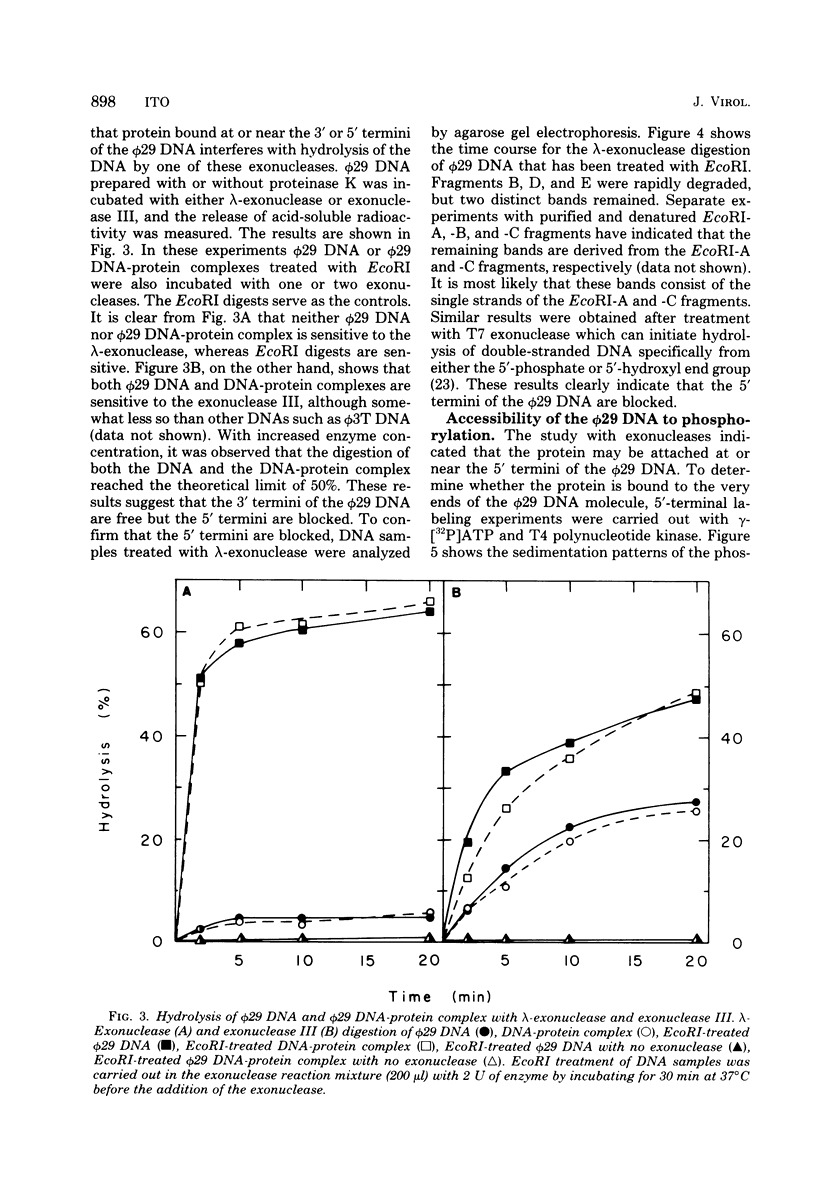
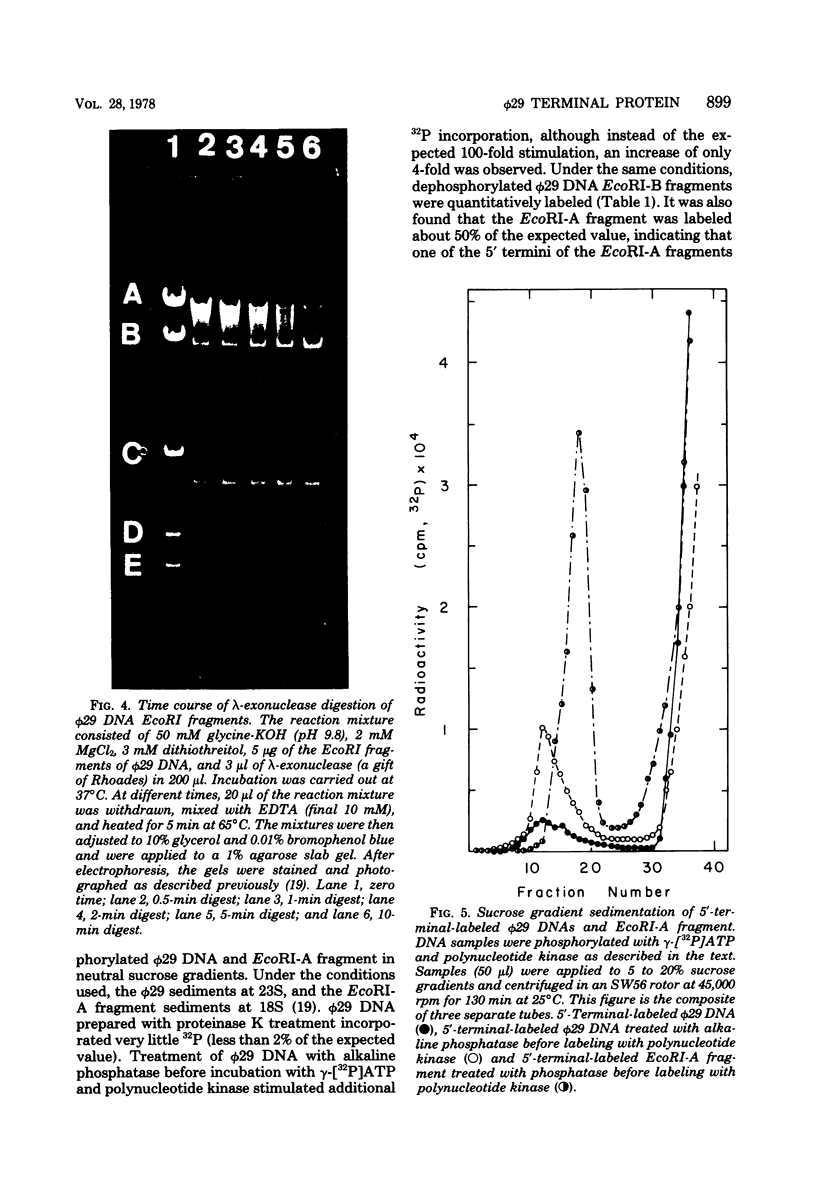
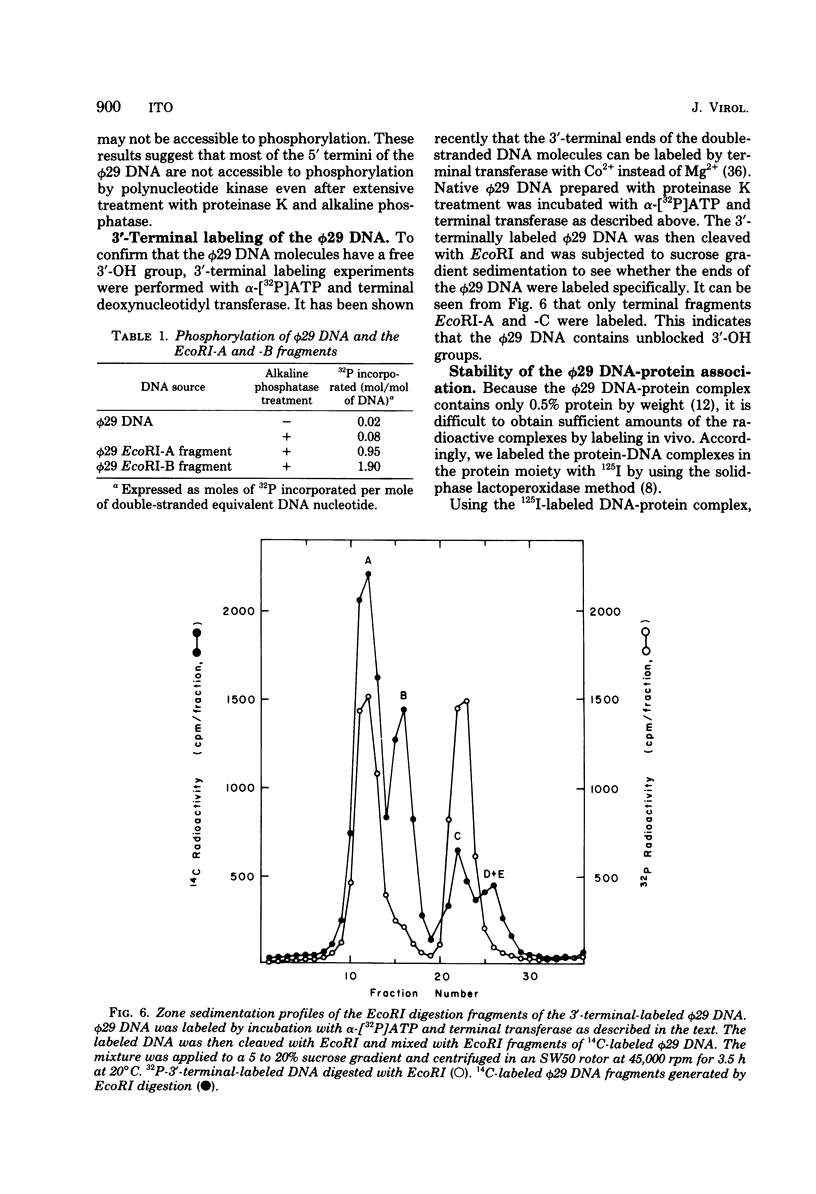
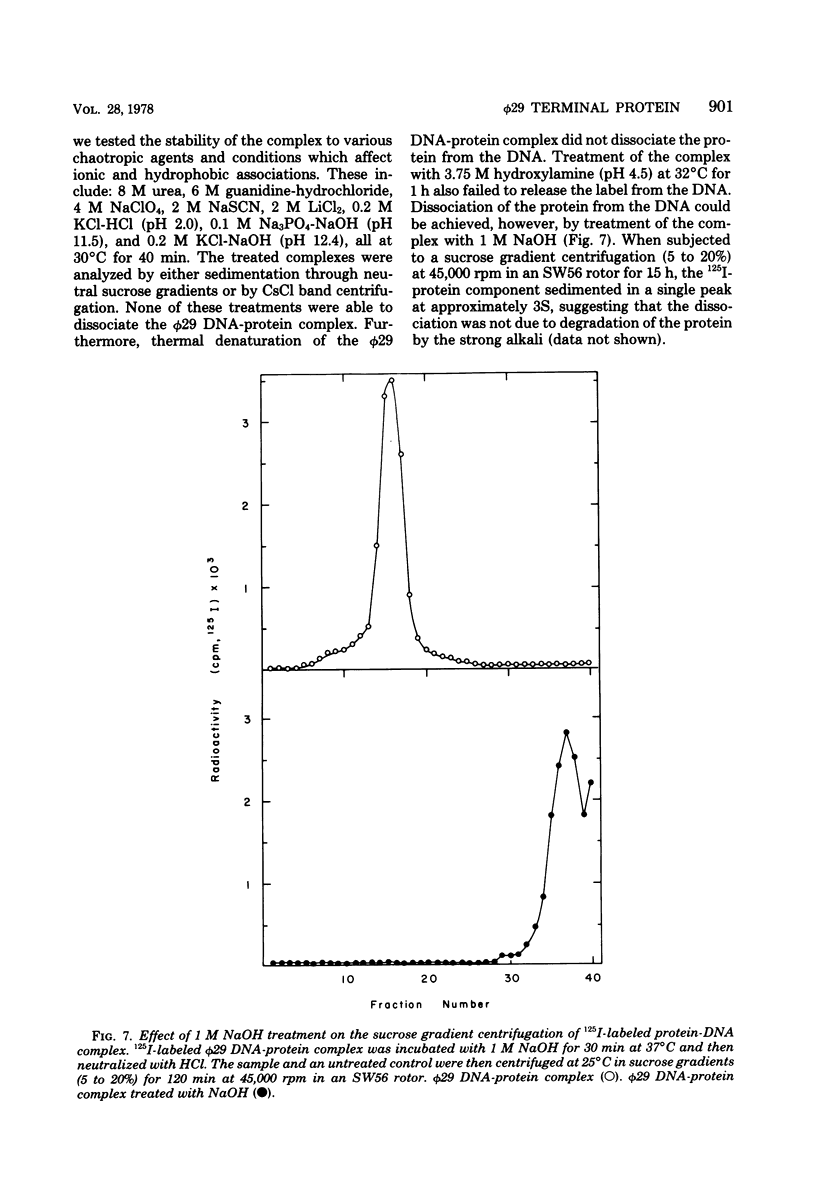
The location of the protein bound to bacteriophage phi29 DNA has been studied with restriction endonucleases, exonucleases, and polynucleotide kinase. The protein is invariably associated with the two terminal DNA fragments generated by restriction endonucleases. The phi29 DNA prepared with or without proteinase K treatment is resistant to the action of the 5'-terminal-specific exonucleases, lambda-exonuclease and T7 exonuclease. The phi29 DNA is also inaccessible to phosphorylation by polynucleotide kinase even after treatment with alkaline phosphatase. On the other hand, phi29 DNA is sensitive to exonuclease III, and the 3' termini of the DNA can be labeled by incubating with alpha-[32P]ATP and terminal deoxynucleotidyl transferase. The protein remains associated with the phi29 DNA after treatment with various chaotropic agents, including 8 M urea, 6 M guanidine-hydrochloride, 4 M sodium perchlorate, 2 M sodium thiocyanate, and 2 M LiCl. These results are consistent with the notion that the protein is linked covalently to the 5' termini of the phi29 DNA.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arwert F., Venema G. Protease-sensitive transfection of Bacillus subtilis with bacteriophage GA-1 DNA: a probable case of heterologous transfection. J Virol. 1974 Mar;13(3):584–589. doi: 10.1128/jvi.13.3.584-589.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. T., Westphal M., Burlingham B. T., Winterhoff U., Doerfler W. Structure and composition of the adenovirus type 2 core. J Virol. 1975 Aug;16(2):366–387. doi: 10.1128/jvi.16.2.366-387.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carusi E. A. Evidence for blocked 5'-termini in human adenovirus DNA. Virology. 1977 Jan;76(1):380–394. doi: 10.1016/0042-6822(77)90310-5. [DOI] [PubMed] [Google Scholar]
- Flanegan J. B., Petterson R. F., Ambros V., Hewlett N. J., Baltimore D. Covalent linkage of a protein to a defined nucleotide sequence at the 5'-terminus of virion and replicative intermediate RNAs of poliovirus. Proc Natl Acad Sci U S A. 1977 Mar;74(3):961–965. doi: 10.1073/pnas.74.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiney D. G., Helinski D. R. Relaxation complexes of poasmid DNA and protein. III. Association of protein with the 5' terminus of the broken DNA strand in the relaxed complex of plasmid ColE1. J Biol Chem. 1975 Nov 25;250(22):8796–8803. [PubMed] [Google Scholar]
- Hagen E. W., Reilly B. E., Tosi M. E., Anderson D. L. Analysis of gene function of bacteriophage phi 29 of Bacillus subtilis: identification of cistrons essential for viral assembly. J Virol. 1976 Aug;19(2):501–517. doi: 10.1128/jvi.19.2.501-517.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding N. E., Ito J. DNA Replication of bacteriophage phi29: isolation of a DNA-protein complex from Bacillus subtilis cells infected with wild-type and with a suppressor-sensitive mutant. Virology. 1976 Sep;73(2):389–401. doi: 10.1016/0042-6822(76)90400-1. [DOI] [PubMed] [Google Scholar]
- Harding N. E., Ito J., David G. S. Identification of the protein firmly bound to the ends of bacteriophage phi 29 DNA. Virology. 1978 Feb;84(2):279–292. doi: 10.1016/0042-6822(78)90248-9. [DOI] [PubMed] [Google Scholar]
- Hirokawa H. Transfecting deoxyribonucleic acid of Bacillus bacteriophage phi 29 that is protease sensitive. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1555–1559. doi: 10.1073/pnas.69.6.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda J. E., Yudelevich A., Hurwitz J. Isolation and characterization of the protein coded by gene A of bacteriophage phiX174 DNA. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2669–2673. doi: 10.1073/pnas.73.8.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
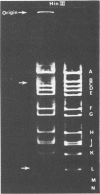
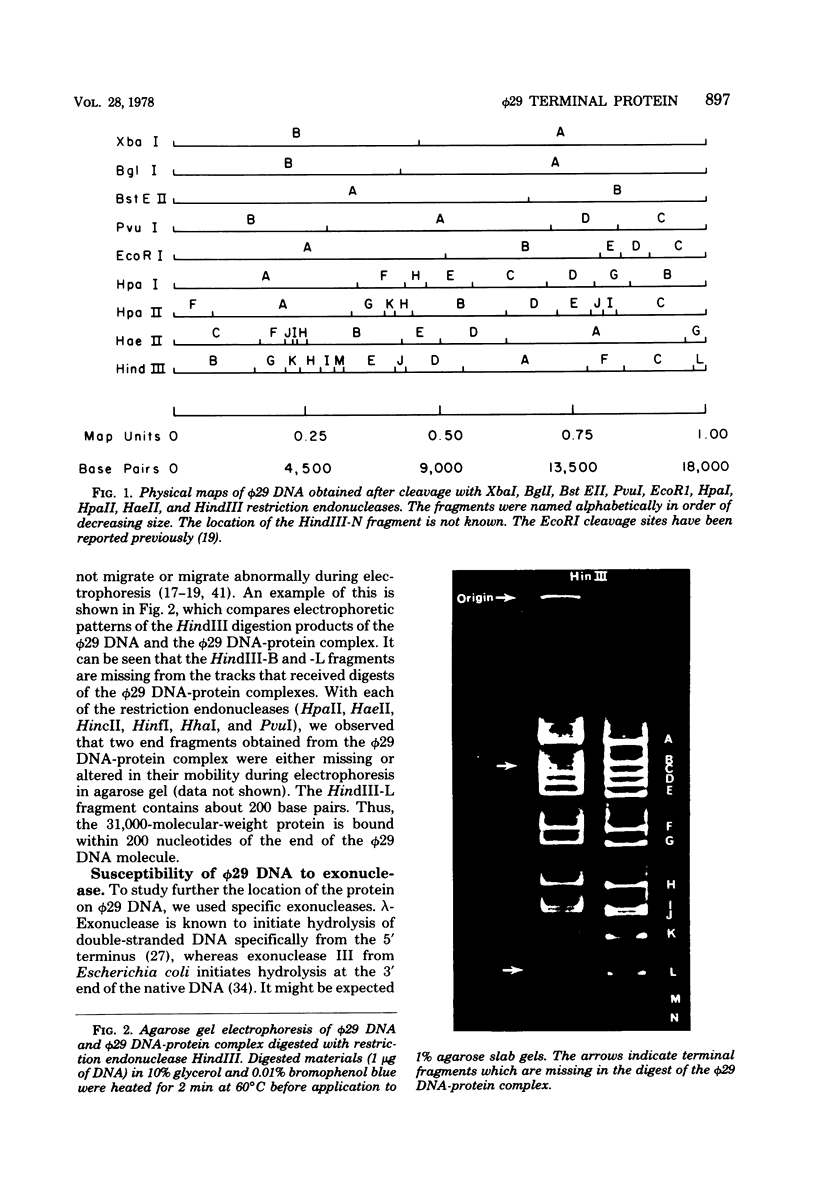
- Inciarte M. R., Lázaro J. M., Salas M., Vińuela E. Physical map of bacteriophage phi29 DNA. Virology. 1976 Oct 15;74(2):314–323. [PubMed] [Google Scholar]
- Ito J., Kawamura F., Yanofsky S. Analysis of phi 29 and phi 15 genomes by bacterial restriction endonucleases, EcoR1 and Hpal. Virology. 1976 Mar;70(1):37–51. doi: 10.1016/0042-6822(76)90234-8. [DOI] [PubMed] [Google Scholar]
- Ito J., Meinke W., Hathaway G., Spizizen J. Studies on Bacillus subtilis bacteriophage phi 15. Virology. 1973 Nov;56(1):110–122. doi: 10.1016/0042-6822(73)90291-2. [DOI] [PubMed] [Google Scholar]
- Kasamatsu H., Wu M. Structure of a nicked DNA-protein complex isolated from simian virus 40: covalent attachment of the protein to DNA and nick specificity. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1945–1949. doi: 10.1073/pnas.73.6.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura F., Ito J. Studies of Bacillus subtilis phage M2: a physical map of the M2 genome. Virology. 1977 Dec;83(2):233–245. doi: 10.1016/0042-6822(77)90168-4. [DOI] [PubMed] [Google Scholar]
- Kerr C., Sadowski P. D. Gene 6 exonuclease of bacteriophage T7. II. Mechanism of the reaction. J Biol Chem. 1972 Jan 10;247(1):311–318. [PubMed] [Google Scholar]
- Larsen S. H., Nathans D. Mouse adenovirus: growth of plaque-purified FL virus in cell lines and characterization of viral DNA. Virology. 1977 Oct 1;82(1):182–195. doi: 10.1016/0042-6822(77)90041-1. [DOI] [PubMed] [Google Scholar]
- Little J. W., Lehman I. R., Kaiser A. D. An exonuclease induced by bacteriophage lambda. I. Preparation of the crystalline enzyme. J Biol Chem. 1967 Feb 25;242(4):672–678. [PubMed] [Google Scholar]
- McGuire J. C., Pène J. J., Barrow-Carraway J. Gene expression during the development of bacteriophage phi 29. 3. Analysis of viral-specific protein synthesis with suppressible mutants. J Virol. 1974 Mar;13(3):690–698. doi: 10.1128/jvi.13.3.690-698.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuire J. C., Gilpatrick M. W., Pène J. J. DNA replication of bacteriophage phi29. Effect of two viral genes on the association of phage chromosomes with the host cell membrane. Virology. 1977 May 1;78(1):234–240. doi: 10.1016/0042-6822(77)90094-0. [DOI] [PubMed] [Google Scholar]
- Ortin J., Viñuela E., Salas M., Vasquez C. DNA-protein complex in circular DNA from phage phi-29. Nat New Biol. 1971 Dec 29;234(52):275–277. doi: 10.1038/newbio234275a0. [DOI] [PubMed] [Google Scholar]
- Rekosh D. M., Russell W. C., Bellet A. J., Robinson A. J. Identification of a protein linked to the ends of adenovirus DNA. Cell. 1977 Jun;11(2):283–295. doi: 10.1016/0092-8674(77)90045-9. [DOI] [PubMed] [Google Scholar]
- Roychoudhury R., Jay E., Wu R. Terminal labeling and addition of homopolymer tracts to duplex DNA fragments by terminal deoxynucleotidyl transferase. Nucleic Acids Res. 1976 Apr;3(4):863–877. doi: 10.1093/nar/3.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas M., Mellado R. P., Viñuela E. Characterization of a protein covalently linked to the 5' termini of the DNA of Bacillus subtilis phage phi29. J Mol Biol. 1978 Feb 25;119(2):269–291. doi: 10.1016/0022-2836(78)90438-2. [DOI] [PubMed] [Google Scholar]
- Sangar D. V., Rowlands D. J., Harris T. J., Brown F. Protein covalently linked to foot-and-mouth disease virus RNA. Nature. 1977 Aug 18;268(5621):648–650. doi: 10.1038/268648a0. [DOI] [PubMed] [Google Scholar]
- Schachtele C. F., Reilly B. E., De Sain C. V., Whittington M. O., Anderson D. L. Selective replication of bacteriophage phi29 deoxyribonucleic acid in 6-(p-hydroxyphenylazo)-uracil-treated Bacillus subtilis. J Virol. 1973 Jan;11(1):153–155. doi: 10.1128/jvi.11.1.153-155.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabarova Z. A. Synthetic nucleotide-peptides. Prog Nucleic Acid Res Mol Biol. 1970;10:145–182. doi: 10.1016/s0079-6603(08)60564-4. [DOI] [PubMed] [Google Scholar]
- Streeck R. E., Philippsen P., Zachau H. G. Cleavage of small bacteriophage and plasmid DNAs by restriction endonucleases. Eur J Biochem. 1974 Jun 15;45(2):489–499. doi: 10.1111/j.1432-1033.1974.tb03574.x. [DOI] [PubMed] [Google Scholar]
- Talavera A., Salas M., Viñuela E. Temperature-sensitive mutants affected in DNA synthesis in phage phi29 of Bacillus subtilis. Eur J Biochem. 1972 Dec 4;31(2):367–371. doi: 10.1111/j.1432-1033.1972.tb02542.x. [DOI] [PubMed] [Google Scholar]
- Wu M., Roberts R. J., Davidson N. Structure of the inverted terminal repetition of adenovirus type 2 DNA. J Virol. 1977 Feb;21(2):766–777. doi: 10.1128/jvi.21.2.766-777.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]