Abstract
Objective:
To investigate the potential use of cone beam CT (CBCT) in adaptive radiotherapy (ART) planning process for non-small-cell lung cancer (NSCLC).
Methods:
17 retrospective patients with NSCLC Stage T1–T4, who had completed a course of radiotherapy with weekly CBCT imaging were selected for the study. The patients had been delineated and planned for three-dimensional (3D) conformal treatment (prescription: 55 Gy in 20 fractions) based on free-breathing four-dimensional CT data. Of these initial 17 patients, 12 had full quantitative data on gross tumour volume (GTV) position and volume throughout treatment. GTV delineation was carried out on weekly CBCT by a clinical oncologist. For each patient, mean percentage change in GTV and centre of mass (COM) displacement (based on 3D vectors) were calculated throughout treatment. Volume overlap between GTVs was calculated. Correlation of the COM displacement and planning GTV (pGTV) was assessed. A linear mixed model with patients as random effects was fitted to the data to assess potential benefit from using ART for these patients.
Results:
Comparison of CBCT-based GTV acquired prior to Fraction 1 (cbctGTV1) to pGTV showed mean 20 ± 19% volume increase using a related sample Wilcoxon signed rank test p = 0.04. Correlation was identified between volume reductions and dose delivered (beta = −0.003, p < 0.001)—a highly statistically significant association. Compared with cbctGTV1, the mean ratios ± standard deviation were cbctGTV2, 0.93 ± 0.08; cbctGTV3, 0.84 ± 0.12; and cbctGTV4, 0.75 ± 0.14. The dice similarity coefficient was 0.81 ± 0.14, 0.78 ± 0.17, 0.73 ± 0.19, respectively. The COM was consistent throughout treatment (mean 0.35 ± 0.24 cm). A fitted model predicts that a mean change of 30% volume relative to cbctGTV1 occurs at a dose of approximately 50 Gy.
Conclusion:
Using a 30% reduction in volume, ART would not be of benefit for all radiotherapy-alone-treated patients with NSCLC assessed in this study. For individual patients and patients with atelectasis, CBCT imaging was able to identify volume change.
Advances in knowledge:
For patients treated with 55 Gy in 20 fractions, target volume changes throughout treatment have been demonstrated using CBCT and can be used to highlight patients who may benefit from ART.
INTRODUCTION
Lung cancer is the second most common diagnosis of cancer for both males and females in the UK and causes the highest number of cancer deaths.1–3 This is often due to patients presenting with later-stage disease, often at an older age and often with pre-existing comorbidities. There is indeed a great scope for improvement in the treatment of lung cancer which is most commonly treated using a combination of surgery, chemotherapy and radiotherapy (RT).
Non-small-cell lung cancer (NSCLC) is often treated using RT either as a stand-alone modality or in combination with surgery or a chemotherapy regimen. Numerous studies have reported on the response of NSCLC to radiation treatment and its significant role in patient care.4,5 Radiation can cause many toxic side effects for the patient including pneumonitis and pulmonary fibrosis6 and is an important consideration in designing treatment approaches.
The challenge for successful RT treatment is primarily to correctly identify disease in relation to surrounding normal tissue. This allows optimization of the planning and dose calculation so that dose to nearby normal tissue can be minimized. Patients being planned for a course of RT may present with atelectasis (lung collapse), and identifying the primary disease site can be difficult on diagnostic CT and RT planning images. Target delineation (and management) may be improved by using additional image modalities such as positron emission tomography (PET).7
Advances in planning techniques such as intensity-modulated radiotherapy (IMRT) and volumetric arc therapy (VMAT) allow improved target conformality and more efficient delivery. However, treatment arcs/fields obviously still pass through healthy normal tissue. Common practice in current RT treatment delivery is to generate a three-dimensional (3D) conformal plan (3D conformal radiotherapy) and deliver it daily, with no alterations made to the original plan throughout treatment.
Although corrections to take account of set-up errors may be made using on-treatment image-guided radiotherapy, anatomical, intrafractional and physiological changes are not accounted for by this method. These latter issues may cause geographical miss so that delivered dose deviates from the planned dose. This may in turn result in a possible underdosage of target volume dose and increased dose to normal tissue.
A review in 2010 by Sonke and Belderbos8 discusses the benefit of adaptive radiotherapy (ART) for the lung. ART is the use of additional, patient-specific information which can be used as feedback in the planning process. This can reduce geometric uncertainties and allow an up-to-date treatment plan to be created based on changes. They report evidence of tumour regression, at rates ranging from −0.39% to −2.4% per day.
There are a number of issues raised by the review article when assessing geometric uncertainties.8 Firstly, the studies9,10 they reviewed have small sample sizes with highly selected patients. There are differences in the type of serial imaging performed to assess geometric uncertainties, e.g. cone beam CT (CBCT),10,11 four-dimensional CT (4DCT)12 and PET CT.13 In some instances, these modalities required additional scanning away from the treatment room, with additional imaging-related radiation dose and inconvenience. Some studies14 used in-room megavoltage CT imaging which has poorer image quality than kilovoltage CT. The frequency of scanning to assess the uncertainty varies considerably, e.g. Juhler-Nøttrup et al9 only acquired scans in the middle and at the end of a 6-week course. Finally, there were other issues raised such as heterogeneous disease stage, RT dose and fractionation, and delivery of chemotherapy.
Using megavoltage CT, Woodford et al14 very usefully showed that a 30% reduction in gross tumour volume (GTV) correlated with a potential benefit with replanning.
Other studies give useful additional information on tumour regression with treatment. In an American Society for Therapeutic Radiation Oncology conference report in 2008, van Zwienen et al11 showed tumour regression in approximately 40% of 114 patients. Importantly, they showed changes in patients due to reduced atelectasis in response to treatment. More recently, Lim et al15 described a complex study of 60 patients with images reconstructed offline to produce a respiratory correlated CBCT at full exhalation. Of 31 patients assessed, 40% showed levels of tumour volume regression >30% at mid-treatment. Targets could not be outlined on another 29 patients who were only scored visually.
There are a few small-scale studies looking more closely at the impact of ART on delivered dose to tumour and normal tissues. In 2008, Harsolia et al16 used a time-consuming technique with four-dimensional CBCT (4D-CBCT) on eight patients. This showed potentially significant reduction in lung dose. Hugo et al17 described a deformable registration technique with repeat CT scans on 13 patients. This provided a method of adding doses throughout a course of treatment to changing volumes. Guckenberger et al18 used a similar technique on 13 patients with advanced disease and showed average tumour regression of 1.2% per day. They further suggest that adaptive planning could reduce mean lung dose by 5–8% and allow an increased dose to be given to the target.
In summary, the literature shows evidence of tumour regression with treatment, but there is great variety in patient selection, treatment and evaluation methodologies.
CT and PET CT give best quality images for on-treatment assessment. However, if used throughout the course of treatment, they would require extra imaging sessions with additional dose and inconvenience to the patient. CBCT is already commonly used as online verification of patient position. As this is performed in the treatment setting within a routine appointment, its use in adaptive planning will be evaluated in this study.
The aim of this study was to assess positional and volume changes to GTV during a course of conformal lung RT by using on-treatment CBCT and its potential for assessing the need to replan. A series of patients undergoing RT alone were included in this study.
METHODS AND MATERIALS
This study was carried out retrospectively on a group of patients already treated between 21 March 2011 and 30 December 2012. Patients were treated according to standard protocols and no additional study visits or intervention occurred. A total of 17 patients who had completed radical RT for lung cancer were selected for inclusion in this study. For 12 of these patients, volume and position changes of GTV could be quantitatively analysed. Patients had pathologically confirmed NSCLC, tumour classification 1–4. Planning target volumes (PTVs) of varying size were distributed in various lobes of the lung (for patient details, see Table 1). None of these patients were receiving sequential or concurrent systemic chemotherapy.
Table 1.
Patient characteristics
| Characteristics | n = 17 |
|---|---|
| Gender | |
| Male | 8 |
| Female | 9 |
| Age (years) | |
| Median | 68 |
| Range | 54–86 |
| Tumour location | |
| RUL | 6 |
| RLL | 3 |
| LUL | 4 |
| LLL | 4 |
| T-classification | |
| 1 | 5 |
| 2 | 6 |
| 3 | 5 |
| 4 | 1 |
| Initial GTV volume (cm3) | |
| Range | 2.7–266.9 |
| Mean (SD) | 58.6 (72.0) |
GTV, gross tumour volume; LLL, left lower lobe; LUL, left upper lobe; RLL, right lower lobe; RUL, right upper lobe; SD, standard deviation.
Patient selection
All patients had completed a course of RT prescribed to a total dose of 55 Gy in 20 fractions, treated daily over 4 weeks. Patients were only selected and included for analysis once it was confirmed that they had not received chemotherapy sequentially or concurrently as part of their disease management. All data were collected and analysed retrospectively, with no alterations being made to the patient's treatment.
Planning image acquisition
Prior to RT planning, each patient underwent a PET scan (GE Discovery™ 690; GE Medical Systems, Waukesha, WI) for diagnosis and staging. This scan was used to aid structure delineation in the planning process. This was viewed as per local planning guidelines, alongside the planning CT, to ensure that all active tumour and nodes are identified (no image registration with CT was performed). This scan was performed with the patient positioned diagnostically. Time between PET-CT scan to planning scan was a median of 28 days (range 9–50 days).
Patients were immobilized using an indexed Posirest™ board (Civco Medical Solutions, Kalona, IA) with headrest and arm supports. A Sinmed knee rest (Civco Medical Solutions) was used to enhance patient comfort and maintain stability. Patients had a CT scan acquired using a standard departmental free-breathing 4DCT scanning protocol with 2.5-mm slices on a GE LightSpeed® 16 helical CT scanner (GE Medical Systems) with Real-Time Position Management™ (RPM; Varian Medical Systems, Palo Alto, CA). Images were acquired and binned to show motion at 10 separate phases of the breathing cycle correlated to the trace acquired by RPM. These images were imported into the Eclipse™ TPS v. 8.6 (Varian Medical Systems) for 3D conformal RT planning. A dose of 55 Gy in 20 daily treatments over 4 weeks was prescribed as per International Commission on Radiation Units and Measurements guidelines19 to the PTV.
Structure delineation and planning
All planning volumes were delineated by one of two experienced clinical oncologist (CO). All CT data sets were viewed using the lung window setting. The planning 4DCT scan was used to delineate the initial planning GTV (pGTV) by identifying the tumour throughout the different phases of the breathing cycle.20 The available PET scan was used for visual guidance in this process. This volume was copied onto the average intensity projection (Ave-IP) and was expanded to create a clinical target volume (CTV) (+5 mm), creating an internal target volume (ITV) and then grown to a PTV (+0 – 20 mm, depending on individual assessment) used for treatment planning. All patients were planned using a conformal three-field isocentric planning technique with 6-MV photons.
Treatment and verification
RT treatment was delivered using a Varian Clinac® 2100EX (Varian Medical Systems) with patients being set up in the treatment position as described above.
Pre-treatment CBCT verification was performed throughout the treatment schedule to verify treatment position immediately before delivery. The departmental protocol was initially to perform the online verification on Fractions 1, 2, 3 and weekly. This changed to Fractions 1 and weekly during acquisition of the data. This change was implemented due to isocentric kV images giving adequate information for the radiographer match on Fractions 2 and 3. This was acquired using Varian on-board imager v. 1.4 (OBI®; Varian Medical Systems) low-dose thorax setting with a half-fan bowtie filter. CBCT images were registered to the planning CT by two radiographers with image-matching competencies. The match consisted of a bony match using a region of interest in close proximity to the PTV and further checking of GTV coverage with the PTV structure. Following registration, these images were automatically stored in the record and verify system (Varian ARIA®; Varian Medical Systems).
Regular quality assurance (QA) checks are carried out on the imaging equipment to ensure matching registration is accurate. Quantitative QA checks of fiducial matching show an accuracy of ≤1 mm.
Image assessment: volumes–cone beam CT
Retrospectively, the pGTV was copied into each of the acquired CBCT image data sets. This structure was then modified by one of the two COs to create a new GTV for each weekly interval to represent any changes before commencing treatment, cbctGTV1 (0 Gy) and throughout treatment cbctGTV2 (13.75–19.25 Gy), cbctGTV3 (27.5–33 Gy) and cbctGTV4 (41.25–46.75 Gy). Dose ranges here indicate the integrated treatment dose delivered at the point of imaging. For patients imaged 1, 2, 3 and weekly, this would be prior to Fractions 1, 8, 13 and 18 and in following patients this would be Fractions 1, 6, 11 and 16.
Data analysis
The following data were collected from the TPS for pGTV and each cbctGTV: (a) volume (cm3) and (b) centre of mass (COM) using 3D vector.
Volume differences
On the first day of their RT course, patients had CBCT acquired immediately prior to treatment. This CBCT data set obviously shows no radiation-induced differences and is retrospectively used to make a comparison between planned GTV and on-treatment GTV. As there were differences between pGTV and cbctGTV1, which may (at least in part) be due to systematic differences in the imaging technique, cbctGTV1 was used as a baseline to investigate changes with radiation dose during the treatment phase. A related sample Wilcoxon signed rank test was used to test for statistical significance.
Furthermore, weekly cbctGTVs were measured and used to compare volumes between baseline (cbctGTV1) and particular dose being delivered. Statistically, data were assessed by fitting mixed models, which takes repeated observations into account with patients as random effects; volume change of 20/30% was predicted at 30/50 Gy, respectively.
Volumes were expressed in cubic centimetres with ratios and means being calculated between the compared volumes.
Geometric shifts
The x, y and z co-ordinates of structure COM were recorded for each GTV including pGTV, cbctGTV1, cbctGTV2, cbctGTV3 and cbctGTV4. These co-ordinates were used to calculate the 3D vector displacement of the following structures: pGTV to cbctGTV1 and then cbctGTV1 to each of the cbctGTVs from subsequent fractions. The shift in COM, between pGTV and each cbctGTV, was also calculated.
A Pearson correlation coefficient was used to assess the relationship of the COM displacement and the pGTV volume. For COM throughout treatment, a fitted model with patients as random variable was used.
Volume overlap
Boolean operators were used to determine the volume of overlap between pGTV and each analysed cbctGTV. A linear regression analysis was used to illustrate this. All data were entered into SPSS® v. 20 (IBM Corp., New York, NY; formerly SPSS Inc., Chicago, IL) for statistical analysis.
To quantitatively compare any two structures, the dice similarity coefficient (DICE) was calculated.
RESULTS
17 patients were suitable for inclusion in the study, and the patient characteristics can be seen in Table 1. 5 of the 17 patients could not be quantitatively analysed using GTV delineation owing to 3 having the presence of atelectasis, 1 having uncertainty about the entirety of the volume being captured on CBCT and 1 patient having a central tumour which caused difficulty in delineation. The 12 patients analysed are quantitatively described below.
Planning gross tumour volume to cone beam CT-based gross tumour volume 1
Volume differences
The volumes from pGTV and all cbctGTV structures are shown in Table 2. Nine patients' cbctGTV1 (mean 77.4 ± 93.2 cm3) showed an increase in volume when compared with the pGTV (mean 66.0 ± 85.3 cm3). The ratio of cbctGTV1 to pGTV has a range of 0.91–1.45, mean 1.20 ± 0.19 standard deviation (SD), indicating a mean 20 ± 19% increase in volume between planning CT and CBCT pre-treatment volume (statistically significant at p = 0.04).
Table 2.
All gross tumour volumes (GTVs) (cm3), ratios and dice similarity coefficient (DICE) for each patient
| Structure | pGTV | cbctGTV1 |
cbctGTV2 |
cbctGTV3 |
cbctGTV4 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Volume | Volume | Ratio to pGTV | DICE | Volume | Ratio to cbctGTV1 | DICE | Volume | Ratio to cbctGTV1 | DICE | Volume | Ratio to cbctGTV1 | DICE |
| 1 | 7.7 | 9.1 | 1.18 | 0.46 | 8.8 | 0.97 | 0.59 | 7.5 | 0.82 | 0.47 | 7.7 | 0.84 | 0.76 |
| 2 | 2.7 | 3.0 | 1.13 | 0.65 | 2.8 | 0.93 | 0.88 | 2.7 | 0.90 | 0.82 | 1.5 | 0.50 | 0.57 |
| 3 | 266.9 | 242.1 | 0.91 | 0.90 | 225.6 | 0.93 | 0.94 | 223.9 | 0.92 | 0.94 | 219.5 | 0.91 | 0.90 |
| 4 | 7.7 | 11.1 | 1.45 | 0.73 | 11.0 | 0.99 | 0.62 | 8.9 | 0.80 | 0.81 | 7.8 | 0.70 | 0.78 |
| 5 | 21.6 | 28.7 | 1.33 | 0.76 | 25.4 | 0.88 | 0.86 | 24.4 | 0.85 | 0.85 | 19.6 | 0.68 | 0.77 |
| 6 | 116.3 | 126.7 | 1.09 | 0.93 | 126.1 | 1.00 | 0.92 | 102.8 | 0.81 | 0.86 | 91.9 | 0.73 | 0.83 |
| 7 | 6.1 | 6.1 | 0.99 | 0.86 | 4.3 | 0.71 | 0.82 | 3.1 | 0.52 | 0.43 | 3.2 | 0.53 | 0.52 |
| 8 | 96.2 | 139.4 | 1.45 | 0.76 | 125.3 | 0.90 | 0.64 | 118.4 | 0.85 | 0.78 | 98.0 | 0.70 | 0.77 |
| 9 | 58.6 | 79.6 | 1.36 | 0.84 | 75.3 | 0.95 | 0.88 | 70.6 | 0.89 | 0.77 | 64.9 | 0.81 | 0.81 |
| 10 | 184.1 | 256.1 | 1.39 | 0.80 | 235.9 | 0.92 | 0.92 | 237.1 | 0.93 | 0.91 | 225.2 | 0.88 | 0.87 |
| 11 | 16.9 | 20.0 | 1.18 | 0.87 | 19.9 | 1.00 | 0.96 | 20.2 | 1.01 | 0.96 | 18.2 | 0.91 | 0.87 |
| 12 | 7.2 | 7.1 | 0.98 | 0.47 | 6.8 | 0.96 | 0.65 | 5.8 | 0.82 | 0.72 | 5.4 | 0.76 | 0.26 |
| Mean | 66.0 | 77.4 | 1.20 | 0.75 | 72.3 | 0.93 | 0.81 | 68.8 | 0.84 | 0.78 | 63.6 | 0.75 | 0.73 |
| Standard deviation | 85.29 | 93.24 | 0.19 | 0.16 | 86.59 | 0.08 | 0.14 | 85.46 | 0.12 | 0.17 | 81.76 | 0.14 | 0.19 |
cbctGTV, cone beam CT-based GTV; pGTV, planning GTV.
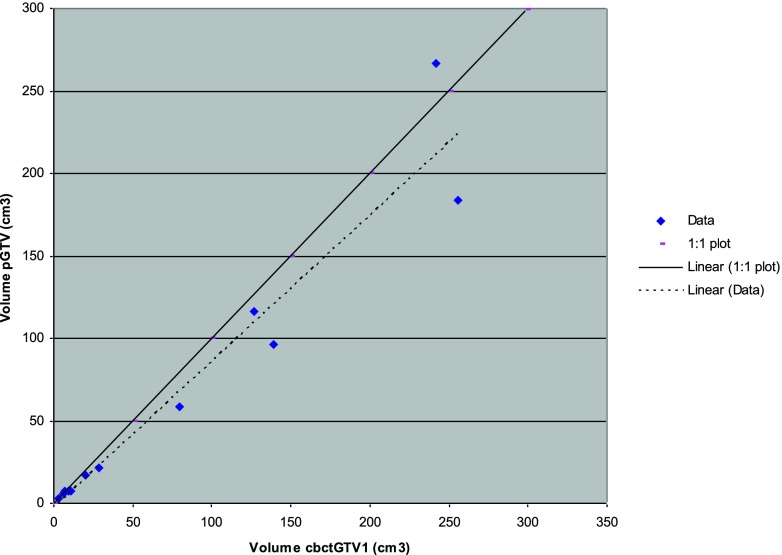
A correlation between pGTV and cbctGTV1 volume was evident (r2 = 0.932) (Figure 1).
Figure 1.
Planning gross tumour volume (pGTV) plotted against cone beam CT-based gross tumour volume 1 (cbctGTV1). r2 linear = 0.932.
Volume overlap
When comparing the volume of pGTV included within the cbctGTV1 volume (Table 3), there were no pGTVs fully encompassed within the CBCT volume or vice versa. On average, 82.9 ± 18.0% of pGTV was within cbctGTV1 and only 69.8 ± 16.1% of cbctGTV1 was within pGTV. This suggests that volumes were not only bigger, but they were also not coincident. This is confirmed by mean DICE (±SD) 0.75 ± 0.16.
Table 3.
Overlap of planning gross tumour volume (pGTV) and cone beam CT-based gross tumour volume acquired prior to fraction 1 (cbctGTV1) volume (cm3), percentage (%) of structure within other structure and dice similarity coefficient (DICE)
| Patient | pGTV and cbctGTV1 (cm3) | % pGTV within cbctGTV1 | % cbctGTV1 within pGTV | DICE |
|---|---|---|---|---|
| 1 | 3.9 | 50.2 | 42.6 | 0.46 |
| 2 | 1.9 | 69.7 | 61.8 | 0.65 |
| 3 | 229.9 | 86.1 | 95.0 | 0.90 |
| 4 | 6.9 | 89.6 | 61.9 | 0.73 |
| 5 | 19.2 | 88.7 | 66.7 | 0.76 |
| 6 | 113.4 | 97.5 | 89.5 | 0.93 |
| 7 | 5.2 | 85.3 | 86.5 | 0.86 |
| 8 | 88.9 | 92.4 | 63.8 | 0.76 |
| 9 | 57.9 | 98.9 | 72.7 | 0.84 |
| 10 | 175.9 | 95.6 | 68.7 | 0.80 |
| 11 | 16.1 | 95.0 | 80.6 | 0.87 |
| 12 | 3.3 | 46.2 | 47.4 | 0.47 |
| Mean | 60.2 | 82.9 | 69.8 | 0.75 |
| Standard deviation | 77.0 | 18.0 | 16.1 | 0.16 |
Centre of mass displacement
Calculated 3D displacements of the following structures: pGTV to cbctGTV1 and then cbctGTV1 to each of the cbctGTVs from subsequent fractions are summarized in Table 4.
Table 4.
Centre of mass displacement between gross tumour volumes (GTVs) (cm) analysed as a three-dimensional vector
| Patient | pGTV to cbctGTV1 | cbctGTV1–2 | cbctGTV1–3 | cbctGTV1–4 |
|---|---|---|---|---|
| 1 | 0.88 | 0.66 | 0.74 | 0.29 |
| 2 | 0.22 | 0.05 | 0.13 | 0.37 |
| 3 | 0.26 | 0.21 | 0.19 | 0.48 |
| 4 | 0.38 | 0.69 | 0.21 | 0.09 |
| 5 | 0.22 | 0.14 | 0.33 | 0.20 |
| 6 | 0.13 | 0.12 | 0.37 | 0.50 |
| 7 | 0.14 | 0.24 | 0.39 | 0.35 |
| 8 | 0.40 | 1.16 | 0.26 | 0.20 |
| 9 | 0.40 | 0.21 | 0.55 | 0.55 |
| 10 | 0.56 | 0.19 | 0.21 | 0.23 |
| 11 | 0.26 | 0.10 | 0.08 | 0.26 |
| 12 | 0.71 | 0.50 | 0.31 | 0.94 |
| Mean | 0.38 | 0.36 | 0.31 | 0.37 |
| Standard deviation | 0.22 | 0.32 | 0.18 | 0.22 |
cbctGTV, cone beam CT-based GTV; pGTV, planning gross tumour volume.
3D COM displacement between pGTV and cbctGTV1 show a displacement range of 0.13–0.88 cm, mean 0.38 ± 0.22 cm; 5 mm is a commonly used threshold for set-up correction, as it is included within the PTV; three patients had a displacement of >5 mm.
No correlation was found between COM displacement and the pGTV (p = 0.709).
All further analysis will describe the weekly CBCT volumes. At these points, radiation had been delivered and effects of this could be investigated.
On-treatment volume comparison
Cone beam CT-based gross tumour volumes
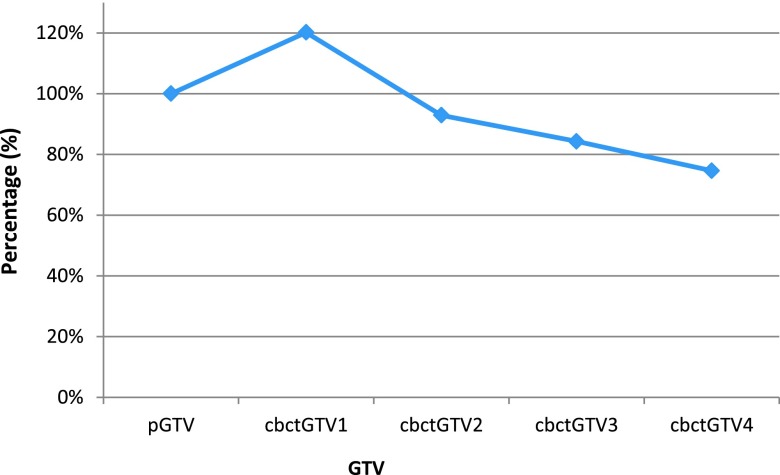
Subsequent image volumes were expressed as a ratio of the cbctGTV1 for each patient, and DICE was included. These data are presented in Table 2 and plotted in Figure 2.
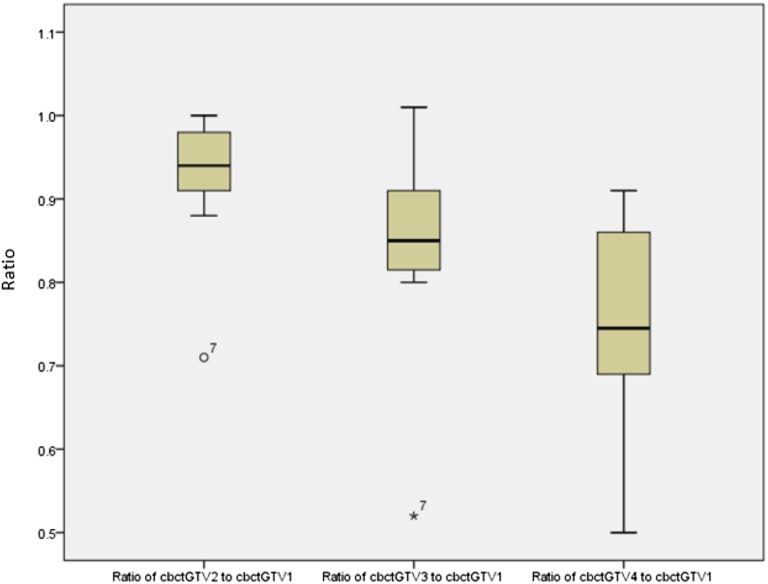
Figure 2.
Ratio of weekly cone beam CT-based gross tumour volume (cbctGTV2–4) to baseline cone beam CT-based gross tumour volume 1 (cbctGTV1) showing range/interquartile range and mean.
Mean volumes for cbctGTV1, cbctGTV2, cbctGTV3 and cbctGTV4 were 77.4 ± 93.2, 72.3 ± 86.6, 68.8 ± 85.5 and 63.6 ± 81.8 cm3, respectively. Comparing weekly volumes to cbctGTV1, the mean ratios (±SD) were cbctGTV2, 0.93 ± 0.08; cbctGTV3, 0.84 ± 0.12; and cbctGTV4, 0.75 ± 0.14 showing a trend towards volume reduction with increasing dose. The DICE for these volumes was 0.81 ± 0.14, 0.78 ± 0.17 and 0.73 ± 0.19, respectively.
On completion of 10–12 fractions of treatment (cbctGTV3), a dose of 27.50–33.0 Gy had been delivered with changes in volume of −1 to 48%, mean 16% ± 12. There was one patient indicating a 30% decrease in GTV, Patient 7 who had a 48% reduction at this time point and can be seen as an outlier in Figure 2.
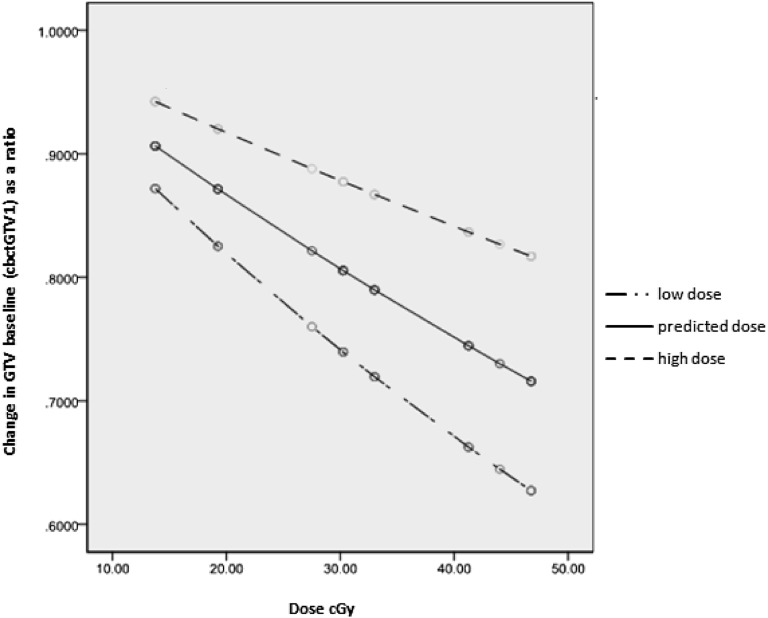
5 patients had ≥30% reduction after receiving 41.25–46.75 Gy, which relates to cbctGTV4, range 9–50% reduction, mean 25 ± 14%. The mean predicted dose required to achieve a 20% reduction in GTV is around 30 Gy and for a 30% reduction around 50 Gy (Figure 3).
Figure 3.
Mean estimated change relative to baseline (centre line) from the fitted model together with upper and lower 95% confidence interval (top and bottom line, respectively). Baseline is cone beam CT-based gross tumour volume 1 (cbctGTV1) volume.
A correlation was identified between volume reductions and dose delivered (beta = −0.003, p < 0.001)—a highly statistically significant association.
The overlap between weekly volumes is displayed in Table 5. Since COM displacement does not change as dose increases, the overlap of the new weekly GTV is consistent with volume reduction. The DICE is shown in Table 2.
Table 5.
This table shows volume overlap of baseline cone beam CT (CBCT)-based gross tumour volume (GTV) acquired prior to fraction 1 (cbctGTV1) with weekly CBCT-based GTV (cbctGTVs) (cm3)
| Patient | Volume of overlap (cm3) |
||
|---|---|---|---|
| cbctGTV1 and 2 | cbctGTV1 and 3 | cbctGTV1 and 4 | |
| 1 | 5.3 | 4.4 | 6.4 |
| 2 | 2.6 | 2.3 | 1.3 |
| 3 | 220.1 | 218.9 | 208.5 |
| 4 | 6.9 | 8.1 | 7.4 |
| 5 | 23.2 | 22.5 | 18.5 |
| 6 | 115.9 | 99.1 | 90.7 |
| 7 | 4.2 | 2.0 | 2.4 |
| 8 | 85.0 | 100.1 | 91.8 |
| 9 | 68.4 | 58.2 | 58.5 |
| 10 | 225.2 | 224.5 | 210.1 |
| 11 | 19.2 | 19.2 | 16.7 |
| 12 | 4.5 | 4.6 | 1.6 |
| Mean | 65.0 | 63.7 | 59.5 |
| Standard deviation | 82.45 | 81.97 | 77.43 |
Centre of mass
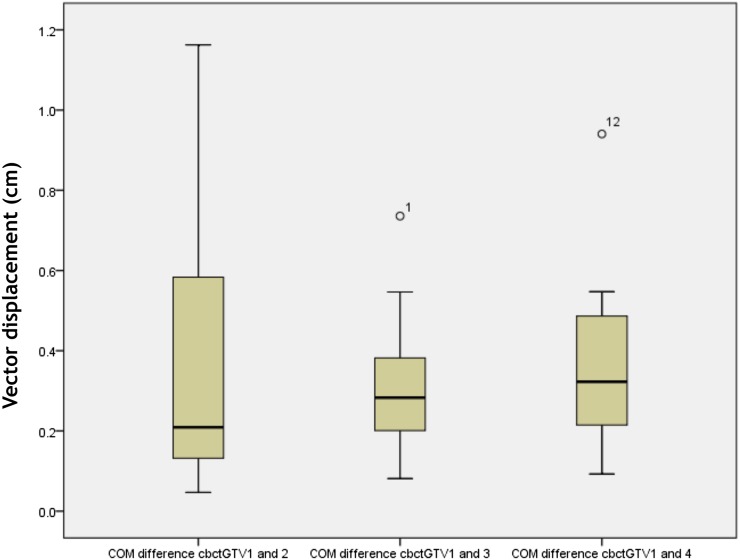
COM displacement for cbctGTV1 to cbctGTV2, cbctGTV3 and cbctGTV4 showed a mean of 0.36 ± 0.32, 0.31 ± 0.18 and 0.37 ± 0.22 cm. Displacements of >5 mm were seen for a total of 3, 2 and 2 out of 12 patients at each respective weekly comparison (Table 4).
Figure 4 shows a consistent trend in the COM displacement throughout the course of radiation being delivered.
Figure 4.
Centre of mass (COM) displacement between each of cone beam CT-based gross tumour volume at 2, 3 and 4 (cbctGTV2–4) with baseline cone beam CT-based gross tumour volume 1 (cbctGTV1). Displacement expressed as three-dimensional vector (cm).
There was no correlation between COM displacement and volume change.
Atelectasis
Three patients had atelectasis and mean lung volume increased as treatment was delivered (Table 6).
Table 6.
Patients with atelectasis. Normal lung included in planning target volume (PTV) on planning CT (pCT) and cone beam CT (CBCT) data set
| Patient | Volume (cm3) |
Normal lung in PTV (cm3) |
|||||
|---|---|---|---|---|---|---|---|
| PTV | pCT | CBCT1 | CBCT2 | CBCT3 | CBCT4 | ||
| 13 | 289.1 | 38.3 | 14.02 | 15.04 | 20.97 | 53.42 | |
| 14 | 363 | 41.81 | 18.6 | 18.1 | 23.2 | 36.5 | |
| 15 | 109.1 | 54.6 | 47.2 | 59.7 | 62.1 | 71.7 | |
| Mean | 253.7 | 44.9 | 26.6 | 30.9 | 35.4 | 53.9 | |
| Standard deviation | 130.59 | 8.58 | 17.98 | 24.95 | 23.13 | 17.60 | |
DISCUSSION
The potential value of using on-treatment acquired CBCT images in lung ART was investigated in this study. Between planning and pre-treatment imaging on Day 1, 75% of tumours had increased in volume. There is a statistically significant difference between pGTV and cbctGTV1. This increase may be in part due to differences in volumes being based on differing imaging modalities. In addition, it may also be due to tumour progression. Biederer et al21 found that CBCT volumes from a phantom-based study were significantly larger (up to 7%) than on the planning scan. In our study, two different CT-based imaging methods were used. 4DCT allows definition of the tumour volume at the maximum phases of the breathing cycle, whereas CBCT acquires a time-averaged mean target position. The comparison in this study was between a tumour volume delineated on different phases of the breathing cycle and CBCT. Although Ave-IP was used as a reference image for matching and calculation, the volume change data is not quantified using any comparison to the Ave-IP.
The quality of CBCT for delineation purposes is inferior to that of the planning scan and interobserver variability using this image modality may be an issue.22,23 Biederer et al reported higher interobserver variability using CBCT. This variation was higher in the small volumes included in the study.21 The benefits of using CBCT are its relatively short acquisition time and availability for patients in a treatment set-up position. If used in the ART process, no additional dose to the patient would be required as this is already acquired for the purposes of set-up.
Reports have shown that disease progression between diagnosis/staging/planning to treatment process has been of concern.24,25 Disease progression cannot be ruled out as a contributing factor when explaining the increase in volume from planning CT to Day 1 of treatment. In the UK, there is now a greater focus on working to 31-day targets where the patients' “ready-to-start” date to “start-of-treatment” should not exceed this time with the hope that the opportunity for progression is minimized. Although the “planning-to-treatment” time is kept to a minimum to allow this target to be achieved, “planning scan-to-start of treatment” can still take up to 3 weeks in our department.
For pGTV and cbctGTV1 COM, we have found positional changes as well as volume changes with 3 out of 12 patients showing a gross displacement in this time, i.e. >5 mm.
Comparison of cbctGTVs demonstrated volume reduction throughout the course of dose delivery. The difference between pGTV and cbctGTV1 is not likely to be related to the use of PET. As PET is used qualitatively to ensure adequate inclusion of nodal disease, this factor would not be responsible for any increase in primary volume at baseline cbctGTV1.
Weekly overlap of these volumes were calculated and showed a slight reduction in volume overlap. When we reviewed the COM displacement for GTVs on CBCT, the majority (78%) of measurements were within the 5-mm tolerance. This displacement would still ensure that the GTV is within our set-up margin. Trends were identified showing a reduction in volumes as radiation dose increased. This is consistent with other studies10–14,26,27 that reported on tumour regression and migration. CBCT data proved to be a useful tool in quantifying the change in GTV and has potential in assessing individual patient's suitability for ART. Although we would not recommend using CBCT data sets to replan treatments, we would encourage the use of its data to identify those patients who are responding well to treatment and may benefit from an adaptive process. Woodford et al14 recommended identifying a 30% change in GTV as a useful indicator of patients who may benefit from ART. This is based on volume change occurring within the first 20 fractions; however, they used a longer fractionation schedule of 30 fractions of 2 Gy over a 6-week period compared with 20 fractions of 2.75 Gy over 4 weeks in our study.
To identify patients who may benefit from ART, on-treatment volume changes are typically compared with planned volumes. For all patients, the mean total volume changes from planning to end of treatment can be seen in Figure 5. The initial volume increases, of up to 20%, counteract volume reductions on-treatment. From our data, a mean 30% reduction in GTV from the first fraction is predicted by the last week of treatment. However, taking into account the initial volume increases between planning and the first fraction of treatment, resultant volume reductions are of the order of 10%, which falls below the 30% threshold in volume change suggested by Woodford et al.14
Figure 5.
Mean ratio volume change on each data set with planning gross tumour volume (pGTV) 100%.
This study does not reach a 30% average volume reduction across patient population. As predicted by our model, the 30% change is likely to happen across the patient population once the patient's treatment is near completion, approximately 50 Gy. From pGTV, we identified a 30% decrease in GTV for 5 of the 12 patients (42%) by cbctGTV4, which is the CBCT data set acquired prior to Fractions 16–18 of 20 (maximum dose of 46.25 Gy/17 fractions being delivered). Practically, to revolume, replan and initiate a new plan for treatment, a minimum time period of 48 h would be required in our department. As the patient would have little remaining treatment, this would reduce the benefit from replanning. Dose-escalated regimes with longer fractionation schedules may provide a better opportunity to assess and implement change, but this would need further research. There would be little benefit from increasing the image frequency to assess volume reduction as tumour volume change appears to happen slowly.
Both COs in this study did not have great confidence in using CBCT to define tumour volumes due to quality of images. They expressed concern at using a non-contrast CBCT as the sole imaging modality when redefining the target volume, particularly where there was mediastinal nodal involvement. The introduction of greater uncertainty could have encouraged the CO to over compensate and be overly conservative when delineating tumours, resulting in larger volumes. As a consequence, this may in turn result in higher doses to normal tissue. We would like to further investigate CBCT as a tool in ART, and respiratory-gated CBCT may offer benefit.
For two patients, a visual assessment was employed where the weekly CBCT images did not allow contouring of GTV. In one patient, their disease was in a central location whereby identification of the tumour boundary within the mediastinum was difficult to detect on non-contrast CBCT. Visual assessment indicated the volumes were impossible to match as they looked so different. The other remaining patient had two areas identified as PTV, and there was uncertainty as to whether the most inferior aspect of the volume was captured on the CBCT data sets. Clear volume reduction was evident by CBCT3. The visible portion of GTV had reduced by around 25% on this CT data set. This was delineated using anatomical reference for inferior measurements and comparison on each data set. Owing to the uncertainty of the full GTV being delineated, it is not evaluated quantitatively.
One of the main challenges of our study was finding a homogeneous group of patients. Our centre treats a large number of patients for NSCLC using combination chemotherapy (sequential or concurrent) or RT alone. Treatment is individualized based on the stage and patient fitness. In order to find a group which would not include confounding factors, it was necessary to use the patients in the RT-alone group. When RT alone is used, our preference is to treat using a hyperfractionated accelerated regime, continuous hyperfractionated accelerated radiotherapy. A minority of patients have a conventionally fractionated regime over 4 weeks, as the clinicians feel this is an inferior option to continuous hyperfractionated accelerated radiotherapy.
There are also resource issues where at the time of analysis, not every patient being treated for lung cancer in our centre was verified using CBCT imaging, something which is improving with the replacement programme of old machines and increased imaging capabilities with new ones.
The tumour classification and location varied within this study. This has led to analysis of a heterogeneous group of patients which reflects the “real life” situation in our department.
Our data showed that by the time a significant reduction in tumour volume was evident, it was too late to introduce an adapted plan. To make a single recommendation for the group of patients receiving RT alone is not possible. Tumours in medial locations are difficult to delineate on CBCT and patient response to treatment varies. However, one of the benefits of looking at RT alone is that tumour regression from chemotherapy effects is eliminated.
In the treatment of lung cancer, chemotherapy may not be an option because of comorbidities and performance status. If this is the case, the RT-alone group is one of the most important groups to assess, with obvious benefits from reducing normal tissue dose. If considering dose escalation, the reduction of normal tissue toxicity is of interest.
Other studies have mainly looked at patients having chemotherapy. This includes the group which recommends the 30% reduction.14 However, Fox et al26 did not see a significant difference in GTV reduction by chemoradiation vs RT alone, treating with 2-Gy fractions over 5–7.5 weeks. Studies already published on lung ART typically discuss the use of 2 Gy per fraction doses with a typical dose and fractionation being around 60 Gy/30 fractions.10–14 This study only looks at a dose and fractionation of 55 Gy/20 fractions, 2.75 Gy/fraction in 4 weeks. This is not a biologically equivalent dose comparable to that of other studies. For this reason, the timescale for dose delivery, tumour regression and even the possibility of tumour repopulation (growth) will differ.
Given that PET aids the delineation of the target volume at the planning stage, it is reasonable to suggest repeat PET scanning in the event that the treatment plan is to be amended. Fluorine-18 fludeoxyglucose (18F-FDG) PET scans are performed on all patients prior to embarking on radical treatment. 18F-FDG PET scans are helpful in identifying nodal involvement and defining the extent of disease at the planning stage. Some groups have evaluated serial 18F-FDG PET scans taken during treatment. Ding et al28 report significant change to avidity using PET/CT after 40 Gy. Their study included patients receiving chemotherapy and RT. Analysis of patient receiving both chemotherapy and RT results in additional uncertainty of tumour change during RT treatment. A study with larger patient numbers would be required to validate any recommendations. Another study by Edet-Sanson et al29 investigated the use of serial PET images acquired throughout treatment. This was a small study which evaluated 10 patients, half of whom also had chemotherapy. A 50% decrease in maximum standardized uptake value was reported near 40–45 Gy. Feng et al27 highlight the caution necessary on reducing GTV when disease has become PET negative.
There is also concern that if any adaptive plan is adopted partway through treatment based on changes in gross disease, this could result in microscopic disease being missed or not treated adequately. Previous models suggested using a CTV margin of 9 mm.30 The reduction in visible primary volume may not be representative of what happens to the microscopic disease: does it shrink with this volume or does it stay in the original location. Guckenberger et al31 described two possible ways of dealing with microscopic disease, including infiltrative growth pattern or an expansive growth pattern. By modelling tumour control probability for microscopic disease, this group was able to assess that dosimetrically, by adapting a plan, they did not compromise dose coverage. Also, since the adaptive process could help dose escalation, this did improve tumour control probability. There is limited information on the total dose required to treat microscopic disease, but it is thought this may be lower than required for GTV. It is certainly not something which should prevent further research into adaptive strategies.
Patients with atelectasis were assessed by recording the volume of normal lung present within the PTV. As a weekly GTV could not be identified on CBCT due to collapse, it was necessary to use the PTV to assess change within the treatment area. These data are presented in Table 6. The mean volume of normal lung within PTV on CBCT1, CBCT2, CBCT3 and CBCT4 is 26.6, 30.9, 35.4 and 53.9 cm3, respectively. The increase in normal lung confirms that there is a change in anatomy around the GTV.
CBCT was useful in this study in identifying change to normal lung within the planned PTV for patients with atelectasis. It would be useful to conduct further work to evaluate where a repeat PET should be requested and used alongside the CBCT data set to assess an adaptive approach. As lung reinflation could significantly change the location of the GTV and the dosimetric reliability of the plan, these patients could benefit from adaptive intervention.
Investigation of dose distributions to PTVs is beyond the scope of this study. This study was purely to assess GTVs and so any implications on CTVs and PTVs resulting from a change in GTV were not assessed. Currently, we would only replan a patient if a repeat planning CT was acquired to allow delineation and calculation.
Since beginning this study, RT treatment planning and delivery developments have evolved significantly in our department, particularly with the implementation of IMRT/VMAT. An evaluation of a midway contrast-enhanced planning CT scans in patients treated with VMAT IMRT is under way. It will be interesting to see if this agrees with our prediction that very few patients will demonstrate significant tumour regression half-way through their course of RT. It may be that CBCT can filter patients who are likely to benefit from a repeat planning scan. This requires further evaluation.
Low numbers of patients with mixed staging is a limitation in this study which will have an impact on SDs and achieving statistical significance. There was no image registration between PET and CT planning data. However, PET data were only used qualitatively to ensure adequate nodal disease coverage and not used quantitatively for target delineation of primary volume.
The time between imaging procedures reflect the practicalities of managing patients within a busy department. Ideally, this should be minimized where possible, and following this analysis, recommendations have been made to reduce the patient pathway.
pGTVs were defined using the different phases of the breathing cycle to ensure full motion was included in the ITV. This was grown to create a PTV, then dose distributions were calculated on the Ave-IP. The Ave-IP is used for dose calculation and as a reference image for on-treatment verification, not solely to define ITV. A limitation in this study is the known differences between imaging modalities and the differences in acquisition principals.22,23,32,33
Assessing volumes on CBCT could have been improved using 4D-CBCT; however, this study made volume assessments using standard free-breathing CBCT which is more readily available in the UK. There are known problems using free-breathing CBCT such as underestimation of ITV where breathing patterns are irregular.33 Benefits of 4D-CBCT include the improvement of defining the shape of a moving structure, as well as reducing artefacts;32 however, 3D-CBCT is commonly used in centres for on-treatment verification and is often carried out routinely.
CONCLUSION
This study assessed serial CBCT imaging for 17 patients with NSCLC undergoing RT-alone treatment.
Results have shown mean volume increases of 20 ± 19% between planned and first fraction GTVs. Volumes decreased throughout treatment, with volumes in the last week of treatment being a mean of 25 ± 14% smaller than those at first fraction. For a small subgroup of patients with atelectasis, CBCT imaging was able to identify changes in lung volume during treatment.
When using CBCT scans, we recommend that all analysis should be carried out using the baseline GTV measurement acquired immediately before Day 1 of treatment. We have shown that CBCT imaging can help us understand how the tumour changes as it responds to treatment. Although, our data suggest that volume changes for patients in this study are not large enough or not occurring sufficiently early in treatment to identify patients who may benefit from the use of ART. Minimizing the gap between planning CT and the start of treatment will reduce volume increase due to progression. Ideally, quantifying differences in imaging volume measurement (between conventional CT and CBCT) would help to reduce volume errors. Interobserver variability should be quantified locally.
Serial imaging can be used as a screening tool to filter patients who are demonstrating significant changes following radiation being delivered. By highlighting the patients who display a good response to treatment, further CT imaging may be beneficial.
For patients with atelectasis, CBCT can enable changes in lung volume to be identified, but it is often not possible to quantify changes to target volumes, limiting its usefulness.
Acknowledgments
ACKNOWLEDGMENTS
The author(s) acknowledge the advice on early drafts of this manuscript from Dr Stuart McNee, formerly of Department of Clinical Physics and Bio-Engineering Beatson West of Scotland Cancer Centre.
Contributor Information
Aileen Duffton, Email: aduffton@hotmail.com, aileen.duffton@ggc.scot.nhs.uk.
Stephen Harrow, Email: stephen.harrow@ggc.scot.nhs.uk.
Carolynn Lamb, Email: carolynn.lamb@ggc.scot.nhs.uk.
Mark McJury, Email: mark.mcjury@ggc.scot.nhs.uk.
REFERENCES
- 1. Cancer Registration Statistics, England: 2014. http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/lung-cancer/incidence#ref-0.
- 2. National records of Scotland, Scotland: March 2014. http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/lung-cancer/incidence#ref-0.
- 3. Northern Ireland Cancer Registry, NI: December 2013. http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/lung-cancer/incidence#ref-0.
- 4.Rowell N, Williams C. Radical radiotherapy for stage I/II non-small cell lung cancer in patients not sufficiently fit for or declining surgery (medically inoperable): a systematic review. Thorax 2001; 56: 628–38. doi: 10.1136/thorax.56.8.628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kong FM, Ten Haken RK, Schipper MJ, Sullivan MA, Chen M, Lopez C, et al. High-dose radiation improved local tumor control and overall survival in patients with inoperable/unresectable non-small-cell lung cancer: long-term results of a radiation dose escalation study. Int J Radiat Oncol Biol Phys 2005; 63: 324–33. doi: 10.1016/j.ijrobp.2005.02.010 [DOI] [PubMed] [Google Scholar]
- 6.Movsas B, Raffin TA, Epstein AH, Link CJ, Jr. Pulmonary radiation injury. Chest 1997; 111: 1061–76. [DOI] [PubMed] [Google Scholar]
- 7.Bradley J, Thorstad WL, Mutic S, Miller TR, Dehdashti F, Siegel BA, et al. Impact of FDG-PET on radiation therapy volume delineation in non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2004; 59: 78–86. doi: 10.1016/j.ijrobp.2003.10.044 [DOI] [PubMed] [Google Scholar]
- 8.Sonke JJ, Belderbos J. Adaptive radiotherapy for lung cancer. Semin Radiat Oncol 2010; 20: 94–106. doi: 10.1016/j.semradonc.2009.11.003 [DOI] [PubMed] [Google Scholar]
- 9.Juhler-Nøttrup T, Korreman SS, Pedersen AN, Persson GF, Aarup LR, Nyström H, et al. Interfractional changes in tumour volume and position during entire radiotherapy courses for lung cancer with respiratory gating and image guidance. Acta Oncol 2008; 47: 1406–13. doi: 10.1080/02841860802258778 [DOI] [PubMed] [Google Scholar]
- 10.Britton KR, Starkschall G, Liu H, Chang JY, Bilton S, Ezhil M, et al. Consequences of anatomic changes and respiratory motion on radiation dose distributions in conformal radiotherapy for locally advanced non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2009; 73: 94–102. doi: 10.1016/j.ijrobp.2008.04.016 [DOI] [PubMed] [Google Scholar]
- 11.van Zwienen M, van Beek S, Belderbos J, van Kranen S, Rasch C, van Herk M, et al. Anatomical changes during radiotherapy of lung cancer patients. Int J Radiat Oncol Biol Phys 2008; 72(Suppl.): S111. [Google Scholar]
- 12.Underberg RW, Lagerwaard FJ, Slotman BJ, Cuijpers JP, Senan S. Benefit of respiration-gated stereotactic radiotherapy for stage I lung cancer: an analysis of 4DCT datasets. Int J Radiat Oncol Biol Phys 2005; 62: 554–60. doi: 10.1016/j.ijrobp.2005.01.032 [DOI] [PubMed] [Google Scholar]
- 13.Bosmans G, van Baardwijk A, Dekker A, Ollers M, Wanders S, Boersma L, et al. Time trends in nodal volumes and motion during radiotherapy for patients with stage III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2008; 71: 139–44. doi: 10.1016/j.ijrobp.2007.08.071 [DOI] [PubMed] [Google Scholar]
- 14.Woodford C, Yartsev S, Dar AR, Bauman G, Van Dyk J. Adaptive radiotherapy planning on decreasing gross tumor volumes as seen on megavoltage computed tomography images. Int J Radiat Oncol Biol Phys 2007; 69: 1316–22. doi: 10.1016/j.ijrobp.2007.07.2369 [DOI] [PubMed] [Google Scholar]
- 15.Lim G, Bezjak A, Higgins J, Moseley D, Hope AJ, Sun A, et al. Tumor regression and positional changes in non-small cell lung cancer during radical radiotherapy. J Thorac Oncol 2011; 6: 531–6. doi: 10.1097/JTO.0b013e31820b8a52 [DOI] [PubMed] [Google Scholar]
- 16.Harsolia A, Hugo GD, Kestin LL, Grills IS, Yan D. Dosimetric advantages of four-dimensional adaptive image-guided radiotherapy for lung tumors using online cone-beam computed tomography. Int J Radiat Oncol Biol Phys 2008; 70: 582–9. doi: 10.1016/j.ijrobp.2007.08.078 [DOI] [PubMed] [Google Scholar]
- 17.Hugo GD, Weiss E, Badawi A, Orton M. Localization accuracy of the clinical target volume during image-guided radiotherapy of lung cancer. Int J Radiat Oncol Biol Phys 2011; 81: 560–7. doi: 10.1016/j.ijrobp.2010.11.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guckenberger M, Wilbert J, Richter A, Baier K, Flentje M. Potential of adaptive radiotherapy to escalate the radiation dose in combined radiochemotherapy for locally advanced non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2011; 79: 901–8. doi: 10.1016/j.ijrobp.2010.04.050 [DOI] [PubMed] [Google Scholar]
- 19.ICRU. Prescribing, recording, and reporting photon beam therapy. ICRU report. Volume 50. Bethesda, MD: International Commission on Radiation Units and Measurements; 1993. [Google Scholar]
- 20.Muirhead R, McNee SG, Featherstone C, Moore K, Muscat S. Use of maximum intensity projections (MIPs) for target outlining in 4DCT radiotherapy planning. J Thorac Oncol 2008; 3: 1433–8. doi: 10.1097/JTO.0b013e31818e5db7 [DOI] [PubMed] [Google Scholar]
- 21.Biederer J, Dinkel J, Remmert G, Jetter S, Nill S, Moser T, et al. 4D-imaging of the lung: reproducibility of lesion size and displacement on helical CT, MRI, and cone beam CT in a ventilated ex vivo system. Int J Radiat Oncol Biol Phys 2009; 73: 919–26. doi: 10.1016/j.ijrobp.2008.09.014 [DOI] [PubMed] [Google Scholar]
- 22.Guckenberger M, Meyer J, Wilbert J, Richter A, Baier K, Mueller G, et al. Intra-fractional uncertainties in cone-beam CT based image-guided radiotherapy (IGRT) of pulmonary tumors. Radiother Oncol 2007; 83: 57–64. doi: 10.1016/j.radonc.2007.01.012 [DOI] [PubMed] [Google Scholar]
- 23.Altorjai G, Fotina I, Lütgendorf-Caucig C, Stock M, Pötter R, Georg D, et al. Cone-beam CT-based delineation of stereotactic lung targets: the influence of image modality and target size on interobserver variability. Int J Radiat Oncol Biol Phys 2012; 82: e265–72. doi: 10.1016/j.ijrobp.2011.03.042 [DOI] [PubMed] [Google Scholar]
- 24.O'Rourke N, Edwards R. Lung cancer treatment waiting times and tumour growth. Clin Oncol (R Coll Radiol) 2000; 12: 141–4. doi: 10.1053/clon.2000.9139 [DOI] [PubMed] [Google Scholar]
- 25.Everitt S, Herschtal A, Callahan J, Plumridge N, Ball D, Kron T, et al. High rates of tumor growth and disease progression detected on serial pretreatment fluorodeoxyglucose-positron emission tomography/computed tomography scans in radical radiotherapy candidates with nonsmall cell lung cancer. Cancer 2010; 116: 5030–7. doi: 10.1002/cncr.25392 [DOI] [PubMed] [Google Scholar]
- 26.Fox J, Ford E, Redmond K, Zhou J, Wong J, Song DY. Quantification of tumor volume changes during radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2009; 74: 341–8. doi: 10.1016/j.ijrobp.2008.07.063 [DOI] [PubMed] [Google Scholar]
- 27.Feng M, Kong FM, Gross M, Fernando S, Hayman JA, Ten Haken RK. Using fluorodeoxyglucose positron emission tomography to assess tumor volume during radiotherapy for non-small-cell lung cancer and its potential impact on adaptive dose escalation and normal tissue sparing. Int J Radiat Oncol Biol Phys 2009; 73: 1228–34. doi: 10.1016/j.ijrobp.2008.10.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ding X, Li H, Wang Z, Huang W, Li B, Zang R, et al. A clinical study of shrinking field radiation therapy based on (18)F-FDG PET/CT for stage III non-small cell lung cancer. Technol Cancer Res Treat 2013; 12: 251–7. doi: 10.7785/tcrt.2012.500310 [DOI] [PubMed] [Google Scholar]
- 29.Edet-Sanson A, Dubray B, Doyeux K, Back A, Hapdey S, Modzelewski R, et al. Serial assessment of FDG-PET FDG uptake and functional volume during radiotherapy (RT) in patients with non-small cell lung cancer (NSCLC). Radiother Oncol 2012; 102: 251–7. doi: 10.1016/j.radonc.2011.07.023 [DOI] [PubMed] [Google Scholar]
- 30.Grills IS, Fitch DL, Goldstein NS, Yan D, Chmielewski GW, Welsh RJ, et al. Clinicopathologic analysis of microscopic extension in lung adenocarcinoma: defining clinical target volume for radiotherapy. Int J Radiat Oncol Biol Phys 2007; 69: 334–41. doi: 10.1016/j.ijrobp.2007.03.023 [DOI] [PubMed] [Google Scholar]
- 31.Guckenberger M, Richter A, Wilbert J, Flentje M, Partridge M. Adaptive radiotherapy for locally advanced non-small-cell lung cancer does not underdose the microscopic disease and has the potential to increase tumor control. Int J Radiat Oncol Biol Phys 2011; 81: e275–82. doi: 10.1016/j.ijrobp.2011.01.067 [DOI] [PubMed] [Google Scholar]
- 32.Sonke JJ, Zijp L, Remeijer P, van Herk M. Respiratory correlated cone beam CT. Med Phys 2005; 32: 1176–86. doi: 10.1118/1.1869074 [DOI] [PubMed] [Google Scholar]
- 33.Vergalasova I, Maurer J, Yin FF. Potential underestimation of the internal target volume (ITV) from free-breathing CBCT. Med Phys 2011; 38: 4689–99. doi: 10.1118/1.3613153 [DOI] [PMC free article] [PubMed] [Google Scholar]