Abstract
Objective:
The aim of the study was to compare epicardial adipose tissue (EAT) characteristics assessed with coronary calcium score (CS) and CT coronary angiography (CTCA) image data sets.
Methods:
In 76 patients (mean age 59 ± 13 years) who underwent CS and CTCA owing to suspected coronary artery disease (CAD), EAT was quantified in terms of density (Hounsfield units), thickness and volume. The EAT volume was extracted with a semi-automatic software.
Results:
A moderate correlation was found between EAT density in CS and CTCA image data sets (−100 ± 19 HU vs −70 ± 24 HU; p < 0.05, r = 0.55). The distribution of EAT was not symmetrical with a maximal thickness at the right atrioventricular groove (14.2 ± 5.3 mm in CS, 15.7 ± 5 mm in CTCA; p > 0.05, r = 0.76). The EAT volume resulted as 122 ± 50 cm3 in CS and 86 ± 40 cm3 in CTCA (Δ = 30%, p < 0.05, r = 0.92). After adjustment for post-contrast EAT attenuation difference (Δ = 30 HU), the volume was 101 ± 47 cm3 (Δ = 17%, p < 0.05, r = 0.92). Based on EAT volume median values, no differences were found between groups with smaller and larger volumes in terms of Agatston score and CAD severity.
Conclusion:
CS and CTCA image data sets may be equally employed for EAT assessment; however, an underestimation of volume is found with the latter acquisition even after post-contrast attenuation adjustment.
Advances in knowledge:
EAT may be measured by processing either the CS or CTCA image data sets.
INTRODUCTION
The epicardial adipose tissue (EAT) is a deposit of visceral intrathoracic fat located within the pericardium. EAT surrounds the coronary arteries for most of their anatomic path.1
Recent articles have shown that EAT may contribute to the development of coronary atherosclerosis owing to the local production of pro-inflammatory cytokines with paracrine effect.2–6 Therefore, the distribution of EAT in humans may also contribute to an unfavourable cardiovascular risk profile.7 Several studies have proposed methods to measure the EAT characteristics with non-invasive imaging techniques, demonstrating an association with coronary artery disease (CAD),8,9 myocardial ischaemia,10,11 atrial fibrillation12 and major adverse cardiovascular events.13 EAT has been assessed with echocardiography, MRI and CT using computer applications.14–19 Calcium score (CS) and CT coronary angiography (CTCA) may be employed separately, according to clinical indications with radiation dose reduction.20
The aim of our study was to provide a systematic comparison of density values, thickness and volume, extracted from CS and CTCA image data sets in a cohort of patients with suspected CAD.
METHODS AND MATERIALS
Patients
76 consecutive patients (53 males, mean age 58 ± 13 years, and 23 females, mean age 59 ± 13 years) who underwent both CS and CTCA at our institution between March 2012 and June 2012 for suspected CAD were included in the study. Population characteristics are summarized in Table 1.
Table 1.
Baseline characteristics
| Age (years) (mean ± SD) | 59 ± 13 |
| Male (%) | 53 (70%) |
| BMI (kg m−2) | 28 ± 5 |
| Family history (%) | 32 (42) |
| Smoking (%) | 18 (24) |
| Hypertension (%) | 51 (67) |
| Dyslipidemia (%) | 31 (41) |
| Obesity (BMI > 30) (%) | 25 (34) |
| Diabetes (%) | 20 (26) |
| Heart rate (bpm) | 61 ± 8 |
| Agatston calcium score | 142 ± 313 |
| Mass calcium score (mg) | 26 ± 57 |
| Volume calcium score (mm3) | 133 ± 290 |
| Normal coronary arteries (%) | 32 (42) |
| Not obstructive CAD (%) | 29 (38) |
| Obstructive CAD (%) | 15 (20) |
BMI, body mass index; bpm, beats per minute; CAD, coronary artery disease; SD, standard deviation.
The exclusion criteria were: severe renal impairment (creatininaemia >120 mmol l−1), known allergy to iodinated contrast media, pregnancy, respiratory failure, known CAD including revascularizations with percutaneous interventions and coronary artery bypass grafting and atrial fibrillation. Institutional review board approval and written informed consent were not required, since retrospective data of standard cardiac CT examinations were used.
Scan and reconstruction parameters
Examinations were performed with a 128-slice CT scanner (SOMATOM® Definition AS, Siemens Healthcare, Forchheim, Germany). Firstly, CS was acquired with a standard protocol (120 kV; slice thickness 3 mm, prospective gating). Then, CTCA scans were performed with the following parameters: slice/collimation 64 × 2/0.6 mm, with z-flying focal spot technique; rotation time 300 ms, effective temporal resolution (with 180° algorithm) 150 ms; 120 kV; 160 mAs per rotation; pitch 0.22; table feed 28.2 mm s−1, field of view 140–180 mm from the carina to the diaphragm; retrospective electrocardiogram gating with prospective modulation of the dose.
All patients with a heart rate of >65 beats per minute (without known contraindications to β-blockers such as asthma, bronchospasm and systolic blood pressure <100 mmHg) were given a dose of 20–40 mg of propranolol orally 1 h prior to the scan or a 5-mg i.v. dose of atenolol prior to the examinations to lower the heart rate.
A bolus of 80 ml of non-ionic iodinated contrast agent (iomeprol) (Iomeron 400 mgI ml−1; Bracco, Milan, Italy) was administered at an injection rate of 5 ml s−1 using a dual-head power automatic injector (Stellant®; MedRAD®, Pittsburgh, PA) connected to an 18-gauge needle cannula placed in the right antecubital vein. The iodine delivery rate (IDR) was 2000 mgI s−1 for each patient. Saline flushing of 40 ml was administered. Intracoronary enhancement was optimized by using the bolus-tracking technique. A region of interest (ROI) was placed at the level of the ascending aorta in order to synchronize the beginning of the scan with the arrival of the contrast agent in the scan target. The scan started automatically with a 7-s delay after a threshold of 110 HU was reached within the ROI.
Image data sets were reconstructed with an effective slice thickness of 0.75 mm, increment of 0.4 mm and standard smooth convolution filter. Temporal windows with the best image quality were chosen with the motion-mapping technique (BestPhase, Siemens Healthcare) in the telediastolic or in telesystolic phase or in additional phases according to operator experience.
Image analysis
The examinations were evaluated by two radiologists with 10 years’ in cardiac CT (LLG and MM), who transferred the image data sets to a workstation (Leonardo, Siemens Healthcare) equipped with dedicated software for post-processing (Syngo Via Cardiac, Siemens Healthcare). Coronary calcifications were defined on CT images as the presence of >2 contiguous pixels with density >130 HU. These lesions were automatically identified and marked in terms of colour at the workstation. The coronary calcium of each patient was quantified using the Agatston, mass and volume methods.
The Agatston score is obtained by multiplying the area of calcification with its maximum attenuation.21
Data from CTCA scans were analyzed with the following post-processing techniques: multiplanar reconstructions, curved multiplanar reconstructions, maximum intensity projections and volume rendering.
Quantification of epicardial adipose tissue
The EAT is defined as the deposit of the visceral intrathoracic adipose tissue within the pericardium and surrounding coronary arteries.1 EAT was assessed in CS and CTCA data sets in terms of density values (Hounsfield units), thickness (mm) and volume (cm3). Attenuation values of EAT were measured by tracing a constant ROI of 20 mm2 in the following anatomic regions: anterosuperior mediastinum at the level of the pulmonary trunk; close to the proximal right coronary artery, left main trunk, proximal left anterior descending artery and proximal circumflex artery; and posterior mediastinum. In addition, a ROI was placed in the subcutaneous fat of the anterior chest wall. We calculated the IDR per kilogram (kg) of individual body weight in order to assess the precise effect of contrast enhancement in a quantifiable manner. We assessed the milligram per kg per second iodine given to each patient in relation to individual arterial enhancement of the aorta (difference between pre- and post-contrast enhancement) and epicardial fat volume (difference between pre- and post-contrast enhancement).
Maximal thickness was measured from the myocardial wall to the pericardium.19 Measurements of thickness were performed in a standard horizontal long-axis plane (right atrioventricular groove, anterior interventricular groove and left atrioventricular groove) in the basal short-axis plane (superior and inferior interventricular grooves) and over the right ventricular free wall at three equally spaced points to obtain an average.19 Thoracic subcutaneous fat was measured anteriorly to the sternum at the four-chamber heart view level. All the measurements were performed at the same point both on the CS and CTCA image data sets in consensus by two radiologists.
A dedicated OsiriX plug-in was developed to calculate the EAT volume. The range of attenuation for EAT segmentation was set between −30 and −190 HU.19 The image processing started at the level of the pulmonary trunk and ended at the level of the inferior diaphragmatic surface of the heart. The operator manually drew the pericardial contours every 15 mm (5 slices in CS and 20 slices in CTCA image data set, respectively). Then, the plug-in automatically generated the missing contours by interpolating the image samples (Figure 1). In the end, images were checked and reviewed by operators to correct potential errors. The volume was measured in cubic centimetres on both the CS and CTCA data sets. The voxels with attenuation values in the range of the adipose tissue were multiplied for pixel spacing of the digital imaging and communications in medicine header file. Based on the difference of post-contrast EAT attenuation, a correction of density range was applied to the plug-in extraction, in order to obtain an adjusted volume. The CTCA image data set based on the adjustment for density range was defined as CTCA-A.
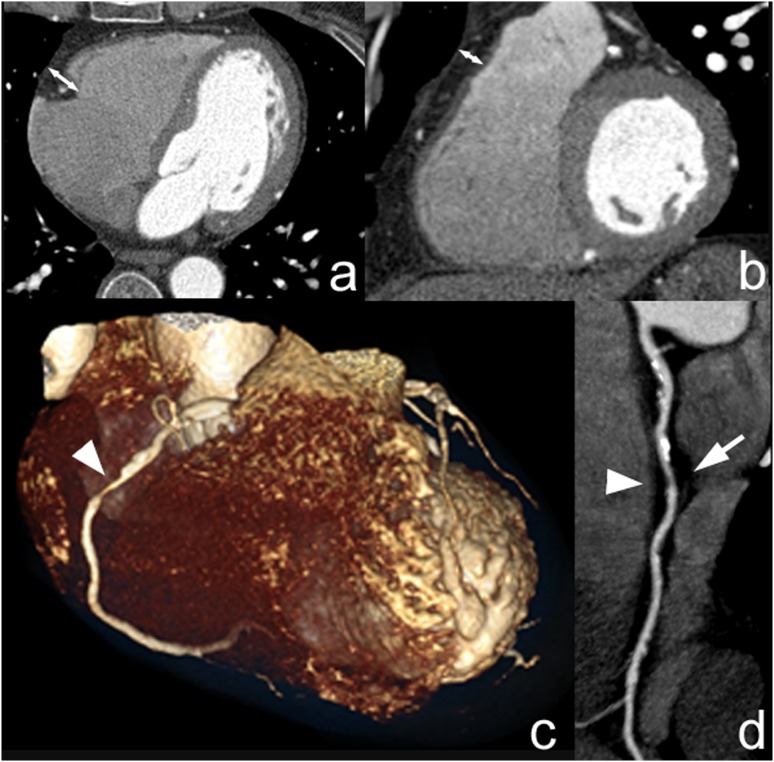
Figure 1.
Examples of pericardial tracing generated with the plug-in in the calcium score and in CT coronary angiography images (a, b) and three-dimensional models as reference images of the isolated epicardial adipose tissue (c, d).
Statistical analysis
Data were presented as means ± standard deviations. Statistical evaluation was performed with dedicated software (SPSSH 11.5; IBM Corp., New York, NY; formerly SPSS® Inc., Chicago, IL)). Paired attenuation values, thickness and volume of CS and CTCA image data sets, even after adjustment of the density range (data set CTCA-A), were compared with the Student's t test or with the one-way analysis of variance and were correlated with the Pearson's test. EAT volumes were grouped according to the median value; the severity of disease in terms of Agatston CS, absolute or by risk category (0, 1–100, 101–400 and >401), and CAD presence at CTCA (normal, <50% stenosis and ≥50% stenosis) were compared with Student's t test or with χ2 analysis. A p-value of <0.05 was set for statistical significance.
RESULTS
38 (34.5%) patients in our population presented an Agatston CS of 0. The mean Agatston CS was 142 ± 313 (16 patients with score 1–100, 12 patients with score 101–400 and 10 patients with score >400). The mean mass and volume scores were 26 ± 57 mg and 133 ± 290 mm3, respectively. 32 (42%) patients with suspected CAD had normal coronary arteries at CTCA. 29 (38%) patients did not have obstructive CAD, and 15 (20%) patients had obstructive CAD at CTCA.
The increment of aortic enhancement (429 ± 97 HU) was 11.3 times with respect to baseline (38 ± 11 HU) and its correlation with IDR per kg of patient body weight was moderate (r = 0.53) (Figure 2). Mean attenuation values of EAT were higher in CTCA image data sets than in CS data sets, with greater difference in mediastinal and juxtacoronary measurements than that in the subcutaneous fat of the anterior chest wall (p < 0.05) (Table 2). A moderate correlation was found between overall mean attenuation values of EAT in CS and CTCA (−100 ± 19 HU vs −70 ± 24 HU; p < 0.05, r = 0.55). The increment of epicardial fat enhancement was just 0.7 times during the arterial phase with respect to baseline, and its correlation with IDR per kg of patient body weight was low (r = 0.11) (Figure 3).
Figure 2.
Correlation between iodine delivery rate per kilogram (kg) of patient body weight and aortic enhancement. HU, Hounsfield units; mgl, milligram iodine; s, seconds.
Table 2.
Comparison between adipose tissue attenuation values in calcium score (CS) and CT coronary angiography (CTCA) image data sets
| Measurement of the adipose tissue | Mean HU ± SD in CS data set | Mean HU ± SD in CTCA data set | Δ (HU) | p-value | r |
|---|---|---|---|---|---|
| Anterosuperior mediastinum | −98 ± 16 | −76 ± 23 | 22 | <0.05 | 0.58 |
| Close to RCA | −97 ± 16 | −66 ± 22 | 31 | <0.05 | 0.44 |
| Close to LM | −97 ± 16 | −66 ± 27 | 31 | <0.05 | 0.42 |
| Close to LAD | −99 ± 16 | −68 ± 19 | 31 | <0.05 | 0.56 |
| Close to CX | −98 ± 18 | −66 ± 20 | 32 | <0.05 | 0.47 |
| Posterior mediastinum | −109 ± 26 | −77 ± 28 | 32 | <0.05 | 0.69 |
| Overall epicardial | −100 ± 19 | −70 ± 24 | 30 | <0.05 | 0.55 |
| Anterior chest wall | −104 ± 14 | −92 ± 18 | 12 | <0.05 | 0.72 |
CX, circumflex artery; HU, Hounsfield units; LAD, left anterior descending artery; LM, left main trunk; r, Pearson's correlation coefficient; RCA, right coronary artery; SD, standard deviations.
Figure 3.
Correlation between iodine delivery rate per kilogram (kg) of patient body weight and epicardial adipose tissue enhancement. HU, Hounsfield units; mgl, milligram iodine; s, seconds.
The distribution of EAT was not symmetrical, with a maximal thickness at the right atrioventricular groove (14.2 ± 5.3 mm in CS and 15.7 ± 5 mm in CTCA) (Table 3). The thickness of the grooved segments (10.1 ± 2.3 mm in CS and 10.2 ± 2.4 mm in CTCA) was greater (p < 0.05) than that of the right ventricular free wall (5.7 ± 2.6 mm in CS and 5.9 ± 2.9 mm in CTCA). No differences were found between the thickness measured in CS and CTCA image data sets (p > 0.05). A high correlation was found between the thickness measured in CS and CTCA with reference to the right ventricular free wall (r = 0.88) and the mean of the grooved segments (r = 0.88).
Table 3.
Comparison between epicardial adipose tissue thickness in calcium score (CS) and CT coronary angiography (CTCA) image data sets
| Thickness | Mean ± SD (mm) in CS data set | Mean ± SD (mm) in CTCA data set | Δ (mm) | p-value | r |
|---|---|---|---|---|---|
| Right atrioventricular groove | 14.2 ± 5.3 | 15.7 ± 5 | 1.5 | >0.05 | 0.76 |
| Anterior interventricular groove | 6.6 ± 2.5 | 7.2 ± 2.9 | 0.6 | >0.05 | 0.86 |
| Left atrioventricular groove | 10.6 ± 2.9 | 10.7 ± 2.8 | 0.1 | >0.05 | 0.73 |
| Superior interventricular groove | 11.8 ± 3.7 | 12.1 ± 3.5 | 0.3 | >0.05 | 0.76 |
| Inferior interventricular groove | 5.6 ± 2 | 5.4 ± 1.7 | 0.2 | >0.05 | 0.78 |
| Overall grooved | 10.1 ± 2.3 | 10.2 ± 2.4 | 0.1 | >0.05 | 0.88 |
| Right ventricular free wall | 5.6 ± 2.6 | 5.9 ± 2.9 | 0.3 | >0.05 | 0.88 |
| Thoracic subcutaneous fat | 17.4 ± 8 | 17.5 ± 8 | 0.1 | >0.05 | 0.99 |
r, Pearson's correlation coefficient; SD, standard deviation.
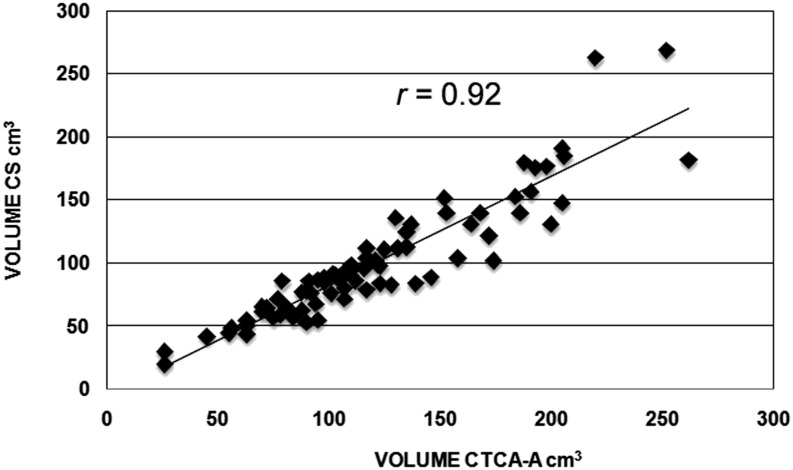
The EAT volume in the CS data set was 122 ± 50 cm3, whereas the volume in the CTCA data set was 86 ± 40 cm3 (p < 0.05, r = 0.92) (Figure 4). Time processing of volumes slightly differed between CS and CTCA image data sets (5 ± 1 vs 7 ± 2 min; p < 0.05). After adjustment for the post-contrast EAT attenuation difference (Δ = 30 HU, density range set from −175 to −15 HU), the volume resulted as 101 ± 47 cm3 (p < 0.05, r = 0.92 (Figure 5). Even though it was low and occurred early, as discussed above, EAT enhancement determined a reduction of post-contrast volume (Δ = 30%), also after adjustment of the density range (Δ = 17%).
Figure 4.
Correlation between the epicardial adipose tissue volume processed in calcium score (CS) and CT coronary angiography (CTCA) image data sets. r, Pearson's correlation coefficient.
Figure 5.
Correlation between the epicardial adipose tissue volume processed in calcium score (CS) and CT coronary angiography image data set based on the adjustment for density range (CTCA-A). r, Pearson's correlation coefficient.
EAT volumes were weakly correlated with age (r = 0.35 in CS, r = 0.32 in CTCA and r = 0.29 in CTCA-A) and body mass index (r = 0.38 in CS, r = 0.27 in CTCA and r = 0.26 in CTCA-A). Males presented a larger EAT volume than females (CS: 128 ± 53 cm3 vs 108 ± 40 cm3, p > 0.05; CTCA: 94 ± 43 cm3 vs 69 ± 28 cm3, p < 0.05; and CTCA-A: 110 ± 51 cm3 vs 82 ± 31 cm3, p < 0.05).
Although not significant, patients with an Agatston score of 0 had a smaller EAT volume than patients with an Agatston score ≠0 (118 ± 53 cm3 vs 126 ± 48 cm3 for the CS data set, p > 0.05; 79 ± 37 cm3 vs 93 ± 43 cm3 for the CTCA data set, p > 0.05; and 95 ± 48 cm3 vs 108 ± 47 cm3 for CTCA-A, p > 0.05).
The population was divided into two subgroups according to the median EAT volume (smaller and larger than median values: 112 cm3 in the CS data set, 77.5 cm3 in the CTCA data set and 89.5 cm3 in CTCA-A, respectively). No differences were found between groups with smaller and larger EAT volumes in terms of Agatston score, absolute or by risk category, and CAD severity (Table 4).
Table 4.
Comparison of groups divided according to the median values of epicardial adipose tissue volume
| Disease parameter | CS data set | CTCA | CTCA-A | |||
|---|---|---|---|---|---|---|
| EAT volume | <111.5 cm3 | >111.5 cm3 | <77.5 cm3 | >77.5 cm3 | <89.5 cm3 | >89.5 cm3 |
| CS value | 126 ± 310 | 158 ± 321 | 128 ± 316 | 156 ± 314 | 119 ± 312 | 165 ± 317 |
| CS = 0 (%) | 20 (52.7) | 18 (47.4) | 20 (52.7) | 18 (47.4) | 20 (52.7) | 18 (47.4) |
| CS, 1–100 (%) | 9 (23.7) | 7 (18.4) | 10 (26.3) | 6 (15.8) | 11 (28.9 | 5 (13.1) |
| CS, 101–400 (%) | 5 (13.1) | 7 (18.4) | 3 (7.9) | 9 (23.7) | 3 (7.9) | 9 (23.7) |
| CS > 401 (%) | 4 (10.5) | 6 (15.8) | 5 (13.1) | 5 (13.1) | 4 (10.5) | 6 (15.8) |
| No CAD | 15 (39.5) | 17 (44.7) | 15 (39.5) | 17 (44.7) | 15 (39.5) | 17 (44.7) |
| CAD < 50% (%) | 17 (44.7) | 12 (31.6) | 16 (42.1) | 13 (34.2) | 17 (44.7) | 12 (31.6) |
| CAD > 50% (%) | 6 (15.8) | 9 (23.7) | 7 (18.4) | 8 (21.1) | 6 (15.8) | 9 (23.7) |
CAD, coronary artery disease; CS, calcium score; CTCA, CT coronary angiography; CTCA-A, CTCA image data set based on adjustment for density range.
p > 0.05 was found for absolute Agatston CS value, risk categories of CS and CAD severity, according to CTCA findings.
DISCUSSION
According to literature, EAT differs from other adipose tissues because of its specific anatomic, histological and biochemical pattern.4,16,19 EAT may contribute to the progression of coronary atherosclerosis owing to the onsite production of pro-inflammatory cytokines.2–7 Non-invasive imaging techniques such as echocardiography, MRI and CT may reliably quantify the distribution and volume of EAT.8–11,14–16 In particular, cardiac CT may simultaneously provide the evaluation of CAD findings and EAT in a non-invasive manner.15–20 Computer applications may be used to select, process and quantify EAT, based on the known adipose tissue attenuation range in image data sets. Typically, the attenuation limits for EAT are identified from −250 to −190 HU for the lower limit and from −50 to −30 HU for the upper limit.19,22,23 In our study, we analyzed the relationship between EAT measurements in CS and CTCA image data sets. Our study confirms a systematic underestimation of EAT volume (Δ = 30%) in CTCA owing to contrast media enhancement. Therefore, we suggested a modified range between −175 and −15 HU, based on the attenuation difference between baseline and post-contrast values of EAT (Δ = 30 HU), to mitigate the underestimation of volume (Δ = 17%). Nonetheless, the contrast enhancement of EAT may be influenced by contrast media choice and individual factors such as ejection fraction.24,25
According to literature, the time processing of EAT volume extracted from image data sets is small.8–13 The distribution of EAT is not symmetrical and uniform, and it is prevalent at grooved segments for both data sets1,26 (Figures 6 and 7). The thickness of EAT at the left atrioventricular groove is reported to be predictive of atherogenic risk, even more than the total volume.22,27 Mahabadi et al demonstrated that the thickness of EAT is often associated with the presence of atherosclerotic plaques in the proximal grooved coronary segments, although these regions of the coronary tree typically show the first signs of atherosclerosis, with a possible overlapping effect.1 On the other side, the quantification of EAT volume is reported to be more reproducible than the thickness measurement,28 despite the absence of reference values for volume being a limitation for clinical use.1 Given that CS and CTCA may be used singularly in order to reduce the radiation dose, a systematic comparison of EAT assessment with those two acquisitions is useful to define the interchangeability of the method.
Figure 6.
Example of pericoronary fat (arrow) adjacent to a stenotic lesion (arrowheads) of the mid-right coronary artery (RCA): horizontal long-axis plane (a), basal short-axis plane (b), volume rendering (c) and multiplanar reconstruction of the RCA (d). The stenosis of the mid-RCA is associated with the maximal epicardial adipose tissue (EAT) thickness (doubleheaded arrow, a) of the right atrioventricular groove (20 mm), while EAT thickness over the right ventricular free wall (doubleheaded arrow, b) and the volume are average (8 mm and 81 cm3, respectively).
Figure 7.
Example of pericoronary fat (arrows) adjacent to a stenotic lesion (arrowheads) of the mid-left anterior descending artery (LAD): horizontal long-axis plane in calcium score (a) and CT coronary angiography image data sets (b) and multiplanar reconstructions of the LAD (c, d). The stenosis of the mid-LAD is associated with the maximal epicardial adipose tissue (EAT) thickness of the anterior interventricular groove (10 mm), while EAT volume is average (77 cm3).
According to literature,1,29 EAT volume is weakly correlated with male gender, age and body mass index. It is still controversial whether CS and EAT values are correlated.28,30 Bettencourt et al28 demonstrated a linear correlation, while Mahabadi et al30 showed that the association is not significant, but it is influenced by cardiovascular risk factors. According to our study, the volumes of EAT are independent of CS values. Both findings are risk factors of atherosclerosis, but, they probably refer to different pathogenic pathways of CAD.23,30,31 The severity of CAD assessed with CTCA is also not affecting EAT volumes, even in case of obstructive disease.28,32
Some limitations occurred in our study. The first is that we did not perform a follow-up of patients. EAT is correlated with the progression of CAD9 and major adverse cardiovascular events, including sudden cardiac death, myocardial infarction, stroke and coronary revascularization.1,13,16 However, the primary aim of our study was to compare measurements and quantification of EAT in CS and CTCA image data sets. The second limitation is that our sample did not include patients with acute presentation. The analysis of patients with acute presentation and dysmetabolic syndrome could provide an explanation of the potential association between EAT volume, CS and coronary plaque composition, including non-calcified plaques and positive remodelling.13,33–35 The increase of C-reactive protein and the reduction of adiponectin in the adipose tissue may contribute to the progression of atherosclerosis.36 Atrial fibrillation is also reported to be correlated with EAT volume;37 however, we did not include patients with atrial fibrillation.
CONCLUSION
CS and CTCA image data sets may be equally employed for EAT quantification, but an underestimation of volume is found with the latter acquisition even after post-contrast attenuation adjustment. The assessment of EAT in large population studies should be encouraged to elucidate its pathophysiology and prognostic role in CAD.
Contributor Information
Ludovico La Grutta, Email: ludovicolagrutta@hotmail.it.
Patrizia Toia, Email: toiapatrizia@gmail.com.
Alfonso Farruggia, Email: alfarruggia@gmail.com.
Domenico Albano, Email: albanodomenico@icloud.com.
Emanuele Grassedonio, Email: egrassedonio@gmail.com.
Antonella Palmeri, Email: antonellapalmeri@hotmail.it.
Erica Maffei, Email: ericamaffei@gmail.com.
Massimo Galia, Email: mgalia@yahoo.com.
Salvatore Vitabile, Email: salvatore.vitabile@gmail.com.
Filippo Cademartiri, Email: filippocademartiri@gmail.com.
Massimo Midiri, Email: massimo.midiri@unipa.it.
REFERENCES
- 1.Marwan M, Achenbach S. Quantification of epicardial fat by computed tomography: why, when and how? J Cardiovasc Comput Tom 2013; 7: 3–10. doi: 10.1016/j.jcct.2013.01.002 [DOI] [PubMed] [Google Scholar]
- 2.Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation 2003; 108: 2460–6. doi: 10.1161/01.CIR.0000099542.57313.C5 [DOI] [PubMed] [Google Scholar]
- 3.Baker AR, Silva NF, Quinn DW, Harte AL, Pagano D, Bonser RS, et al. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc Diabetol 2006; 5: 1. doi: 10.1186/1475-2840-5-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosito GA, Massaro JM, Hoffmann U, Ruberg FL, Mahabadi AA, Vasan RS, et al. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation 2008; 117: 605–13. doi: 10.1161/CIRCULATIONAHA.107.743062 [DOI] [PubMed] [Google Scholar]
- 5.Mahabadi AA, Massaro JM, Rosito GA, Levy D, Murabito JM, Wolf PA, et al. Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: the Framingham Heart Study. Eur Heart J 2009; 30: 850–6. doi: 10.1093/eurheartj/ehn573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taguchi R, Takasu J, Itani Y, Yamamoto R, Yokoyama K, Watanabe S, et al. Pericardial fat accumulation in men as a risk factor for coronary artery disease. Atherosclerosis 2001; 157: 203–9. doi: 10.1016/S0021-9150(00)00709-7 [DOI] [PubMed] [Google Scholar]
- 7.Iacobellis G, Pistilli D, Gucciardo M, Leonetti F, Miraldi F, Brancaccio G, et al. Adiponectin expression in human epicardial adipose tissue in vivo is lower in patients with coronary artery disease. Cytokine 2005; 29: 251–5. doi: 10.1016/j.cyto.2004.11.002 [DOI] [PubMed] [Google Scholar]
- 8.Dey D, Wong ND, Tamarappoo B, Nakazato R, Gransar H, Cheng VY, et al. Computer-aided non-contrast CT-based quantification of pericardial and thoracic fat and their associations with coronary calcium and Metabolic Syndrome. Atherosclerosis 2010; 209: 136–41. doi: 10.1016/j.atherosclerosis.2009.08.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakanishi R, Rajani R, Cheng VY, Gransar H, Nakazato R, Shmilovich H, et al. Increase in epicardial fat volume is associated with greater coronary artery calcification progression in subjects at intermediate risk by coronary calcium score: a serial study using non-contrast cardiac CT. Atherosclerosis 2011; 218: 363–8. doi: 10.1016/j.atherosclerosis.2011.07.093 [DOI] [PubMed] [Google Scholar]
- 10.Tamarappoo B, Dey D, Shmilovich H, Nakazato R, Gransar H, Cheng VY, et al. Increased pericardial fat volume measured from noncontrast CT predicts myocardial ischemia by SPECT. JACC Cardiovasc Imaging 2010; 3: 1104–12. doi: 10.1016/j.jcmg.2010.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janik M, Hartlage G, Alexopoulos N, Mirzoyev Z, McLean DS, Arepalli CD, et al. Epicardial adipose tissue volume and coronary artery calcium to predict myocardial ischemia on positron emission tomography-computed tomography studies. J Nucl Cardiol 2010; 17: 841–7. doi: 10.1007/s12350-010-9235-1 [DOI] [PubMed] [Google Scholar]
- 12.Wong CX, Abed HS, Molaee P, Nelson AJ, Brooks AG, Sharma G, et al. Pericardial fat is associated with atrial fibrillation severity and ablation outcome. J Am Coll Cardiol 2011; 57: 1745–51. doi: 10.1016/j.jacc.2010.11.045 [DOI] [PubMed] [Google Scholar]
- 13.Cheng VY, Dey D, Tamarappoo B, Nakazato R, Gransar H, Miranda-Peats R, et al. Pericardial fat burden on ECG-gated noncontrast CT in asymptomatic patients who subsequently experience adverse cardiovascular events. JACC Cardiovasc Imaging 2010; 3: 352–60. doi: 10.1016/j.jcmg.2009.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eroglu S, Sade LE, Yildirir A, Bal U, Ozbicer S, Ozgul AS, et al. Epicardial adipose tissue thickness by echocardiography is a marker for the presence and severity of coronary artery disease. Nutr Metab Cardiovasc Dis 2009; 19: 211–17. doi: 10.1016/j.numecd.2008.05.002 [DOI] [PubMed] [Google Scholar]
- 15.Elming MB, Lønborg J, Rasmussen T, Kühl JT, Engstrøm T, Vejlstrup N, et al. Measurements of pericardial adipose tissue using contrast enhanced cardiac multidetector computed tomography–comparison with cardiac magnetic resonance imaging. Int J Cardiovasc Imaging 2013; 29: 1401–7. doi: 10.1007/s10554-013-0218-6 [DOI] [PubMed] [Google Scholar]
- 16.Dey D, Nakazato R, Li D, Berman DS. Epicardial and thoracic fat—noninvasive measurement and clinical implications. Cardiovasc Diagn Ther 2012; 2: 85–93. doi: 10.3978/j.issn.2223-3652.2012.04.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sarin S, Wenger C, Marwaha A, Qureshi A, Go BD, Woomert CA, et al. Clinical significance of epicardial fat measured using cardiac multislice computed tomography. Am J Cardiol 2008; 102: 767–71. doi: 10.1016/j.amjcard.2008.04.058 [DOI] [PubMed] [Google Scholar]
- 18.Spearman JV, Meinel FG, Schoepf UJ, Apfaltrer P, Silverman JR, Krazinski AW, et al. Automated quantification of epicardial adipose tissue using CT angiography: evaluation of a prototype software. Eur Radiol 2014; 24: 519–26. doi: 10.1007/s00330-013-3052-2 [DOI] [PubMed] [Google Scholar]
- 19.Wang TD, Lee WJ, Shih FY, Huang CH, Chang YC, Chen WJ, et al. Relations of epicardial adipose tissue measured by multidetector computed tomography to components of the metabolic syndrome are region-specific and independent of anthropometric indexes and intraabdominal visceral fat. J Clin Endocrinol Metab 2009; 94: 662–9. doi: 10.1210/jc.2008-0834 [DOI] [PubMed] [Google Scholar]
- 20.Di Cesare E, Carbone I, Carriero A, Centonze M, De Cobelli F, De Rosa R, et al. Clinical indications for cardiac computed tomography. From the Working Group of the Cardiac Radiology Section of the Italian Society of Medical Radiology (SIRM). Radiol Med 2012; 117: 901–38. doi: 10.1007/s11547-012-0814-x [DOI] [PubMed] [Google Scholar]
- 21.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990; 15: 827–32. doi: 10.1016/0735-1097(90)90282-T [DOI] [PubMed] [Google Scholar]
- 22.Mahabadi AA, Reinsch N, Lehmann N, Altenbernd J, Kälsch H, Seibel RM, et al. Association of pericoronary fat volume with atherosclerotic plaque burden in the underlying coronary artery: a segment analysis. Atherosclerosis 2010; 211: 195–9. doi: 10.1016/j.atherosclerosis.2010.02.013 [DOI] [PubMed] [Google Scholar]
- 23.Alexopoulos N, McLean DS, Janik M, Arepalli CD, Stillman AE, Raggi P. Epicardial adipose tissue and coronary artery plaque characteristics. Atherosclerosis 2010; 210: 150–4. doi: 10.1016/j.atherosclerosis.2009.11.020 [DOI] [PubMed] [Google Scholar]
- 24.La Grutta L, Galia M, Gentile G, Lo Re G, Grassedonio E, Coppolino F, et al. Comparison of iodinated contrast media for the assessment of atherosclerotic plaque attenuation values by CT coronary angiography: observations in an ex vivo model. Br J Radiol 2013; 86: 20120238. doi: 10.1259/bjr.20120238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hachiya K, Fukuta H, Wakami K, Goto T, Tani T, Ohte N. Relation of epicardial fat to central aortic pressure and left ventricular diastolic function in patients with known or suspected coronary artery disease. Int J Cardiovasc Imaging 2014; 30: 1393–8. doi: 10.1007/s10554-014-0472-2 [DOI] [PubMed] [Google Scholar]
- 26.Saura D, Oliva MJ, Rodriguez D, Pascual-Figal DA, Hurtado JA, Pinar E, et al. Reproducibility of echocardiographic measurements of epicardial fat thickness. Int J Cardiol 2010; 141: 311–3. doi: 10.1016/j.ijcard.2008.11.127 [DOI] [PubMed] [Google Scholar]
- 27.Wang TD, Lee WJ, Shih FY, Huang CH, Chen WJ, Lee YT, et al. Association of epicardial adipose tissue with coronary atherosclerosis is region-specific and independent of conventional risk factors and intra-abdominal adiposity. Atherosclerosis 2010; 213: 279–87. doi: 10.1016/j.atherosclerosis.2010.07.055 [DOI] [PubMed] [Google Scholar]
- 28.Bettencourt N, Toschke AM, Leite D, Rocha J, Carvalho M, Sampaio F, et al. Epicardial adipose tissue is an independent predictor of coronary atherosclerotic burden. Int J Cardiol 2012; 158: 26–32. doi: 10.1016/j.ijcard.2010.12.085 [DOI] [PubMed] [Google Scholar]
- 29.Thanassoulis G, Massaro JM, Hoffmann U, Mahabadi AA, Vasan RS, O'Donnell CJ, et al. Prevalence, distribution, and risk factor correlates of high pericardial and intrathoracic fat depots in the Framingham Heart Study. Circ Cardiovasc Imaging 2010; 3: 559–66. doi: 10.1161/CIRCIMAGING.110.956706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahabadi AA, Berg MH, Lehmann N, Kälsch H, Bauer M, Kara K, et al. Association of epicardial fat with cardiovascular risk factors and incident myocardial infarction in the general population. J Am Coll Cardiol 2013; 61: 1388–95. doi: 10.1016/j.jacc.2012.11.062 [DOI] [PubMed] [Google Scholar]
- 31.Ito T, Suzuki Y, Ehara M, Matsuo H, Teramoto T, Terashima M, et al. Impact of epicardial fat volume on coronary artery disease in symptomatic patients with a zero calcium score. Int J Cardiol 2013; 167: 2852–8. doi: 10.1016/j.ijcard.2012.07.026 [DOI] [PubMed] [Google Scholar]
- 32.Ueno K, Anzai T, Jinzaki M, Yamada M, Jo Y, Maekawa Y, et al. Increased epicardial fat volume quantified by 64-multidetector computed tomography is associated with coronary atherosclerosis and totally occlusive lesions. Circ J 2009; 73: 1927–33. doi: 10.1253/circj.CJ-09-0266 [DOI] [PubMed] [Google Scholar]
- 33.Motoyama S, Sarai M, Harigaya H, Anno H, Inoue K, Hara T, et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol 2009; 54: 49–57. doi: 10.1016/j.jacc.2009.02.068 [DOI] [PubMed] [Google Scholar]
- 34.Oka T, Yamamoto H, Ohashi N, Kitagawa T, Kunita E, Utsunomiya H, et al. Association between epicardial adipose tissue volume and characteristics of non-calcified plaques assessed by coronary computed tomographic angiography. Int J Cardiol 2011; 161: 45–9. doi: 10.1016/j.ijcard.2011.04.021 [DOI] [PubMed] [Google Scholar]
- 35.Cademartiri F, La Grutta L, Palumbo A, Maffei E, Aldrovandi A, Malagò R, et al. Imaging techniques for the vulnerable coronary plaque. Radiol Med 2007; 112: 637–59. doi: 10.1007/s11547-007-0170-4 [DOI] [PubMed] [Google Scholar]
- 36.Ouchi N, Kihara S, Funahashi T, Nakamura T, Nishida M, Kumada M, et al. Reciprocal association of c-reactive protein with adiponectin in blood stream and adipose tissue. Circulation 2003; 107: 671–4. doi: 10.1161/01.CIR.0000055188.83694.B3 [DOI] [PubMed] [Google Scholar]
- 37.Al Chekakie MO, Welles CC, Metoyer R, Ibrahim A, Shapira AR, Cytron J, et al. Pericardial fat is independently associated with human atrial fibrillation. J Am Coll Cardiol 2010; 56: 784–8. doi: 10.1016/j.jacc.2010.03.071 [DOI] [PubMed] [Google Scholar]