Abstract
Objective:
To compare dosimetric parameters and acute toxicity rates between whole-pelvic (WP) and prostate-only (PO) volumetric-modulated arc therapy (VMAT) in patients with localized prostate cancer.
Methods:
A total of 224 consecutive patients treated with definitive VMAT to 78 Gy in 39 fractions were enrolled. Of these, 119 patients received initial WP VMAT at 46.8 Gy in 26 fractions using a simultaneous integrated boost technique, and 105 patients received PO VMAT. Image-guided radiotherapy was practised with daily cone beam CT.
Results:
The mean rectal dose, the rectal volume receiving ≥30 Gy (V30Gy), rectal V50Gy, the mean bladder dose, bladder V30Gy and bladder V50Gy were significantly increased in the WP group (p < 0.05 each); however, the rectal V70Gy did not differ between groups (p = 0.101), and the bladder V70Gy was significantly lower in the WP group (p = 0.029). The WP group experienced a significantly increased frequency of acute grade 2 diarrhoea relative to the PO group (5.9% vs 0%; p = 0.015). No differences were seen between the WP and PO groups in terms of acute grade 2 proctitis (10.1% vs 6.7%; p = 0.360) and genitourinary (GU) toxicity (12.6% vs 10.5%; p = 0.620).
Conclusion:
Despite larger rectum and bladder volumes at low- and medium-dose levels, WP VMAT resulted in no significant increase in acute proctitis or GU toxicity when compared with PO VMAT.
Advances in knowledge:
This study demonstrates that whole-pelvic radiotherapy has comparable acute toxicity to those observed with prostate-only radiotherapy when VMAT with daily image guidance is used.
INTRODUCTION
The benefit of whole-pelvic radiotherapy (WPRT) in patients at high risk for involvement of the pelvic lymph nodes is the subject of ongoing debate, since its effect on survival rates has not yet been clarified. In addition, there is a concern that WPRT could lead to additional toxicity compared with prostate-only radiotherapy (PORT). Two randomized Phase III trials have evaluated WPRT in comparison with PORT for patients with localized prostate cancer using conventional techniques.1,2 A subset analysis of the Radiation Therapy Oncology Group (RTOG) 9413 trial demonstrated that a larger field size resulted in a significant increase in acute grade 2 gastrointestinal (GI) and genitourinary (GU) toxicity and late grade 3 or higher GI toxicity among patients treated to the whole-pelvic (WP), mini-pelvis and prostate-only (PO) fields.1 The GETUG-01 trial from the Groupe D'Etude des Tumeurs Uro-génitales showed that WPRT resulted in a significant increase in acute grade 3 or higher GU toxicity and no difference in late GI and GU toxicity in comparison with PORT.2 Thus, these studies have yielded mixed results. Furthermore, in these studies, the prostate dose was restricted to approximately 70 Gy with conventional fractionation, which has been shown to be suboptimal by current standards in the treatment of localized prostate cancer.3–5
Previous studies have demonstrated that intensity-modulated radiotherapy (IMRT) provides superior target coverage and organ at risk (OAR) sparing over three-dimensional conformal radiotherapy when performing WPRT.6–8 However, in the context of dose escalation to the prostate, dosimetric and clinical results from the literature comparing WP IMRT with PO IMRT are still limited. In a planning study, Guckenberger et al9 predicted similar risks of rectal, bladder and small bowel toxicity in both WP IMRT and PO IMRT. A retrospective study by Deville et al10 comparing the toxicity of WP IMRT and PO IMRT (30 patients in each group) showed a clinically significant increase in acute GI toxicity with WP IMRT, whereas no difference was seen in GU or late GI toxicity.
Volumetric-modulated arc therapy (VMAT) is now widely used clinically,11 since it is capable of delivering IMRT-quality plans with superior treatment delivery efficiency. Although several recent studies have reported early clinical results of VMAT for prostate cancer,12–14 to the best of our knowledge, there are no published clinical studies comparing WP VMAT with PO VMAT. Therefore, there is a need to demonstrate whether the use of WPRT could result in increased toxicity when VMAT is performed. Previously, we reported that VMAT for WPRT in prostate cancer is dosimetrically feasible and results in an acceptable rate of acute toxicity.14 In this study, we further investigated the differences in dosimetric parameters and acute toxicity rates between WP VMAT and PO VMAT with daily cone beam CT (CBCT)-based image-guided radiotherapy (IGRT) for localized prostate cancer.
METHODS AND MATERIALS
Patient characteristics
This study was based on 224 consecutive patients with clinically localized prostate cancer undergoing definitive VMAT to a total dose of 78 Gy, using daily image guidance, between July 2011 and June 2014. Of these patients, 105 patients received PORT and 119 patients received WPRT. Written informed consent was obtained from all patients, and the Tane General Hospital ethics committee approved the study.
All patients had a biopsy-proven adenocarcinoma that was classified according to the Gleason grading system and were clinically staged according to the 2009 American Joint Committee on Cancer staging classification.15 The staging evaluation revealed no patients with metastatic disease. The patients were also stratified according to the 2009 National Comprehensive Cancer Network (NCCN) prognostic risk grouping (www.nccn.org). As per our institutional protocol, patients classified as low or intermediate risk as defined by NCCN criteria were treated to the PO fields. Patients at high risk were mainly treated to the WP fields; however, patients more than 80 years of age or those who did not wish to receive WPRT were treated to the PO fields. Androgen deprivation therapy (ADT) was recommended for 2 years or longer for patients at high risk and for 6 months for patients at intermediate risk. ADT was not recommended for patients at low risk. However, the actual duration of ADT was dependent on the clinical judgment, based on the aggressiveness of the disease. The duration of neoadjuvant ADT was at least 3 months.
Simulation, organ contouring and planning
All patients underwent CT scanning with 1.25-mm-thick slices in the supine position after immobilization with a vacuum-based device (VacLock™; CIVCO Medical Solutions, Kalona, IA). The patients were instructed to have a comfortably full bladder and an empty rectum for the CT scan and for each treatment. In patients at low risk, the prostate clinical target volume (CTV) was defined as the entire prostate, while for those at intermediate and high risk, the prostate CTV was defined as the entire prostate with a 15-mm area proximal to the seminal vesicles and any visible tumour extension. The prostate planning target volume (PTV) was the prostate CTV with a margin of 8 mm in all directions except posteriorly, where the margin was reduced to 5 mm. Pelvic lymph node volumes were standardized according to RTOG recommendation.16 The nodal CTV included a 7-mm expansion volume on the obturator vessels in addition to the common, external and internal iliac vessels, while excluding the adjacent bone, muscle, bowel and bladder. Differing from the RTOG recommendation where the pre-sacral nodes were included in the nodal CTV, the pre-sacral nodes were excluded in nodal CTV in the current study. The nodal PTV consisted of a 5-mm geometric expansion on the nodal CTV. Contouring of the OARs, including the rectum, bladder, femoral heads and bowel bag, was performed in accordance with RTOG contouring guidelines for the normal pelvic tissue.17 All OARs were contoured as solid organs. For the rectum, segmentation was performed from the level of the ischial tuberosities to the rectosigmoid flexure. The entire bladder was contoured from the apex to the dome. In terms of the femoral heads, these were delineated to the level of the ischial tuberosities. The bowel bag was defined as the total volume of the peritoneal space to 10 mm above the nodal PTV.
All patients received 78 Gy in 39 fractions of 2 Gy to the prostate PTV. A simultaneously integrated boost technique was used for the patients undergoing WPRT, who received 46.8 Gy in 1.8-Gy fractions to the nodal PTV for the first 26 fractions. The dose was normalized to the mean dose delivered to the prostate PTV in all patients undergoing WPRT, and the mean dose to the nodal PTV was maintained as close to 46.8 Gy as possible. All VMAT plans were developed in the Eclipse™ Treatment Planning System v. 10.0 (Varian Medical Systems, Palo Alto, CA) using 10-MV photons, and the final dose calculation was performed using the Anisotropic Analytical Algorithm v. 10.0.28 (Varian Medical Systems, Palo Alto, CA). The VMAT plans were generated with a single arc for the PO fields and double arcs for the WP fields. Plans were considered acceptable when at least 95% of the PTV received 95% or more of the prescribed dose. Our institutional OAR dose volume constraints were as follows: V70Gy (the volume receiving ≥ 70 Gy) <20% and V50Gy <45% for the rectum; V70Gy <25% and V50Gy <50% for the bladder; D2% (dose to 2% of the volume) <50 Gy for the femoral heads; D2% <50 Gy and V45Gy <195 ml for the bowel bag.
Online image-guided radiotherapy
The patients were treated with Novalis Tx™ (Varian Medical Systems in collaboration with Brainlab AG, Feldkirchen, Germany). Radiation was delivered with image guidance using ExacTrac® (Brainlab) and CBCT for all patients. After the initial setup according to the marks on the skin of each patient, orthogonal kilovoltage radiographs were obtained using ExacTrac and then matched with the corresponding digitally reconstructed radiographs that were generated from the planning CT. When bone registration was satisfactory, CBCT images were acquired for use in the soft-tissue registration of the target.
Dosimetric analysis
The dose–volume histograms (DVHs) were calculated for the prostate PTV, nodal PTV, rectum, bladder and bowel bag and were averaged over all patients. The parameters chosen for comparison between the PO and WP groups were D2% and D95% (dose to 95% of the volume) for the prostate PTV. For the rectum and bladder, the analysis included the mean dose, D2%, V70Gy, V50Gy and V30Gy. The D2% and V45Gy of the bowel bag were also compared.
Acute toxicity
For all patients, the Common Terminology Criteria for Adverse Events v. 4.0 (CTCAE v4.0) was used to retrospectively score the prospectively recorded GI and GU toxicity. The patients were monitored weekly during radiotherapy (RT) and at 2 weeks and 3 months after RT. Acute toxicity was defined as the reported toxicity originating within 90 days after the end of RT. The maximum toxicity grade rates were compared for each group.
Statistical analysis
The characteristics of the patients were compared using unpaired Student's t-tests for continuous variables and χ2 test for categorical variables. When a categorical variable contained <5 patients, Fischer's exact test was used in place of the χ2 test. The dose–volume parameters were compared using the unpaired Student's t-test. Either χ2 test or Fischer's exact test was used to analyze the differences in the frequency of maximum GI and GU toxicities. All reported p-values were two tailed, and significance was defined at p < 0.05.
RESULTS
The baseline patient characteristics are summarized by treatment modality in Table 1. No significant differences were evident between the groups in terms of age or comorbidities. As a consequence of the patient selection for WPRT, those in the WP group had higher clinical stages, Gleason scores and pre-treatment prostate-specific antigen (PSA) levels and received ADT more frequently.
Table 1.
Patient characteristics and treatment modalities
| Characteristic | PO VMAT | WP VMAT | p-value |
|---|---|---|---|
| Number of patients | 105 | 119 | |
| Age (years) | 0.086 | ||
| Median | 73 | 72 | |
| Range | 56–87 | 50–79 | |
| Gleason score | <0.001 | ||
| 4–6 | 33 | 6 | |
| 7 | 59 | 33 | |
| 8–10 | 13 | 80 | |
| PSA (ng ml−1) | <0.001 | ||
| Median | 9.5 | 28.2 | |
| Range | 3.9–129.2 | 4.0–445.0 | |
| Clinical stage | <0.001 | ||
| T1 | 42 | 14 | |
| T2 | 55 | 34 | |
| T3 | 8 | 63 | |
| T4 | 0 | 8 | |
| NCCN risk group | <0.001 | ||
| Low risk | 21 | 0 | |
| Intermediate risk | 63 | 0 | |
| High risk | 21 | 119 | |
| Diabetes | 9 | 20 | 0.070 |
| Anticoagulants | 18 | 20 | 0.947 |
| Androgen deprivation | 69 | 113 | <0.001 |
NCCN, National Comprehensive Cancer Network; PO, prostate-only; PSA, prostate-specific antigen; VMAT, volumetric-modulated arc therapy; WP, whole-pelvic.
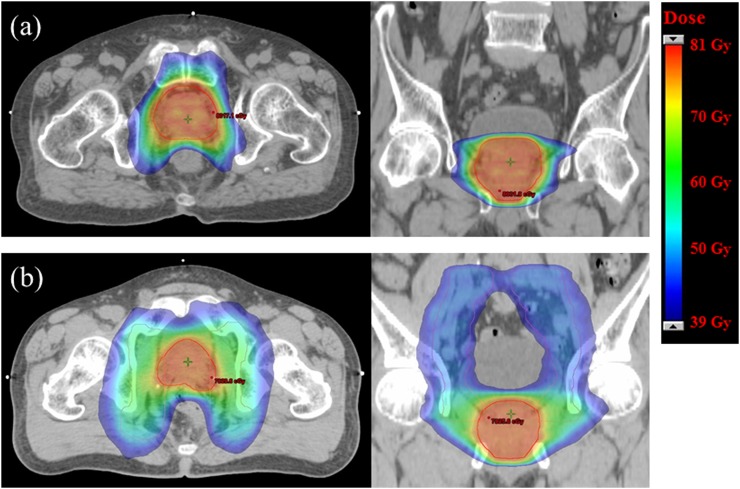
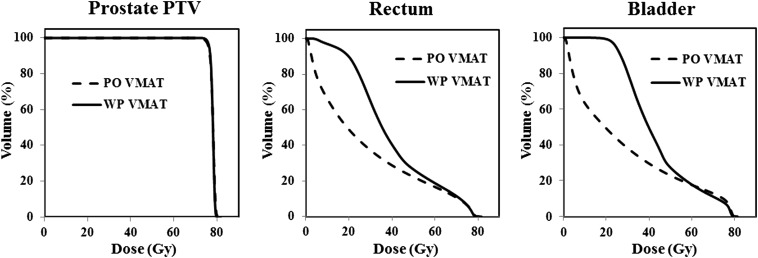
The representative dose distributions for the PO VMAT and WP VMAT groups are illustrated in Figure 1. The average DVHs for the prostate PTV, rectum and bladder are shown in Figure 2. The DVHs for the prostate PTV were similar in both groups. Although WP VMAT resulted in an increased dose to the rectum and bladder at the low- and medium-dose levels, the high-dose part of the DVH curves (60–80 Gy) for the rectum and bladder overlapped between the WP and PO VMAT groups, as seen in Figure 2. All dose–volume constraints were met for all treatment plans. The mean values of the dosimetric parameters of the PTV and OARs are shown in Table 2. Although there were statistically significant differences in target coverage between the WP and PO groups, the absolute differences in dose were very small. The rectal mean dose, V30Gy and V50Gy, and bladder mean dose, V30Gy and V50Gy, were significantly higher in the WP group; however, the rectal V70Gy did not differ between the groups, and the bladder V70Gy was significantly lower in the WP group. In addition, rectal V70Gy in terms of absolute rectal volume did not differ between the WP and PO groups (6.7 ± 2.2 vs 6.4 ± 2.4 ml; p = 0.284). Similarly, bladder V70Gy in terms of absolute bladder volume for the WP and PO groups was 18.6 ± 6.3 ml and 18.3 ± 8.0 ml, respectively, indicating no statistically significant difference between the groups (p = 0.830). The bowel bag D2% and V45Gy were markedly increased in the WP group.
Figure 1.
Representative dose distributions for (a) prostate-only volumetric-modulated arc therapy (VMAT) and (b) whole-pelvic VMAT. Prostate planning target volumes (PTVs) are shown in red and nodal PTVs are shown in pink. The dose colour wash is from 39 Gy (dark blue) to approximately 81 Gy (red).
Figure 2.
Average dose–volume histograms for prostate-only (PO) volumetric-modulated arc therapy (VMAT) and whole-pelvic (WP) VMAT for the prostate planning target volume (PTV), rectum and bladder.
Table 2.
Dosimetric parameter comparisons of prostate-only volumetric-modulated arc therapy (PO VMAT) and whole-pelvic volumetric-modulated arc therapy (WP VMAT)
| Parameter | PO VMAT (mean ± SD) | WP VMAT (mean ± SD) | p-value |
|---|---|---|---|
| Prostate PTV | |||
| Volume (ml) |
113 ± 40 | 93 ± 23 | <0.001 |
| D95% (Gy) |
76.0 ± 0.5 | 76.4 ± 0.3 | <0.001 |
| Dmean (Gy) |
78 | 78 | – |
| D2% (Gy) |
79.7 ± 0.3 | 79.3 ± 0.2 | <0.001 |
| Nodal PTV | |||
| Volume (ml) |
– | 791 ± 132 | – |
| D95% (Gy) |
– | 44.8 ± 0.4 | – |
| Dmean (Gy) |
– | 46.8 ± 0.4 | – |
| Rectum | |||
| Volume (ml) |
61 ± 19 | 60 ± 15 | 0.62 |
| Dmean (Gy) |
27.7 ± 4.9 | 40.0 ± 2.7 | <0.001 |
| D2% (Gy) |
77.6 ± 0.9 | 77.1 ± 1.1 | <0.001 |
| V70% (Gy) |
10.7 ± 3.4 | 11.5 ± 3.9 | 0.101 |
| V50% (Gy) |
22.3 ± 5.4 | 26.5 ± 5.5 | <0.001 |
| V30% (Gy) |
37.3 ± 8.6 | 64.8 ± 10.9 | <0.001 |
| Bladder | |||
| Volume (ml) |
169 ± 90 | 198 ± 87 | 0.023 |
| Dmean (Gy) |
28.2 ± 9.6 | 43.7 ± 4.0 | <0.001 |
| D2% (Gy) |
78.5 ± 0.7 | 78.0 ± 0.7 | <0.001 |
| V70Gy (%) |
12.9 ± 6.2 | 11.1 ± 5.5 | 0.029 |
| V50Gy (%) |
22.9 ± 10.2 | 27.4 ± 10.8 | 0.003 |
| V30Gy (%) |
38.2 ± 15.9 | 80.3 ± 11.5 | <0.001 |
| Bowel bag | |||
| D2% (Gy) |
14.5 ± 15.4 | 47.1 ± 0.7 | <0.001 |
| V45Gy (ml) | 0.2 ± 0.8 | 76.0 ± 30.0 | <0.001 |
Dmean, mean dose; Dn%, dose to n% of the volume; PTV, planning target volume; Dn%, minimal dose to n% of the structure; SD, standard deviation; VnGy, percentage or absolute structure volume receiving ≥n Gy.
Two-sided p-values calculated using unpaired Student's t test.
All patients completed RT and 3 months of follow-up. There was no acute GI or GU toxicity ≥grade 3. The maximal acute toxicity is detailed in Table 3. Despite a statistically significant increase in the incidence of acute grade 2 diarrhoea in the WP group, only 7 (5.9%) patients experienced this side effect. No significant differences were noted between both groups with regard to acute grade 2 proctitis or GU toxicity.
Table 3.
Comparisons of acute grade 2 toxicity between prostate-only volumetric-modulated arc therapy (PO VMAT) and whole-pelvic volumetric-modulated arc therapy (WP VMAT)
| Toxicity | PO VMAT | WP VMAT | p-value |
|---|---|---|---|
| GI | 7 (6.7%) | 17 (14.3%) | 0.066 |
| Proctitis | 7 (6.7%) | 12 (10.1%) | 0.360 |
| Diarrhoea | 0 | 7 (5.9%) | 0.015 |
| GU | 11 (10.5%) | 15 (12.6%) | 0.620 |
| Frequency | 9 (8.6%) | 14 (11.8%) | 0.432 |
| Retention | 3 (2.9%) | 1 (0.8%) | 0.343 |
| Haematuria | 2 (1.9%) | 1 (0.8%) | 0.601 |
| Urinary tract pain | 2 (1.9%) | 0 | 0.219 |
| Urgency | 1 (1.0%) | 0 | 0.469 |
GI, gastrointestinal; GU, genitourinary.
DISCUSSION
In this study, we have examined the differences in both the dosimetric parameters and the acute toxicities between WP and PO VMAT with daily CBCT-based IGRT in patients with localized prostate cancer. While the volumes of the irradiated rectum and bladder differed at the low- and medium-dose levels, the volumes exposed to high-dose levels were equivalent in both groups. The maximum acute toxicity was limited to grade 2. In addition, there was no difference in the rates of acute grade 2 proctitis and GU toxicity. However, WP VMAT resulted in a statistically significant increase in acute diarrhoea. Although this study was limited by its retrospective nature, it was novel in that all patients were treated in a consistent manner with image-guided VMAT, with dose escalation to the prostate.
There are limited data in the literature with regard to dosimetric comparisons between WP and PO IMRT. In a planning study by Guckenberger et al9 with a series of 10 patients, WP IMRT resulted in increased doses to the rectum at the low-dose level (≤30 Gy) and to the bladder at the low- and medium-dose levels (≤50 Gy) when compared with PO IMRT, whereas no difference was observed at the high-dose levels for the rectum and bladder. In a report by Deville et al,10 60 patients received either WP IMRT or PO IMRT, and there was no dosimetric difference at the high-dose levels (V65Gy and V70Gy) for the rectum and bladder. Similar results were obtained in our study when we compared WP VMAT with PO VMAT. This result can be explained by the fact that the in-field rectum and bladder volumes were greater in WP VMAT owing to the increased overlap with the nodal PTV, which caused an increase at the low- and medium-dose levels assessed for the rectum and bladder.
The dosimetric differences in the volume of the rectum and bladder irradiated at the low- and medium-dose levels did not transfer to statistically significant differences in the rates of acute proctitis and GU toxicity between WP and PO VMAT. In previous reports discussing the relationship between acute proctitis and rectal dose–volume parameters, no clear dose–volume effect for acute proctitis could be found.18–20 In contrast, the Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC) review demonstrated that most rectal dose–volume parameters for doses ≥60 Gy were significantly associated with late rectal toxicity, with the exception of faecal incontinence.21 Because late faecal incontinence has been associated with medium doses to larger volumes of the rectum,22 it is likely that these larger volumes irradiated at medium-dose levels in the WP group of this study may have influenced the late toxicity profile. For the bladder, there is no clear correlation between the DVH parameters and the incidence of acute and late toxicity,23 since the DVH obtained from a single planning CT image is unlikely to represent the true dose distribution delivered to the bladder during the treatment course. Furthermore, GU toxicity may be partly caused by the effects of radiotherapy on the prostatic urethra and part of the bladder neck, which are unavoidable parts of the target volume and are likely to receive similar doses, independent of the radiation field size.24,25 In contrast, WP VMAT resulted in a significant increase in acute grade 2 diarrhoea because most bowel bag volumes were outside the PTV with PO VMAT, while the bowel bag and PTV partially overlapped in cases that underwent WP VMAT. In this study, the bowel bag V45Gy was 0.2 ± 0.8 and 76.0 ± 30.0 ml for PO and WP VMAT, respectively. However, the incidence of grade 2 diarrhoea was only 5.9%.
IMRT has become the standard of treatment for dose-escalated external beam RT in patients with localized prostate cancer. In terms of acute GI and GU toxicities ≥grade 2, the rates reported from PO IMRT series are from 1.9% to 32.4% and 9–64%, respectively.10,24–28 For WP IMRT, acute GI and GU toxicities ≥grade 2 have been reported from 12% to 50% and 44–51.7%, respectively.10,28–30 Although the use of different toxicity scales makes comparison difficult, in our study, both PO and WP VMAT with daily CBCT-based IGRT provided acceptably low rates of acute GI and GU toxicity when compared with the results presented in other studies.10,24–30
Data comparing acute toxicities between WPRT and PORT in the setting of dose escalation to the prostate are limited. Table 4 summarizes the available reports in comparison with our findings. All of the reports showed that there was no difference in acute GU toxicity, as in the present study. However, there was a statistically significant or tendency towards higher rates of acute GI toxicity in the WPRT groups, except for the report by Guckenberger et al.9 Unlike the other reports, the one by Aizer et al31 reported acute grade 3 GI toxicity in the WPRT group. This high-grade toxicity is potentially explained by the fact that the patients in the WPRT group were treated with three-dimensional conformal radiotherapy, whereas IMRT in combination with IGRT, such as CBCT or the ultrasound-guided system for anatomic positioning verification, was used for WPRT in the other studies.10,28 Given these findings, although there is a trend towards increased acute grade 2 GI toxicity in WPRT, it is conceivable that the incidence of severe acute GI and GU toxicities is infrequent and is similar when comparing WPRT with PORT in the setting of IMRT/VMAT using IGRT.
Table 4.
Summary of reports on acute toxicities in whole-pelvic radiotherapy (WPRT) vs prostate-only radiotherapy (PORT) with dose escalation to the prostate
| Study | Technique | Treatment site | Patients (n) | Total dose (Gy)/number of fractions |
Acute GI toxicity (%) |
Acute GU toxicity (%) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostate | Whole pelvis | Grade 3 | Grade 2 | p-value | Grade 3 | Grade 2 | p-value | ||||
| Aizer et al31 | 3D-CRT, IMRT | WPRT | 68 | 75.6/42 | 45/25 | 10 | 34 | 0.048 | 2 | 18 | 0.090 |
| PORT | 209 | – | 6 | 25 | 3 | 7 | |||||
| Guckenberger et al28 | IMRT | WPRT | 25 | 73.91–76.23/32–33 | 46/25 | 0 | 12 | 1.00 | 0 | 44 | 0.81 |
| PORT | 75 | – | 0 | 12 | 5 | 36 | |||||
| Deville et al10 | IMRT | WPRT | 30 | 79.2/44 | 45/25 | 0 | 50 | 0.006 | 3 | 50 | 0.587 |
| PORT | 30 | – | 0 | 13 | 0 | 50 | |||||
| Current study | VMAT | WPRT | 119 | 78/39 | 46.8/26 | 0 | 14 | 0.066 | 0 | 13 | 0.620 |
| PORT | 105 | – | 0 | 7 | 0 | 11 | |||||
3D-CRT, three-dimensional conformal radiotherapy; GI, gastrointestinal; GU, genitourinary; IMRT, intensity-modulated radiotherapy; VMAT, volumetric-modulated arc therapy.
The pelvic lymph node volumes were similar to those recommended by the RTOG consensus,16 except for the exclusion of the pre-sacral nodes. The pre-sacral lymph nodes were not irradiated in many WP IMRT series in the context of conventionally fractionated8,10,30,32 and hypofractionated33,34 RT. Similar to other institutions,10,34 the reason for the nodal CTV definition used at our institution is that inclusion of the pre-sacral nodes could potentially increase the volume of the rectum, bladder and bowel bag irradiated at the low- to medium-dose levels, which could further impact GI and GU toxicities.
There are several limitations in the present study. First, although the pre-treatment patient characteristics, such as age and comorbidities, were similar in both groups, patients in the WP group received ADT more frequently because this cohort consisted only of patients with high-risk prostate cancer. Whether androgen suppression is correlated with increased toxicity is a controversial issue in the literature.35,36 However, we cannot exclude the possibility that prostate volume reduction after ADT likely yielded the low incidence of acute bladder and rectal toxicity in the WP group. Second, there was a short follow-up period. While a lower incidence of acute toxicity might reduce the development of subsequent late toxicity,25,37,38 WPRT could result in worse late toxicity because the field is larger than that irradiated during PORT. Therefore, late toxicity should be assessed during longer follow-up.
CONCLUSION
WP VMAT resulted in larger rectum and bladder volumes at low- and medium-dose levels, but similar or smaller volumes at high-dose levels than PO VMAT. Both WP and PO VMAT with daily image guidance provided acceptably low rates of acute GI and GU toxicity. Although WP VMAT resulted in a significant increase in acute diarrhoea, the larger volumes of irradiated rectum and bladder in the WP group did not lead to a significant increase in acute proctitis and GU toxicity when compared with the PO group. Further follow-up is needed to assess late toxicity.
Contributor Information
Kentaro Ishii, Email: 141kentaro@gmail.com.
Ryo Ogino, Email: oginoryo0313@gmail.com.
Yukinari Hosokawa, Email: yukinari46@nyc.odn.ne.jp.
Chiaki Fujioka, Email: afcmp134@oct.zaq.ne.jp.
Wataru Okada, Email: okada9011@yahoo.co.jp.
Ryota Nakahara, Email: kio23@hotmail.com.
Ryu Kawamorita, Email: kawamorita@tane.or.jp.
Takuhito Tada, Email: m4677694@msic.med.osaka-cu.ac.jp.
Yoshiki Hayashi, Email: hayashi-uro@tane.or.jp.
Toshifumi Nakajima, Email: naka1951@m5.kcn.ne.jp.
REFERENCES
- 1.Roach M, 3rd, DeSilvio M, Valicenti R, Grignon D, Asbell SO, Lawton C, et al. Whole-pelvis, “mini-pelvis,” or prostate-only external beam radiotherapy after neoadjuvant and concurrent hormonal therapy in patients treated in the Radiation Therapy Oncology Group 9413 trial. Int J Radiat Oncol Biol Phys 2006; 66: 647–53. doi: 10.1016/j.ijrobp.2006.05.074 [DOI] [PubMed] [Google Scholar]
- 2.Pommier P, Chabaud S, Lagrange JL, Richaud P, Lesaunier F, Le Prise E, et al. Is there a role for pelvic irradiation in localized prostate adenocarcinoma? Preliminary results of GETUG-01. J Clin Oncol 2007; 25: 5366–73. doi: 10.1200/JCO.2006.10.5171 [DOI] [PubMed] [Google Scholar]
- 3.Kuban DA, Tucker SL, Dong L, Starkschall G, Huang EH, Cheung MR, et al. Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys 2008; 70: 67–74. doi: 10.1016/j.ijrobp.2007.06.054 [DOI] [PubMed] [Google Scholar]
- 4.Al-Mamgani A, van Putten WL, Heemsbergen WD, van Leenders GJ, Slot A, Dielwart MF, et al. Update of Dutch multicenter dose-escalation trial of radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys 2008; 72: 980–8. doi: 10.1016/j.ijrobp.2008.02.073 [DOI] [PubMed] [Google Scholar]
- 5.Dearnaley DP, Jovic G, Syndikus I, Khoo V, Cowan RA, Graham JD, et al. Escalated-dose versus control-dose conformal radiotherapy for prostate cancer: long-term results from the MRC RT01 randomized controlled trial. Lancet Oncol 2014; 15: 464–73. doi: 10.1016/S1470-2045(14)70040-3 [DOI] [PubMed] [Google Scholar]
- 6.Wang-Chesebro A, Xia P, Coleman J, Akazawa C, Roach M, 3rd. Intensity-modulated radiotherapy improves lymph node coverage and dose to critical structures compared with three-dimensional conformal radiation therapy in clinically localized prostate cancer. Int J Radiat Oncol Biol Phys 2006; 66: 654–62. doi: 10.1016/j.ijrobp.2006.05.037 [DOI] [PubMed] [Google Scholar]
- 7.Sanguineti G, Cavey ML, Endres EJ, Franzone P, Barra S, Parker BC, et al. Does treatment of the pelvic nodes with IMRT increase late rectal toxicity over conformal prostate-only radiotherapy to 76 Gy?. Strahlenther Onkol 2006; 182: 543–9. doi: 10.1007/s00066-006-1586-9 [DOI] [PubMed] [Google Scholar]
- 8.Muren LP, Wasbø E, Helle SI, Hysing LB, Karlsdottir A, Odland OH, et al. Intensity-modulated radiotherapy of pelvic lymph nodes in locally advanced prostate cancer: planning procedures and early experiences. Int J Radiat Oncol Biol Phys 2008; 71: 1034–41. doi: 10.1016/j.ijrobp.2007.11.060 [DOI] [PubMed] [Google Scholar]
- 9.Guckenberger M, Baier K, Richter A, Vordermark D, Flentje M. Does intensity modulated radiation therapy (IMRT) prevent additional toxicity of treating the pelvic lymph nodes compared to treatment of the prostate only? Radiat Oncol 2008; 3: 3. doi: 10.1186/1748-717X-3-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deville C, Both S, Hwang WT, Tochner Z, Vapiwala N. Clinical toxicities and dosimetric parameters after whole-pelvis versus prostate-only intensity-modulated radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys 2010; 78: 763–72. doi: 10.1016/j.ijrobp.2009.08.043 [DOI] [PubMed] [Google Scholar]
- 11.Teoh M, Clark CH, Wood K, Whitaker S, Nisbet A. Volumetric modulated arc therapy: a review of current literature and clinical use in practice. Br J Radiol. 2011; 84: 967–96. doi: 10.1259/bjr/22373346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pesce GA, Clivio A, Cozzi L, Nicolini G, Richetti A, Salati E, et al. Early clinical experience of radiotherapy of prostate cancer with volumetric modulated arc therapy. Radiat Oncol 2010; 5: 54. doi: 10.1186/1748-717X-5-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall WA, Colbert L, Nickleach D, Shelton J, Marcus DM, Switchenko J, et al. Reduced acute toxicity associated with the use of volumetric modulated arc therapy for the treatment of adenocarcinoma of the prostate. Pract Radiat Oncol 2013; 3: e157–64. doi: 10.1016/j.prro.2013.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishii K, Ogino R, Hosokawa Y, Fujioka C, Okada W, Nakahara R, et al. Whole-pelvic volumetric-modulated arc therapy for high-risk prostate cancer: treatment planning and acute toxicity. J Radiat Res 2015; 56: 141–50. doi: 10.1093/jrr/rru086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Joint Committee on Cancer. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, eds. AJCC cancer staging manual. 7th edn New York, NY: Springer; 2009. [Google Scholar]
- 16.Lawton CA, Michalski J, El-Naqa I, Buyyounouski MK, Lee WR, Menard C, et al. RTOG GU radiation oncology specialists reach consensus on pelvic lymph node volumes for high-risk prostate cancer. Int J Radiat Oncol Biol Phys 2009; 74: 383–7. doi: 10.1016/j.ijrobp.2008.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gay HA, Barthold HJ, O'Meara E, Bosch WR, El Naqa I, Al-Lozi R, et al. Pelvic normal tissue contouring guidelines for radiation therapy: a radiation therapy oncology group consensus panel atlas. Int J Radiat Oncol Biol Phys 2012; 83: e353–62. doi: 10.1016/j.ijrobp.2012.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michalski JM, Purdy JA, Winter K, Roach M, 3rd, Vijayakumar S, Sandler HM, et al. Preliminary report of toxicity following 3D radiation therapy for prostate cancer on 3DOG/RTOG 9406. Int J Radiat Oncol Biol Phys 2000; 46: 391–402. doi: 10.1016/S0360-3016(99)00443-5 [DOI] [PubMed] [Google Scholar]
- 19.Teh BS, Mai WY, Uhl BM, Augspurger ME, Grant WH, 3rd, Lu HH, et al. Intensity-modulated radiation therapy (IMRT) for prostate cancer with the use of a rectal balloon for prostate immobilization: acute toxicity and dose-volume analysis. Int J Radiat Oncol Biol Phys 2001; 49: 705–12. doi: 10.1016/S0360-3016(00)01428-0 [DOI] [PubMed] [Google Scholar]
- 20.Ryu JK, Winter K, Michalski JM, Purdy JA, Markoe AM, Earle JD, et al. Interim report of toxicity from 3D conformal radiation therapy (3D-CRT) for prostate cancer on 3DOG/RTOG 9406, level III (79.2 Gy). Int J Radiat Oncol Biol Phys 2002; 54: 1036–46. doi: 10.1016/S0360-3016(02)03006-7 [DOI] [PubMed] [Google Scholar]
- 21.Michalski JM, Gay H, Jackson A, Tucker SL, Deasy JO. Radiation dose-volume effects in radiation-induced rectal injury. Int J Radiat Oncol Biol Phys 2010; 76(Suppl. 3): S123–9. doi: 10.1016/j.ijrobp.2009.03.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heemsbergen WD, Hoogeman MS, Hart GA, Lebesque JV, Koper PC. Gastrointestinal toxicity and its relation to dose distributions in the anorectal region of prostate cancer patients treated with radiotherapy. Int J Radiat Oncol Biol Phys 2005; 61: 1011–18. doi: 10.1016/j.ijrobp.2004.07.724 [DOI] [PubMed] [Google Scholar]
- 23.Viswanathan AN, Yorke ED, Marks LB, Eifel PJ, Shipley WU. Radiation dose-volume effects of the urinary bladder. Int J Radiat Oncol Biol Phys 2010; 76(Suppl. 3): S116–22. doi: 10.1016/j.ijrobp.2009.02.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zelefsky MJ, Fuks Z, Hunt M, Yamada Y, Marion C, Ling CC, et al. High-dose intensity modulated radiation therapy for prostate cancer: early toxicity and biochemical outcome in 772 patients. Int J Radiat Oncol Biol Phys 2002; 53: 1111–16. doi: 10.1016/S0360-3016(02)02857-2 [DOI] [PubMed] [Google Scholar]
- 25.Michalski JM, Yan Y, Watkins-Bruner D, Bosch WR, Winter K, Galvin JM, et al. Preliminary toxicity analysis of 3-dimensional conformal radiation therapy versus intensity modulated radiation therapy on the high-dose arm of the Radiation Therapy Oncology Group 0126 prostate cancer trial. Int J Radiat Oncol Biol Phys 2013; 87: 932–8. doi: 10.1016/j.ijrobp.2013.07.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Meerleer G, Vakaet L, Meersschout S, Villeirs G, Verbaeys A, Oosterlinck W, et al. Intensity-modulated radiotherapy as primary treatment for prostate cancer: acute toxicity in 114 patients. Int J Radiat Oncol Biol Phys 2004; 60: 777–87. [DOI] [PubMed] [Google Scholar]
- 27.Matzinger O, Duclos F, van den Bergh A, Carrie C, Villà S, Kitsios P, et al. Acute toxicity of curative radiotherapy for intermediate- and high-risk localized prostate cancer in the EORTC trial 22991. Eur J Cancer 2009; 45: 2825–34. doi: 10.1016/j.ejca.2009.07.009 [DOI] [PubMed] [Google Scholar]
- 28.Guckenberger M, Ok S, Polat B, Sweeney RA, Flentje M. Toxicity after intensity-modulated, image-guided radiotherapy for prostate cancer. Strahlenther Onkol 2010; 186: 535–43. doi: 10.1007/s00066-010-2144-z [DOI] [PubMed] [Google Scholar]
- 29.Sanguineti G, Endres EJ, Parker BC, Bicquart C, Little M, Chen G, et al. Acute toxicity of whole-pelvis IMRT in 87 patients with localized prostate cancer. Acta Oncol 2008; 47: 301–10. doi: 10.1080/02841860701558849 [DOI] [PubMed] [Google Scholar]
- 30.Bayley A, Rosewall T, Craig T, Bristow R, Chung P, Gospodarowicz M, et al. Clinical application of high-dose, image-guided intensity-modulated radiotherapy in high-risk prostate cancer. Int J Radiat Oncol Biol Phys 2010; 77: 477–83. doi: 10.1016/j.ijrobp.2009.05.006 [DOI] [PubMed] [Google Scholar]
- 31.Aizer AA, Yu JB, McKeon AM, Decker RH, Colberg JW, Peschel RE. Whole pelvic radiotherapy versus prostate only radiotherapy in the management of locally advanced or aggressive prostate adenocarcinoma. Int J Radiat Oncol Biol Phys 2009; 75: 1344–9. doi: 10.1016/j.ijrobp.2008.12.082 [DOI] [PubMed] [Google Scholar]
- 32.Lilleby W, Stensvold A, Dahl AA. Adding intensity-modulated radiotherapy to the pelvis does not worsen the adverse effect profiles compared to limited field radiotherapy in men with prostate cancer at 12-month follow-up. Acta Oncol 2014; 53: 1380–9. doi: 10.3109/0284186X.2014.916042 [DOI] [PubMed] [Google Scholar]
- 33.Pervez N, Small C, MacKenzie M, Yee D, Parliament M, Ghosh S, et al. Acute toxicity in high-risk prostate cancer patients treated with androgen suppression and hypofractionated intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys 2010; 76: 57–64. doi: 10.1016/j.ijrobp.2009.01.048 [DOI] [PubMed] [Google Scholar]
- 34.Norkus D, Karklelyte A, Engels B, Versmessen H, Griskevicius R, De Ridder M, et al. A randomized hypofractionation dose escalation trial for high risk prostate cancer patients: interim analysis of acute toxicity and quality of life in 124 patients. Radiat Oncol 2013; 8: 206. doi: 10.1186/1748-717X-8-206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feigenberg SJ, Hanlon AL, Horwitz EM, Uzzo RG, Eisenberg D, Pollack A. Long-term androgen deprivation increases grade 2 and higher late morbidity in prostate cancer patients treated with three-dimensional conformal radiation therapy. Int J Radiat Oncol Biol Phys 2005; 62: 397–405. doi: 10.1016/j.ijrobp.2004.10.021 [DOI] [PubMed] [Google Scholar]
- 36.Lawton CA, Bae K, Pilepich M, Hanks G, Shipley W. Long-term treatment sequelae after external beam irradiation with or without hormonal manipulation for adenocarcinoma of the prostate: analysis of radiation therapy oncology group studies 85-31, 86-10, and 92-02. Int J Radiat Oncol Biol Phys 2008; 70: 437–41. doi: 10.1016/j.ijrobp.2007.06.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zelefsky MJ, Levin EJ, Hunt M, Yamada Y, Shippy AM, Jackson A, et al. Incidence of late rectal and urinary toxicities after three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys 2008; 70: 1124–9. doi: 10.1016/j.ijrobp.2007.11.044 [DOI] [PubMed] [Google Scholar]
- 38.Jereczek-Fossa BA, Zerini D, Fodor C, Santoro L, Serafini F, Cambria R, et al. Correlation between acute and late toxicity in 973 prostate cancer patients treated with three-dimensional conformal external beam radiotherapy. Int J Radiat Oncol Biol Phys 2010; 78: 26–34. doi: 10.1016/j.ijrobp.2009.07.1742 [DOI] [PubMed] [Google Scholar]