Abstract
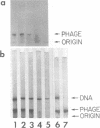
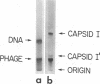
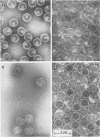
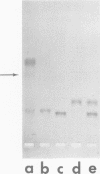
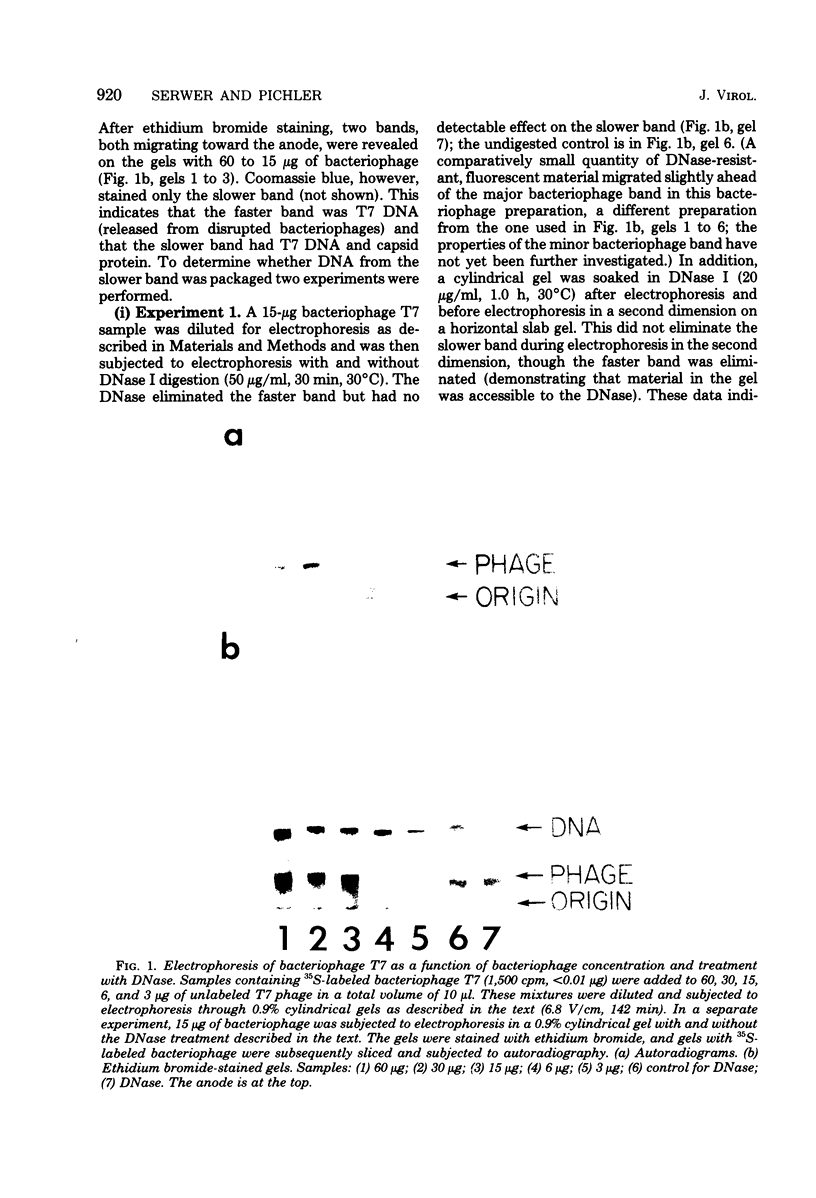
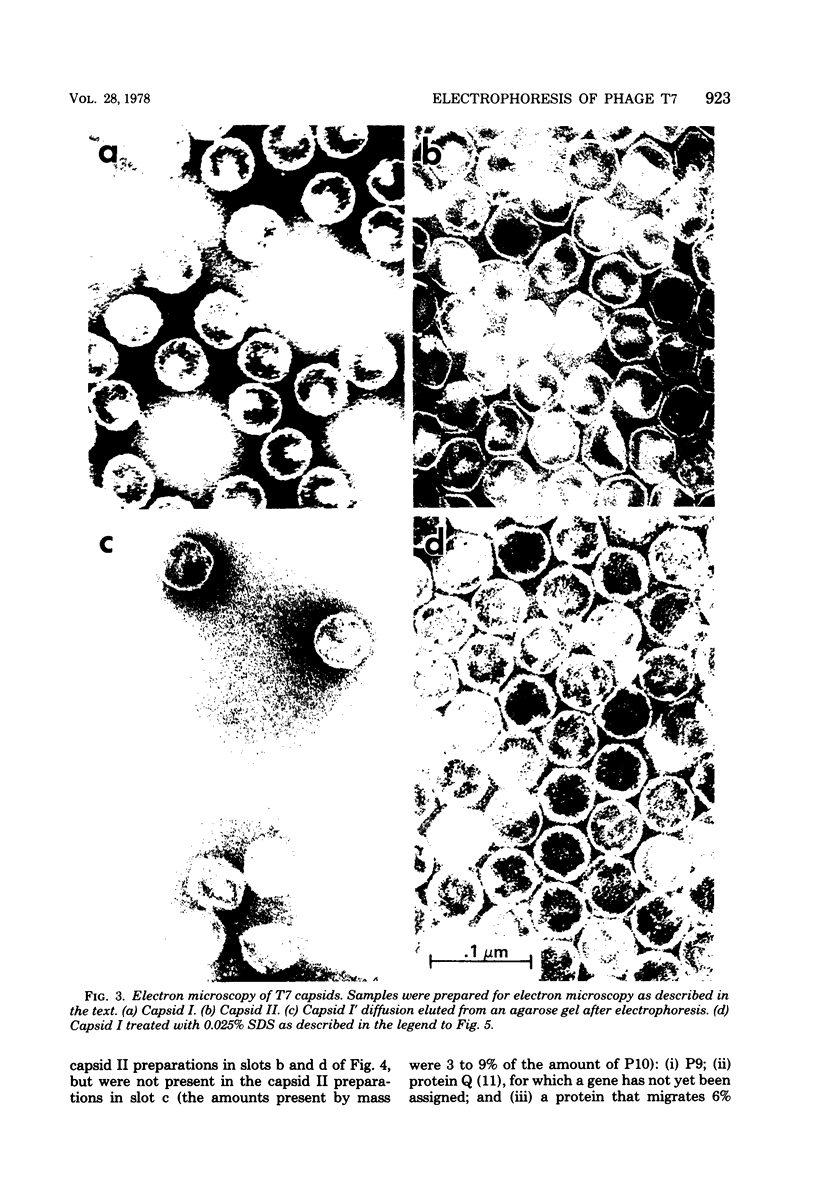
Agarose gel electrophoresis of the following was performed in 0.05 M sodium phosphate-0.001 M MgCl2 (pH 7.4): (i) bacteriophage T7; (ii) a T7 precursor capsid (capsid I), isolated from T7-infected Escherichia coli, which has a thicker and less angular envelope than bacteriophage T7; (iii) a second capsid (capsid II), isolated from T7-infected E. coli, which has a bacteriophage-like envelope; and (iv) capsids (capsid IV) produced by temperature shock of bacteriophage T7. Bacteriophage T7 and all of the above capsids migrated towards the anode. In a 0.9% agarose gel, capsid I had an electrophoretic mobility of 9.1 +/- 0.4 X 10(-5) cm2/V.s; bacteriophage T7 migrated 0.31 +/- 0.02 times as fast as capsid I. The mobilities of different preparations of capsid II varied in such gels: the fastest-migrating capsid II preparation was 0.51 +/- 0.03 times as fast as capsid I and the slowest was 0.37 +/- 0.02 times as fast as capsid I. Capsid IV with and without the phage tail migrated 0.29 +/- 0.02 and 0.42 +/- 0.02 times as fast as capsid I. The results of the extrapolation of bacteriophage and capsid mobilities to 0% agarose concentration indicated that the above differences in mobility are caused by differences in average surface charge density. To increase the accuracy of mobility comparisons and to increase the number of samples that could be simultaneously analyzed, multisample horizontal slab gels were used. Treatment with the ionic detergent sodium dodecyl sulfate converted capsid I to a capsid that migated in the capsid II region during electrophoresis through agarose gels. In the electron microscope, most of the envelopes of these latter capsids resembled the capsid II envelope, but some envelope regions were thicker than the capsid II envelope.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bancroft F. C., Freifelder D. Molecular weights of coliphages and coliphage DNA. I. Measurement of the molecular weight of bacteriophage T7 by high-speed equilibrium centrifugation. J Mol Biol. 1970 Dec 28;54(3):537–546. doi: 10.1016/0022-2836(70)90124-5. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Earnshaw W., Casjens S., Harrison S. C. Assembly of the head of bacteriophage P22: x-ray diffraction from heads, proheads and related structures. J Mol Biol. 1976 Jun 25;104(2):387–410. doi: 10.1016/0022-2836(76)90278-3. [DOI] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Oliver R. M. Negative stain electron microscopy of protein macromolecules. Methods Enzymol. 1973;27:616–672. doi: 10.1016/s0076-6879(73)27029-5. [DOI] [PubMed] [Google Scholar]
- Roeder G. S., Sadowski P. D. Bacteriophage T7 morphogenesis: phage-related particles in cells infected with wild-type and mutant T7 phage. Virology. 1977 Jan;76(1):263–285. doi: 10.1016/0042-6822(77)90302-6. [DOI] [PubMed] [Google Scholar]
- Serwer P. Buoyant density sedimentation of macromolecules in sodium iothalamate density gradients. J Mol Biol. 1975 Mar 5;92(3):433–448. doi: 10.1016/0022-2836(75)90290-9. [DOI] [PubMed] [Google Scholar]
- Serwer P. Fast sedimenting bacteriophage T7 DNA from T7-infected Escherichia coli. Virology. 1974 May;59(1):70–88. doi: 10.1016/0042-6822(74)90207-4. [DOI] [PubMed] [Google Scholar]
- Serwer P., Graef P. R., Garrison P. N. Use of ethidium bromide fluorescence enhancement to detect duplex DNA and DNA bacteriophages during zone sedimentation in sucrose gradients: molecular weight of DNA as a function of sedimentation rate. Biochemistry. 1978 Apr 4;17(7):1166–1170. doi: 10.1021/bi00600a005. [DOI] [PubMed] [Google Scholar]
- Serwer P. Internal proteins of bacteriophage T7. J Mol Biol. 1976 Nov 5;107(3):271–291. doi: 10.1016/s0022-2836(76)80005-8. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Bacteriophage T7. Science. 1972 Apr 28;176(4033):367–376. doi: 10.1126/science.176.4033.367. [DOI] [PubMed] [Google Scholar]