Abstract
Objective:
To compare the reliability of two recently reported MR grading systems of cervical neural foraminal stenosis (CNFS) and their correlation with clinical manifestations.
Methods:
We evaluated 188 patients (male : female = 80 : 108; mean age of 41 years) who visited our institution and underwent oblique sagittal MRI of the cervical spine. Two radiologists evaluated the MRI findings for the presence and grade of CNFS at the narrowest point, with the grading systems (Park, Kim and mKim systems) suggested by Park et al and Kim et al. More than one positive neurologic sign and more than one neurologic clinical symptom was considered a positive neurologic manifestation of each foraminal stenosis. Interobserver agreement between the two readers was analyzed using kappa statistics. Non-parametric correlation analysis (Spearman's correlation) was used to evaluate the correlation coefficients (R) to assess the relationship between CNFS grade and clinical manifestations.
Results:
Both the Park and mKim systems demonstrated a relatively high correlation (R = 0.714–0.764) between the CNFS grade and clinical manifestation, while the Kim system yielded a moderate correlation (by Reader 2). The Park and mKim systems demonstrated higher correlation values at the level of C6–7 than C4–5, while the Kim system showed no difference in correlation at the cervical spine level.
Conclusion:
Both the Park and mKim systems provide a reliable, reproducible CNFS diagnosis, while the Kim system has a slightly inferior reliability. The Park and mKim systems had a similar, relatively high clinical correlation.
Advances in knowledge:
Grades 2 and 3 of the Park system and Grade 2 in the Kim and mKim systems exhibited a similar clinical significance. Patients with a grade of 0 (using each system) consistently exhibit negative neurologic manifestation.
INTRODUCTION
Cervical radiculopathy is a disease process caused by the compression of cervical nerve roots, commonly a result of degenerative osteophytes or lateral disc herniation, otherwise known as cervical neural foraminal stenosis (CNFS).1 MRI-based diagnosis and evaluation of CNFS is important, and precise grading of stenosis may aid neurosurgeons and neurophysicians in determining appropriate treatment options.2 During recent years, two semi-quantitative grading systems regarding CNFS have been reported.2–4 Kim et al3 suggested a grading system based on T2 weighted axial sections and classification of stenoses using three grades (0, 1 and 2) (the Kim system). Kim grades are calculated from the ratio of the narrowest width of the cervical neural foramen to the width of the extraforaminal nerve root. A follow-up study suggested a moderate correlation between the Kim system and clinical manifestation of CNFS, and a modified Kim system was proposed (the mKim system) to enhance clinical correlation.5 In addition, Park et al2 introduced a grading system using oblique views, which allows for the evaluation of narrowed neural foramen (the Park system). Park grades are morphologic diagnoses based on the perineural fat obliteration. The group classified stenoses using four grades (0, 1, 2 and 3). A follow-up study indicated that the Park system provides a reliable, reproducible CNFS assessment and also suggested a moderate correlation between the Park system and clinical manifestations.4 Until now, there have been no studies comparing the Kim and Park grading systems based on the same patient group. The purpose of this study was to compare the reliability, reproducibility and clinical correlation of the Park and Kim systems.
METHODS AND MATERIALS
Case selection
The study was approved by the institutional review board, and the requirement for informed consent was waived owing to the retrospective nature of the study. We reviewed the medical records of 243 consecutive patients who underwent C-spine MRI with neck pain with or without symptom spreading to the upper extremities, weakness of the hand or fingers and tingling or loss of sensation in the upper extremities. MRI was performed in our clinic between January 2014 and May 2014. We excluded 55 patients for the following reasons: central or multilevel stenosis (47 cases), possible spinal cord injury caused by space-occupying lesions such as tumours or cysts (3 cases), stroke (4 cases) and syringomyelia (1 case). Two radiologists who had performed image analyses decided whether the patient had multilevel disease or not by consensus. The patients who had stroke of the brain were excluded because the patients can show similar clinical manifestations such as paraesthesia, numbness and extremity weakness and can be a bias. Our final analysis included 188 patients, including 80 males (mean age: 41 ± 13 years, range: 14–72 years) and 108 females (mean age: 41 ± 16 years, range: 26–81 years).
Clinical correlation
A neurosurgeon who had 25 years' experience and otherwise not involved with CNFS grading assessed the clinical symptoms and performed neurological examinations. Positive neurologic clinical symptoms included paraesthesia, numbness, extremity weakness and radicular pain corresponding to each cervical level.5 The patient was considered as neurologic signs positive, if the patient demonstrated Spurling sign, Lhermitte sign, decreased response to deep tendon reflex or a positive response on electromyogram.4 More than one positive neurologic sign and more than one neurologic clinical symptom was considered a positive neurologic manifestation (PNM) of each foraminal stenosis.5 Clinical symptoms and neurologic signs in the lower extremities or contralateral to the stenosis were not considered a PNM.4,5
MRI parameters
The MR scans were performed using a 1.5-T magnet (Intera™; Philips Medical Systems, Best, Netherlands) using a syn-head coil. Identical MR protocols were used for all patients, and a fast spin-echo imaging technique was employed with patients in the supine position. Axial and sagittal MR scans were acquired and included the following parameters: axial T2 weighted spin echo [repetition time (TR)/echo time (TE), 3000–4000/100 ms; 315 × 250 pixels matrix; field of view (FOV), 17 cm; 3-mm slice thickness; signal average, 1; interslice gap, 0.3 mm]; sagittal T2 weighted fast spin echo (TR/TE, 2000–3000/100 ms; 360 × 280 pixels matrix; FOV, 24 cm; slice thickness, 4 mm; signal average, 3; interslice gap, 0 mm); axial T1 weighted spin echo (TR/TE, 700/10 ms; 315 × 250 pixels matrix; FOV, 17 cm; slice thickness, 3 mm; signal average, 1; interslice gap, 0.3 mm); and sagittal T1 weighted spin echo (TR/TE, 500/10 ms; 360 × 280 pixels matrix; FOV, 24 cm; slice thickness, 4 mm; signal average, 3; interslice gap, 0 mm). Left and right oblique sagittal images were acquired. Seven images from left oblique sagittal and right oblique sagittal images were taken progressing laterally through the foramen at a projection angle of 45° from the medial edge of the neural foramen. The angled projections were taken using the following parameters: FOV, 24 cm; matrix, 360 × 290 pixels; slice thickness, 3 mm; interslice gap, 0 mm; TR/TE = 1300–2600/100 ms; and signal average 3. The images included the lateral edge of the foramen, the isthmus of the foramen and the medial margin of each pedicle.6 The mean scan time of both oblique images was 3.25 min and the total scan time for all sequences was approximately 23 min. The time difference for the two settings was about 3 min.
Image analysis
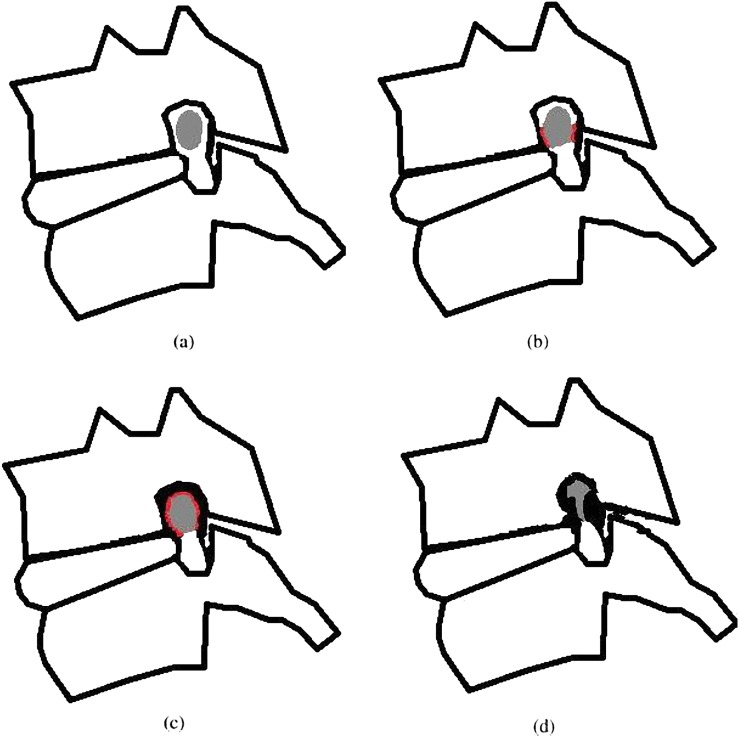
Two readers (musculoskeletal radiologists with 10 and 15 years' experience) interpreted the MR images separately for the three CNFS grading systems. They interpreted MR images independently. Both radiologists assessed the presence and grade of CNFS at the narrowest point, following the grading system suggested by Park et al2 (Park system). Grade 0 refers to the absence of foraminal stenosis. Grade 1 refers to mild foraminal stenosis with partial (<50% of total root circumference) perineural fat obliteration. Grade 2 refers to moderate stenosis with near complete (≥50% of total root circumference) perineural fat obliteration, but no morphological changes in the nerve root. Grade 3 refers to severe foraminal stenosis with nerve root collapse or morphological changes (Figure 1). The Kim and mKim systems3,5 were assessed based on T2 axial scans. Grading of the Kim system is described as follows. Grade 0 signifies no stenosis, with the narrowest portion of the neural foramen wider than the extraforaminal nerve root [foramen-to-root (FR) ratio >100%]. Grade 1 indicates that the narrowest portion of the cervical neural foramen is equal to or less than the width of the extraforaminal nerve (50% < FR ratio ≤ 100%). Grade 2 indicates that the width of the cervical neural foramen is <50% of the width of the extraforaminal nerve root (FR ratio ≤50%). Grading using the mKim system is described as follows. Grade 0 signifies that the narrowest portion of the cervical neural foramen is >80% of the width of the extraforaminal nerve root (FR ratio >80%). Grade 1 indicates that the narrowest portion of the cervical neural foramen is <80% but >50% of the width of the extraforaminal nerve root (50% < FR ratio ≤ 80%). Grade 2 for the mKim system is the same as that for the Kim system (Figures 2–5).
Figure 1.
Illustrations of the Park grading system based on the oblique sagittal plane. (a) Grade 0, no neural foraminal stenosis and an intact perineural fat plane. (b) Grade 1, perineural fat obliteration of <50% of the nerve root circumference. (c) Grade 2, perineural fat obliteration of ≥50% of the nerve root circumference. (d) Grade 3, morphologic distortion of the nerve root. Drawings by HJ Park. Adapted from Park et al2 with permission from British Institute of Radiology.
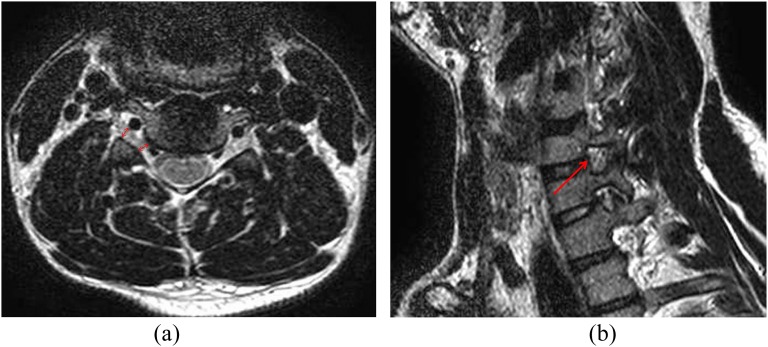
Figure 2.
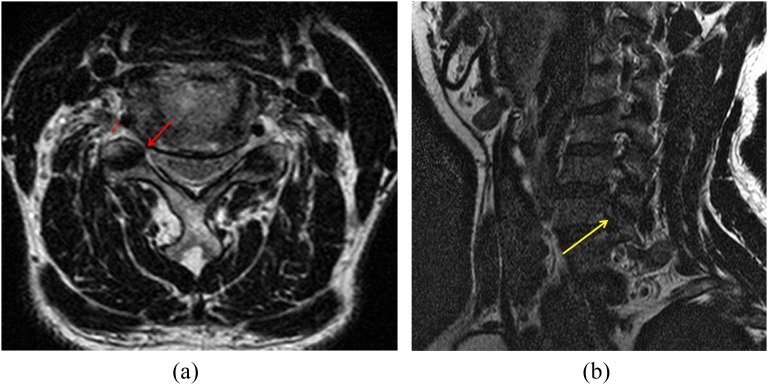
An example of the Kim and modified Kim grading systems: a male with right arm numbness. (a) Width of the narrowest portion of the neural foramen/extraforaminal nerve root = 3.22 mm (lower double-headed arrow)/3.22 mm (upper double-headed arrow), Kim system Grade 1, modified Kim system Grade 0 (repetition time/echo time, 4000/100). The patient exhibited negative neurologic manifestations. (b) This patient showed no significant stenosis (arrow) in right C5–6 on T2 weighted oblique sagittal image (Park Grade 0).
Figure 5.
A 46-year-old male with right forearm weakness. (a) A T2 weighted axial image [repetition time (TR)/echo time (TE), 4000/100]; foramen-to-root ratio = 0.46 (1.90/4.10) (arrow/double-headed arrow). (b) An oblique sagittal T2 weighted turbo spin-echo image (1460/100, TR/TE) showing moderate (>50% of the root circumference) perineural fat obliteration at C5–6 (arrow). Park grade = 2, Kim grade = 2 and modified Kim grade = 2. He demonstrated positive neurologic manifestations, and neuropathy was confirmed by electromyogram. He showed dramatic improvement of symptoms after epidural nerve block on C5–6.
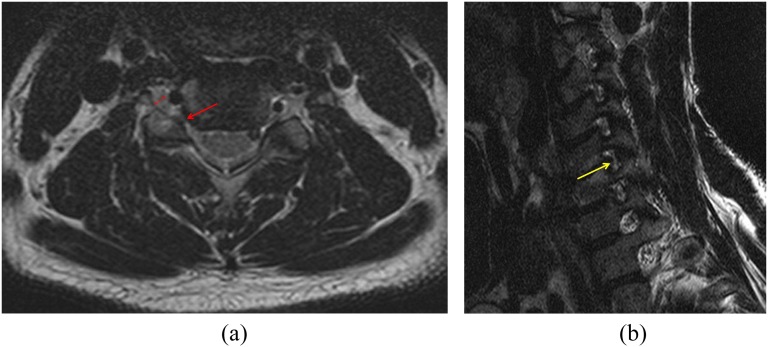
Figure 3.
A 53-year-old male with right arm pain. (a) A T2 weighted axial image [repetition time (TR)/echo time (TE), 4000/100]; foramen-to-root ratio = 0.54 (1.61/2.96) (double-headed arrows). (b) An oblique sagittal T2 weighted turbo spin-echo image (1460/100, TR/TE) showing marked narrowing of the neural foramen and collapsed nerve root at C5–6 (arrow) on the right side. Park grade = 3, Kim grade = 1 and modified Kim grade = 1. He demonstrated positive clinical manifestations. Neuropathy due to compression was confirmed by electromyogram.
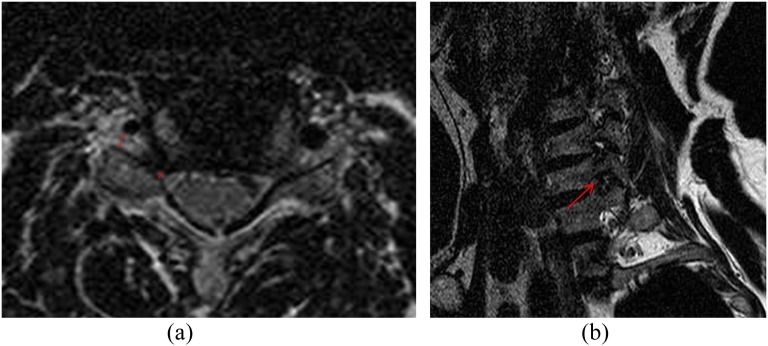
Figure 4.
A 59-year-old male with right shoulder and arm pain. (a) A T2 weighted axial image [repetition time (TR)/echo time (TE), 4000/100]; foramen-to-root ratio = 0.40 (1.06/2.65) (arrow and double-headed arrows). (b) An oblique sagittal T2 weighted turbo spin-echo image (1460/100, TR/TE) showing marked narrowing of the neural foramen and collapsed nerve root at C5–6 (arrow). Park grade = 3, Kim grade = 2 and modified Kim grade = 2. He demonstrated positive clinical manifestations.
Statistical analysis
Kappa statistics were used in the evaluation of interobserver agreement between the two readers. Agreement between the two radiologists was analyzed by the cervical spine level. Strength of the interobserver agreement was interpreted as follows: poor (k < 0.1), slight (0.1 ≤ k ≤ 0.2), fair (0.2 < k ≤ 0.4), moderate (0.4 < k ≤ 0.6), substantial (0.6 < k ≤ 0.8) and nearly perfect (0.8 < k ≤ 1).7 Correlation coefficients (R) were calculated using the non-parametric correlation analysis (Spearman's correlation) to assess the relationship between grade and clinical manifestation. The relationship between grade and neurologic manifestation was analyzed at each cervical level (C4–5, C5–6 and C6–7). A weak correlation was defined as a correlation coefficient between 0.1 and 0.3, a moderate correlation between 0.3 and 0.7, a relatively high correlation between 0.7 and 0.9 and a very high correlation above 0.9.8 We used PASW® software v. 18.0 (IBM, Armonk, NY), and p-values of <0.05 were considered statistically significant.
RESULTS
Schematic illustrations of the Park system are shown in Figure 1. CNFS distributions by spinal cord level according to each grading system are described in Table 1. Grade 0 was the most common grade for all scoring systems (72% in the Park system, 56–58% in the Kim system and 69–72% in the mKim system). Overall, 51 patients exhibited PNMs. The incidence of PNM corresponding to each grade (stratified by the scoring system) is shown in Table 2. The highest score of each grading system (Grade 3 of the Park system, Grade 2 of the Kim and mKim systems) demonstrated a good correlation with clinical manifestation without difference according to the type of reader [93% (13/14, number of PNM/number of the grade) and 87% (13/15) in the Park system, 97% (30/31) and 93% (25/27) in the Kim and mKim systems]. Interobserver reliability is described in Table 3. Whereas the overall interobserver agreement for the Kim system was substantial (κ = 0.769), the agreements for the Park and mKim systems were nearly perfect (κ = 0.913 and 0.823, respectively). Correlation coefficients of each grading system between grades and clinical manifestation according to each reader and CNFS level are shown in Table 4. Both the Park and mKim systems exhibited relatively high correlation (R 0.714–0.764), while the correlation of the Kim system by Reader 2 demonstrated a moderate correlation. Whereas the Park and mKim systems exhibited higher correlation at the level of C6–7 than C4–5, the Kim system showed no difference according to cervical spine level.
Table 1.
Distribution of foraminal stenosis by cervical level and grade
| Cervical level | Grade 0 |
Grade 1 |
Grade 2 |
Grade 3 |
Total |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Reader 1 | Reader 2 | Reader 1 | Reader 2 | Reader 1 | Reader 2 | Reader 1 | Reader 2 | Reader 1 | Reader 2 | |
| Park system | ||||||||||
| C4–5 | 46 (34%) | 46 (34%) | 3 (14%) | 3 (3%) | 2 (12%) | 2 (13%) | 1 (7%) | 1 (7%) | 52 (28%) | 52 (28%) |
| C5–6 | 45 (33%) | 45 (33%) | 13 (62%) | 13 (57%) | 9 (53%) | 8 (53%) | 9 (64%) | 10 (67%) | 76 (40%) | 76 (40%) |
| C6–7 | 45 (33%) | 44 (33%) | 5 (24%) | 7 (30%) | 6 (35%) | 5 (33%) | 4 (29%) | 4 (27%) | 60 (32%) | 60 (32%) |
| Overall | 136 (72%) | 135 (72%) | 21 (11%) | 23 (12%) | 17 (10%) | 15 (8%) | 14 (7%) | 15 (8%) | 188 (100%) | 188 (100%) |
| Kim system | ||||||||||
| C4–5 | 40 (38%) | 43 (39%) | 8 (16%) | 6 (12%) | 4 (13%) | 3 (11%) | – | – | 52 (28%) | 52 (28%) |
| C5–6 | 36 (34%) | 34 (31%) | 25 (49%) | 27 (52%) | 15 (48%) | 15 (56%) | – | – | 76 (40%) | 76 (40%) |
| C6–7 | 30 (28%) | 32 (29%) | 18 (35%) | 19 (37%) | 12 (39%) | 9 (33%) | – | – | 60 (32%) | 60 (32%) |
| Overall | 106 (56%) | 109 (58%) | 51 (27%) | 52 (28%) | 31 (17%) | 27 (14%) | – | – | 188 (100%) | 188 (100%) |
| Modified Kim system | ||||||||||
| C4–5 | 45 (35%) | 45 (33%) | 3 (11%) | 4 (15%) | 4 (13%) | 3 (11%) | – | – | – | 52 (28%) |
| C5–6 | 46 (36%) | 50 (37%) | 15 (54%) | 11 (42%) | 15 (48%) | 15 (56%) | – | – | 76 (40%) | 76 (40%) |
| C6–7 | 38 (29%) | 40 (30%) | 10 (36%) | 11 (42%) | 12 (39%) | 9 (33%) | – | – | 60 (32%) | 60 (32%) |
| Overall | 129 (69%) | 135 (72%) | 28 (15%) | 26 (14%) | 31 (16%) | 27 (14%) | – | – | 188 (100%) | 188 (100%) |
Park system: Grade 0 = absence of foraminal stenosis. Grade 1 = mild foraminal stenosis with partial (<50% of total root circumference) perineural fat obliteration. Grade 2 = moderate stenosis with near complete (≥50% of total root circumference) perineural fat obliteration. Grade 3 = severe foraminal stenosis with nerve root collapse or morphological changes.
Kim system: Grade 0 = no stenosis [foramen-to-root (FR) ratio > 100%]. Grade 1 = (50% < FR ratio ≤ 100%). Grade 2 = (FR ratio ≤50%).
mKim system: Grade 0 = [foramen-to-root (FR) ratio >80%]. Grade 1 = (50% < FR ratio ≤80%). Grade 2 = (FR ratio ≤50%).
Table 2.
Prevalence of grade assignment by radiologist and prevalence of associated neurologic manifestation by grade
| Parameter | Grade 0 | Grade 1 | Grade 2 | Grade 3 | Total | |
|---|---|---|---|---|---|---|
| Park system | Prevalence | 136/135 | 21/23 | 17/15 | 14/15 | 188/188 |
| PNM | 9/8 | 13/16 | 16/14 | 13/13 | 51/51 | |
| NNM | 127/127 | 8/7 | 1/1 | 1/2 | 137/137 | |
| Kim system | Prevalence | 106/109 | 51/52 | 31/27 | – | 188/188 |
| PNM | 0/5 | 21/21 | 30/25 | – | 51/51 | |
| NNM | 106/104 | 30/31 | 1/2 | – | 137/137 | |
| Modified Kim system | Prevalence | 129/135 | 28/26 | 31/27 | – | 188/188 |
| PNM | 7/11 | 14/15 | 30/25 | – | 51/51 | |
| NNM | 122/124 | 14/11 | 1/2 | – | 137/137 | |
NNM, negative neurologic manifestation; PNM, positive neurologic manifestation.
Reader 1/Reader 2.
Table 3.
Interobserver reliability by spinal cord level. Reported values are κ values
| Grades | C4–5 | C5–6 | C6–7 | Overall |
|---|---|---|---|---|
| Park system | 1.000 (1.00–1.00) | 0.911 (0.829–0.993) | 0.922 (0.818–1.00) | 0.913 (0.856–0.970) |
| Kim system | 0.662 (0.422–0.902) | 0.750 (0.620–0.880) | 0.808 (0.662–0.937) | 0.769 (0.688–0.851) |
| Modified Kim system | 0.921 (0.777–1.000) | 0.803 (0.680–0.926) | 0.839 (0.710–0.968) | 0.823 (0.745–0.902) |
Strength of interobserver agreement (κ) was characterized as follows: poor (k < 0.1), slight (0.1 ≤ k ≤ 0.2), fair (0.2 < k ≤ 0.4), moderate (0.4 < k ≤ 0.6), substantial (0.6 < k ≤ 0.8) and almost perfect (0.8 < k ≤ 1).
Data: values in parentheses are 95% confidence intervals.
Table 4.
Correlation coefficients (R) between grade and clinical manifestation
| Grade | C4–5 (52 cases) | C5–6 (76 cases) | C6–7 (60 cases) | Total (188 cases) | |
|---|---|---|---|---|---|
| Park system | Radiologist 1 | 0.582 (<0.001) | 0.772 (<0.001) | 0.802 (<0.001) | 0.741 (<0.001) |
| Radiologist 2 | 0.582 (<0.001) | 0.746 (<0.001) | 0.777 (<0.001) | 0.737 (<0.001) | |
| Kim system | Radiologist 1 | 0.766 (<0.001) | 0.758 (<0.001) | 0.784 (<0.001) | 0.768 (<0.001) |
| Radiologist 2 | 0.456 (<0.001) | 0.668 (<0.001) | 0.716 (<0.001) | 0.658 (<0.001) | |
| Modified Kim system | Radiologist 1 | 0.541 (<0.001) | 0.755 (<0.001) | 0.839 (<0.001) | 0.764 (<0.001) |
| Radiologist 2 | 0.532 (<0.001) | 0.664 (<0.001) | 0.801 (<0.001) | 0.714 (<0.001) | |
The level of correlation significance was 0.01.
Data: values in parentheses are p-values.
Correlation coefficient strength was characterized as follows: weak (0.1 ≤ R < 0.3), moderate (0.3 ≤ R < 0.7), relatively high (0.7 ≤ R < 0.9) and very high (0.9 ≤ R).
DISCUSSION
CNFS, caused by the narrowing of cervical nerve roots at the entrance of foraminal canals, is not uncommon in clinical settings. Significant foraminal narrowing may present as a sharp neck or arm pain, tingling sensation, paraesthesia or numbness with or without symptom spreading to the distal portion of the arms. Motor symptoms such as weakness may accompany sensory symptoms in aggravated cases.9 Clinical management of patients with CNFS is determined primarily based on neurologic symptoms with MRI studies, which may or may not indicate surgical intervention.9 There have been several attempts to quantify the degree of CNFS.2–5 Among these, the Kim and Park systems are simple, reproducible, semi-quantitative grading methods. MR evaluation of CNFS should be accurate and easy, as a labour-intensive, time-consuming analysis is not practical in clinical settings.4 Semi-quantitative methods help eliminate factors leading to variability caused by internal subjective standards.10
In comparison with previous reports, our study reveals similar CNFS distributions of each grade, according to each grading system (Table 1).4,5 In the Park system, >70% of patients were Grade 0 (68% in the previous study, Park et al4) and in the Kim and mKim systems, between 56% and 72% of patients were Grade 0 (53–72% in previous studies, Park et al5). We found PNMs in only 6–7% of Grade 0 cases using the Park system, 0–5% of Grade 0 cases using the Kim system and 5–8% of Grade 0 cases using the mKim system (Table 2). The results of the Kim and mKim systems are similar to the results of a previous study, which found PNMs in 4–6% and 6–7% of Grade 0 cases, respectively.5 The results of the Park system are somewhat different than those of the previous study, which found PNMs in 17–18% of Grade 0 cases.4,5 One possible explanation may be a potential overestimation of the results of the Park et al4 investigation. However, the results of our study comprise more concordant to the initial results of Park because the incidence of the number of Grade 0 cases according to the Kim and mKim system of our study are similar with that of the previous study by Park et al.5
The Park and mKim systems demonstrated nearly perfect interobserver agreement, while the Kim system demonstrated substantial agreement (Table 3). These results are similar to those of previous reports that showed nearly perfect interobserver agreement, irrespective of the grading method.4,5
The three systems revealed relatively high correlation values, except for the moderate correlation value of the Kim system by Reader 2 (Table 4). For the Park system, these results were superior to those of previous studies (R = 0.737 and 0.741 vs 0.63 and 0.65).4,5 We were unable to find significant differences among the three grading systems with respect to clinical correlation of stenosis grade, except in the case of the Kim system (the Kim system by Reader 2 was inferior to other systems). In previous studies, even if we could evaluate the accuracy of the three grading systems (Park system, Kim system and mKim system, 0.63–0.65, 0.53–0.55 and 0.61–0.67), each results were based on different patient populations.4,5 Our results are based on the same patient population and therefore may have a more meaningful clinical applicability. Park et al4 reported that in the Park system, Grades 2 and 3 are most likely associated with PNMs and can predict clinical symptoms, whereas Grade 0 is not strictly free of clinical manifestation. In our study, we found that only 6–7% of Grade 0 cases demonstrated PNMs; therefore, we can conclude that Grade 0 is strongly indicative of a negative neurologic manifestation (NNM), as in the case of the Kim or mKim systems. Park et al5 reported that they could find 4–7% of PNMs from Grade 0 using the Kim or mKim system and concluded that Grade 0 strongly indicates NNMs in their framework.
The Park system is based on oblique scans oriented perpendicular to the entrance of the neural foramen. This simplifies the visualization of the neural foramina, with accurate stenosis evaluation.6 In addition, the oblique scans provide more information regarding stenosis pathology, including facet joint overgrowth, osteophyte formation from the uncinate process and intervertebral disc herniation.11 In two cases of our study, the Park system yielded a grade of 3, whereas the Kim and mKim system yielded a grade of 1. These cases had obvious neuropathies that demonstrated positive electromyogram results at the corresponding vertebral levels. However, in spite of these advantages, the Park system requires additional oblique scans, which add 3–5 min to the MRI protocol.2,11 The three-dimensional (3D) isotropic fast spin-echo T2 weighted MR such as volume isotropic turbo spin-echo acquisition can be used to make multiplanar reformatted images and can reduce the total acquisition time without the need to acquire the same sequence in different planes.12 However, the mean scan time for the 3D source image is about 3 min 20 s, not including the time for reformation. There may be a time benefit, if we use the 3D MR image to replace axial and sagittal views in addition to oblique views.12
Whereas grades of 2 or 3 (of all three grading system) were found in >87% of PNMs, the prevalence of PNM for Grade 1 cases ranged from 19% to 70%. At least 30% of patients with NNM were considered Grade 1 and therefore, these values do not sufficiently support the probability of NNM or PNM. Although the Park system is composed of four grades and the Kim system is composed of three grades, Grade 0 of all three systems implies NNM. In addition, Grades 2 and 3 can predict PNM, irrespective of the grading type.
Our study has several limitations. First, C-spine MR in our institution was acquired in a single, supine position. Postural changes, including neck extension, during scanning could induce narrowing of the neural foramen and can be associated with neurologic symptoms.13,14 However, it is clinically difficult to perform several MR scans on patients in different positions, owing to a lack of compliance and cost. All patients were placed in a neutral neck position to minimize differences in foraminal dimension. Second, this study was not designed to quantitatively evaluate clinical manifestations: the neurologic manifestations were classified as either positive or negative. We assigned the same weight to clinical sign and neurologic examination, which may be regarded as oversimplification. Third, we used 1.5-T MRI in image acquisition. We do not know what the results might have been with 3.0-T MR. However, 1.5-T MR is quite common, particularly in ambulant services where most of the spine examinations are performed. Finally, we excluded patients with severe stenosis, as well as multilevel and central stenoses. We excluded 47 patients with multilevel or central stenosis, with the thought that this population can be biased. Significant CNFS that may reveal definite neurologic signs is sometimes featured to as multilevel stenosis, as multilevel stenoses are commonly caused by degenerative changes of the cervical spine.
In conclusion, Park and mKim systems should be used for CNFS MRI reading because these systems show very high clinical correlation and a strong negative-predictive value. Grades 2 and 3 of the Park system and Grade 2 in the Kim and mKim systems exhibited high PNM incidence and therefore similar clinical significance. Patients with a grade of 0 (using each system) consistently exhibit NNM. As the mKim system does not need additional oblique MR sequences, the mKim system appears faster to interpret. However, the mKim system needs a delicate measurement of the distance and calculation of the ratio, whereas the Park system does not need complicated measurements but depends on morphologic diagnoses only. Therefore, each system has advantages and disadvantages.
To summarize, oblique sequences are very illustrative, but by using them, the Park system needs more time to perform more images. 3D isotropic fast spin-echo T2 weighted MR may overcome such time limitations. In addition, we acknowledge that our research is based on a single-centre patient collective with 1.5-T MR and does not allow extrapolation to patients with the common type of multisegmental stenoses.
Contributor Information
Kyu H Lee, Email: mdcappuccino@daum.net.
Hee J Park, Email: parkhiji@gmail.com.
So Y Lee, Email: capella27@gmail.com.
Eun C Chung, Email: ciochung@naver.com.
Myung H Rho, Email: rmh96@dreamwiz.com.
Hyunchul Shin, Email: nsdrshin@gmail.com.
Young J Kwon, Email: neuriac@skku.edu.
REFERENCES
- 1.Yousem DM, Atlas SW, Goldberg HI, Grossman RI. Degenerative narrowing of the cervical spine neural foramina: evaluation with high-resolution 3DFT gradient-echo MR imaging. AJNR Am J Neuroradiol 1991; 12: 229–36. [PMC free article] [PubMed] [Google Scholar]
- 2.Park HJ, Kim SS, Lee SY, Park NH, Chung EC, Rho MH, et al. A practical MRI grading system for cervical foraminal stenosis based on oblique sagittal images. Br J Radiol 2013; 86: 20120515. doi: 10.1259/bjr.20120515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim SJ, Lee JW, Chai JW. New MRI grading system for cervical neural foraminal stenosis based on T2-axial images. In: Proceedings of the 67th Korean Congress of Radiology and Annual Delegate Meeting of the Korean Society of Radiology; 27–29 October 2011; Seoul, Republic of Korea. p. 282.
- 4.Park HJ, Kim SS, Han CH, Lee SY, Chung EC, Kim MS, et al. The clinical correlation of a new practical MRI method for grading cervical neural foraminal stenosis based on oblique sagittal images. AJR Am J Roentgenol 2014; 203: 412–7. doi: 10.2214/AJR.13.11647 [DOI] [PubMed] [Google Scholar]
- 5.Park HJ, Kim JH, Lee JW, Lee SY, Chung EC, Rho MH, et al. Clinical correlation of a new and practical magnetic resonance grading system for cervical foraminal stenosis assessment. Acta Radiol 2015; 56: 727–32. doi: 10.1177/0284185114537929 [DOI] [PubMed] [Google Scholar]
- 6.Humphreys SC, An HS, Eck JC, Coppes M, Lim TH, Estkowski L. Oblique MRI as a useful adjunct in evaluation of cervical foraminal impingement. J Spinal Disord 1998; 11: 295–9. doi: 10.1097/00002517-199808000-00005 [DOI] [PubMed] [Google Scholar]
- 7.Sim J, Wright CC. The kappa statistics in reliability studies: use, interpretation, and sample size requirements. Phys Ther 2005; 85: 257–68. [PubMed] [Google Scholar]
- 8.Cohen J. Statistical power analysis for the behavioral sciences. 2nd edn. Mahwah, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 9.Ruggieri PM. Cervical radiculopathy. Neuroimaging Clin N Am 1995; 5: 349–66. [PubMed] [Google Scholar]
- 10.Park HJ, Kim SS, Lee SY, Park NH, Rho MH, Hong HP, et al. Clinical correlation of a new MR imaging method for assessing lumbar foraminal stenosis. AJNR Am J Neuroradiol 2012; 33: 818–22. doi: 10.3174/ajnr.A2870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shim JH, Park CK, Lee JH, Choi JW, Lee DC, Kim DH, et al. A comparison of angled sagittal MRI and conventional MRI in the diagnosis of herniated disc and stenosis in the cervical foramen. Eur Spine J 2009; 18: 1109–16. doi: 10.1007/s00586-009-0932-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park HJ, Lee SY, Park NH, Ahn JH, Chung EC, Kim SJ, et al. Three-dimensional isotropic T2-weighted fast spin-echo (VISTA) knee MRI at 3.0 T in the evaluation of the anterior cruciate ligament injury with additional views: comparison with two-dimensional fast spin-echo T2-weighted sequences. Acta Radiol Jan 2015. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 13.Stafira JS, Sonnad JR, Yuh WT, Huard DR, Acker RE, Nguyen DL, et al. Qualitative assessment of cervical spinal stenosis: observer variability on CT and MR images. AJNR Am J Neuroradiol 2003; 24: 766–9. [PMC free article] [PubMed] [Google Scholar]
- 14.Bartlett RJ, Hill CA, Rigby AS, Chandrasekaran S, Narayanamurthy H. MRI of the cervical spine with neck extension: is it useful? Br J Radiol 2012; 85: 1044–51. doi: 10.1259/bjr/94315429 [DOI] [PMC free article] [PubMed] [Google Scholar]