Abstract
Trends in obesity have continued to increase in the developed world over the past few decades, along with related conditions such as metabolic syndrome, which is strongly associated with this epidemic. Novel and innovative methods to assess relevant obesity-related biomarkers are needed to determine the clinical significance, allow for surveillance and intervene if appropriate. Aggregations of specific types of fat, specifically hepatic and visceral adiposity, are now known to be correlated with these conditions, and there are a variety of imaging techniques to identify and quantify their distributions and provide diagnostic information. These methods are particularly salient for metabolic syndrome, which is related to both hepatic and visceral adiposity but currently not defined by it. Simpler non-specific fat measurements, such as body weight, abdominal circumference and body mass index are more frequently used but lack the ability to characterize fat location. In addition, non-alcoholic fatty liver disease (NAFLD) is a related condition that carries relevance not only for obesity-related diseases but also for the progression of the liver-specific disease, including non-alcoholic steatohepatitis and cirrhosis, albeit at a much lower frequency. Recent CT and MRI techniques have emerged to potentially optimize diagnosing metabolic syndrome and NAFLD through non-invasive quantification of visceral fat and hepatic steatosis with high accuracy. These imaging modalities should aid us in further understanding the relationship of hepatic and visceral fat to the obesity-related conditions such as metabolic syndrome, NAFLD and cardiovascular disease.
INTRODUCTION
In the past few decades, obesity has become one of the foremost health concerns for people in the developed world.1–6 Since the 1950s, there has been a three-fold increase in the incidence of obesity in the USA to the point where 66% of adults and 16% of children are now overweight or obese.7–10 These numbers are expected to continue to rise even more in the near future. Owing to this upward trend in obesity, researchers have investigated a variety of ways to measure adiposity in the population. Beyond body weight itself, two of the most common methods to measure obesity are body mass index (BMI) and abdominal circumference, and these measures are commonly used to determine obesity prevalence and incidence.11–13 However, neither of these simple non-imaging biomarkers allows for the quantification and localization of fat into visceral and subcutaneous compartments, which are the metabolic bases for the “apple” vs “pear” body habitus and for the progression of various metabolic conditions. Furthermore, BMI and other basic measures also fail to account for the accumulation of fat within the liver. As we will discuss, non-alcoholic fatty liver disease (NAFLD) is another important related condition that is growing in prevalence and intersects with other obesity-related conditions.
Certain cross-sectional techniques utilizing CT and MRI have shown to be highly effective for both the localization and quantification of fat, which are important considerations in terms of obesity-related conditions and diseases.14–19 Both CT and MRI can easily distinguish and quantify visceral fat from subcutaneous fat and can also accurately quantify liver fat (steatosis). This is important because research has shown that metabolic syndrome, hepatic steatosis, NAFLD, visceral fat and cardiovascular disease are all interrelated and share the common thread of fat aggregations contributing to disease progression (Figures 1 and 2).20–24 More widespread adoption of these imaging techniques into standard practice may be beneficial when investigating the spread of obesity-related and liver-related diseases in the general population, owing to their efficacy in both localization and quantification of adiposity.
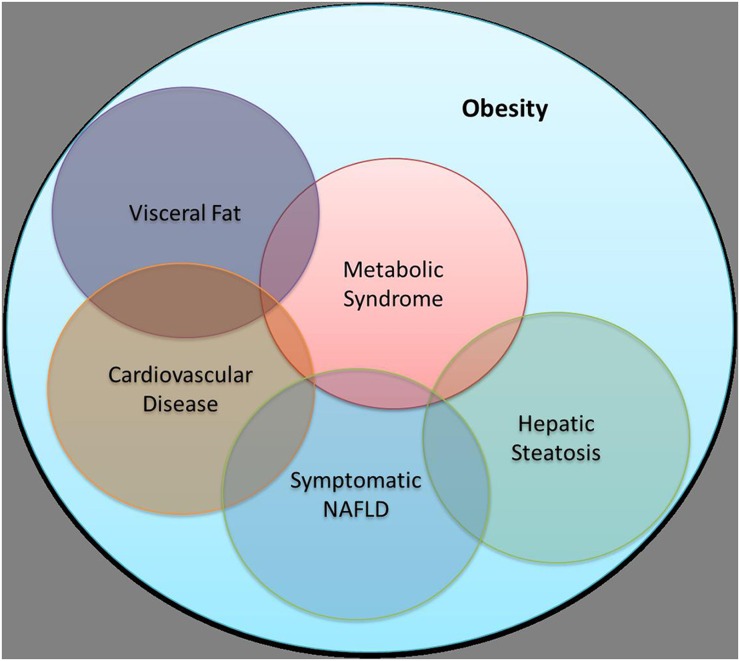
Figure 1.
A simplified overview of the complex association between obesity with visceral fat, cardiovascular disease, hepatic steatosis, metabolic syndrome and non-alcoholic fatty liver disease (NAFLD).
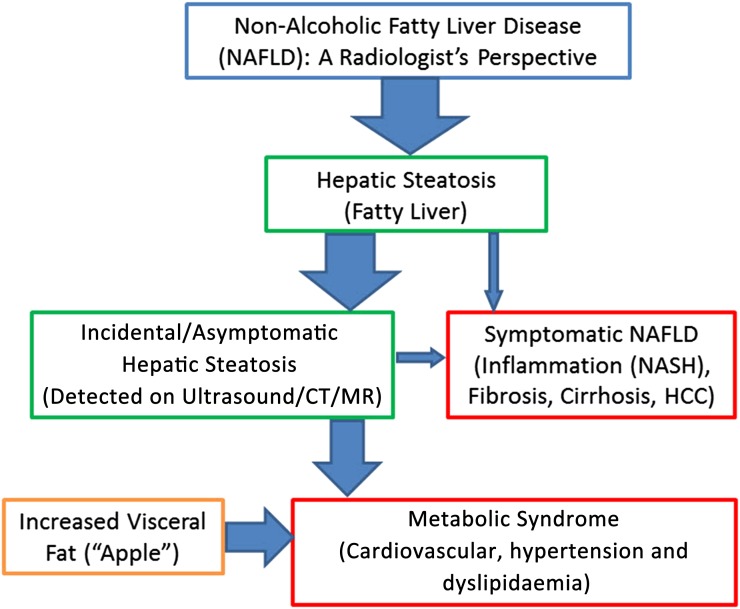
Figure 2.
A simplified flowchart from the radiologist's perspective that demonstrates the complex interplay between the ubiquitous incidental steatosis and increased visceral fat with the important conditions of metabolic syndrome and symptomatic non-alcoholic fatty liver disease (NAFLD). In terms of prevalence, metabolic syndrome is much more common than NAFLD-related non-alcoholic steatohepatitis (NASH), hepatocellular carcinoma (HCC) and cirrhosis.
METABOLIC SYNDROME
Metabolic syndrome is perhaps the most enigmatic leading public health issue affecting the developed world. Although it is relatively easy to define by the current diagnostic criteria, it remains unclear whether the defining elements truly capture this phenomenon. In essence, the metabolic syndrome clusters the cardiovascular risk factors of abdominal obesity with Type 2 diabetes mellitus, hypertension and dyslipidemia. Research has clearly shown that the prevalence of metabolic syndrome is increasing worldwide, as obesity rates continue to grow.25–28 The actual diagnosis of metabolic syndrome is somewhat arbitrary and has changed over time; however, measures of visceral and hepatic fat are not part of the current definition. Despite this, visceral adiposity and hepatic steatosis (and NAFLD in general) have been shown to be key factors in metabolic syndrome.23,29,30 The fact that steatosis and visceral fat are not currently part of the definition of metabolic syndrome is perhaps not all that surprising, given that these measures cannot be reliably obtained from a physical examination or blood draw. CT and MR can rapidly and non-invasively measure both hepatic steatosis and visceral fat, which can provide relevant objective information to providers and patients, both retrospectively and prospectively.
HEPATIC STEATOSIS AND NON-ALCOHOLIC FATTY LIVER DISEASE
Similar to metabolic syndrome, the precise prevalence of both hepatic steatosis and NAFLD is unknown across the globe, but some estimates suggest that there are potentially 100 million cases or more of NAFLD in the USA alone.31–34 However, this is an entity that also suffers from a lack of formalized or uniform definition. There are multiple causes for hepatic steatosis, which simply refers to excess lipid accumulation in the liver, that range from alcohol consumption and medication use to obesity or hyperlipidaemia.35–37 NAFLD is the most common cause of hepatic steatosis by far and is known to be associated with the characteristics of metabolic syndrome and cardiovascular disease, but it has yet to be determined whether it is a cause or an effect.38,39 In terms of liver-specific disease, the natural progression of NAFLD is also not well understood, as many consider simple steatosis to be the required precursor to the more clinically important conditions of non-alcoholic steatohepatitis (NASH) and cirrhosis. However, the actual risk of progression from the very common asymptomatic hepatic steatosis to the symptomatic forms of NAFLD (i.e. symptomatic steatosis, NASH and cirrhosis) is likely to be extremely low.40 Regardless of the actual risk for NASH and cirrhosis, the known association between NAFLD, metabolic syndrome and cardiovascular disease makes the condition of hepatic steatosis relevant.39,41 Although cross-sectional imaging (e.g. with ultrasound, CT or MR) can establish the diagnosis of fatty liver without the need for biopsy, what is still missing is a non-invasive method to differentiate between the ubiquitous benign hepatic steatosis and the much less common but more important condition of NASH. In terms of the full NAFLD spectrum, many unanswered questions remain regarding this rare transition to NASH and cirrhosis.
Methods for the assessment and quantification of hepatic steatosis
In order to investigate the presence of steatosis or NAFLD, there are several biomarkers that can be examined, although in the end, either cross-sectional imaging or liver biopsy is required for a confident diagnosis. There are several imaging techniques that can accurately diagnose fatty liver, but there is currently no reliable means for detecting NASH or early cirrhosis,42–44 although elastography can at least suggest the diagnosis owing to increased liver stiffness.19,45 There are non-imaging methods to investigate hepatic steatosis, ranging from definitive but invasive biopsy to non-invasive but highly non-specific laboratory tests.
Non-imaging methods
Liver fat (steatosis) can be assessed in a variety of different ways. Historically, liver biopsy has served as the “gold standard”, which invasively samples a tiny portion of the liver to get a highly specific but relatively crude quantitative visual estimate of the percent of lipid droplets occupying the histology slide. Liver biopsies are expensive, and their sampling error can be quite high.46–49 Ideally, one would like to limit or avoid liver biopsies for these reasons, but it is currently the only reliable way to show superimposed liver inflammation in patients with NASH. In addition to liver biopsies, another non-imaging method to assess liver adiposity is through laboratory blood tests, such as the serum levels of aspartate transaminase and alanine transaminase. However, the elevation of liver enzymes is highly non-specific and could be related to a wide variety of other causes. In patients with known NAFLD, the aspartate transaminase/alanine transaminase ratio is sometimes helpful for suggesting the degree of superimposed fibrosis, but this is also very non-specific and cannot actually quantify fat, inflammation or fibrosis.50 More complex laboratory panels, such as the enhanced liver fibrosis panel, have shown utility in identifying advanced fibrosis and cirrhosis in the setting of NAFLD, but still do not quantify fat.51
IMAGING METHODS
Ultrasound is a simple non-invasive, inexpensive and widely available method for assessing the presence or absence of hepatic steatosis. Although some advances have been made with transient and shear-wave elastography, ultrasound assessment remains largely subjective and therefore is unreliable for quantification, which requires more advanced CT or MRI.52
Non-contrast CT is a simple and objective method capable of accurately quantifying liver fat. A CT attenuation threshold of 40 or 45 HU has been widely used for the diagnosis of moderate or severe steatosis,18,53 which is defined at histopathology by 30% or more lipid on the specimen slide. We have also found these non-contrast CT HU cut-off values to be highly specific for moderate–severe steatosis at histopathology.40 More recently, our work has shown that there is a strong linear correlation between standard single-energy non-contrast CT and MR proton density fat fractions (PDFF), which is discussed later in this section. Contrast-enhanced CT is more complicated than non-contrast CT in terms of liver fat quantification, owing to superimposed parenchymal liver enhancement.53,54 Nonetheless, contrast-enhanced CT during the portal venous has shown to be useful in diagnosing the presence of at least moderate or severe steatosis. In particular, Kim et al55 showed that an attenuation difference between the liver and spleen of 19 HU or more (spleen > liver) was optimal for diagnosing moderate–severe steatosis. In addition, dual-energy CT (DECT) may be more useful in the setting of i.v. contrast relative to unenhanced CT, where there is little or no added benefit.56
Until recently, the non-invasive imaging reference standard for hepatic fat quantification has been MR spectroscopy (MRS).57–60 However, this technique is somewhat time consuming and, like biopsy, is prone to sampling errors. Standard MRI, in particular chemical-shift imaging with in and opposed phases, has been useful for diagnosing hepatic steatosis in general. However, this MRI technique lacks objective quantification. More recently, more advanced Dixon-based and complex MR-PDFF sequences have been developed that allow for a more accurate quantification of hepatic fat fraction.61,62 Although several vendors now offer the Food and Drug Administration-approved versions of complex PDFF fat quantification, we utilize the IDEAL (iterative decomposition of water with echo asymmetry and least-squares estimation) sequence, which provides global assessment of the liver that is equivalent to MRS in terms of fat quantification. IDEAL uses complex source images which catalogue the chemical shift in the liver based on a water–fat separation method. It provides confounder-corrected PDFF maps and computes a fat fraction for any selected region of the liver. This method has proven to be very robust, with advantages over both spectroscopy and liver biopsy, as it provides rapid, accurate, non-invasive assessment of the entire liver.61,63 In general, we feel that the current MR-PDFF sequences currently offer the best overall technique to assess and quantify hepatic steatosis, owing to its relative ease of use and high accuracy.
COMPARING ULTRASOUND, CT AND MR WITH MR SPECTROSCOPY
In order to assess the accuracy of the various cross-sectional imaging techniques used to diagnose and quantify fat, we recently conducted a prospective trial to compare the correlation of each against the reference standard of MRS.64 In this trial, 50 patients underwent non-contrast single-energy CT (SECT), non-contrast DECT, MR-PDFF (IDEAL) and ultrasound shear-wave elastography, all within a 2-h window. MRS was used as the reference standard owing to its ability to accurately measure fat fraction.58,59,65 Of all the imaging techniques, MR-PDFF and SECT demonstrated the best correlation with MRS. Ultrasound shear-wave elastography showed very poor correlation with MRS, and DECT with material decomposition did not improve the accuracy over SECT. These unpublished results suggest that both MR-PDFF and non-contrast SECT can serve as accurate and non-invasive biomarkers for identifying fatty liver disease through quantifying steatosis. Importantly, it was also shown that SECT and MR-PDFF were linearly correlated, which means that routine non-contrast CT may be used to detect and quantify hepatic fat fraction, and MR-PDFF could then be used for the clinical follow-up of NAFLD (Figure 3).
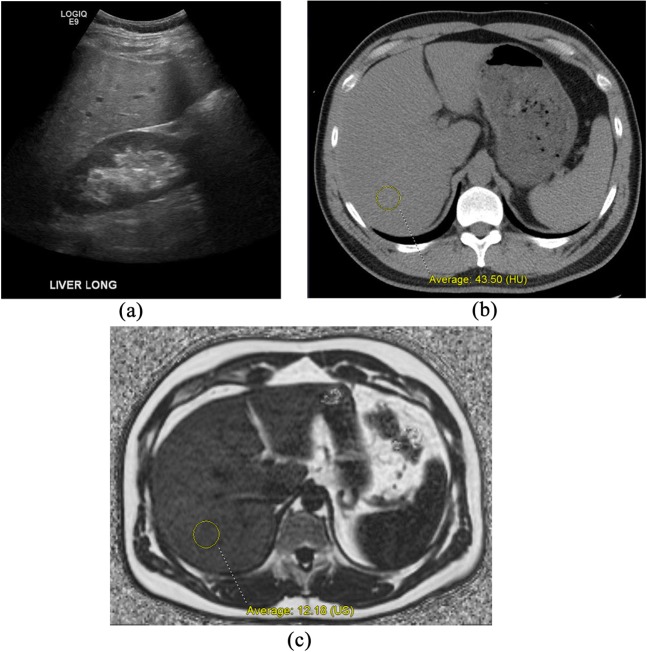
Figure 3.
Comparison of cross-sectional imaging techniques for the assessment of hepatic steatosis in a 44-year-old male. Right upper quadrant ultrasound image (a) shows moderate diffuse increase hepatic parenchymal echogenicity, compatible with steatosis. Objective quantification of fat fraction is not possible with ultrasound. Unenhanced CT image (b) shows diffusely decreased hepatic attenuation, which measures 43.50 HU, compatible with moderate steatosis. Based on our unpublished research, the linear correlation between non-contrast CT Hounsfield unit and MR proton density fat fractions (PDFF) (iterative decomposition of water with echo asymmetry and least-squares estimation) would predict a 10–15% fat fraction, which is confirmed at MR-PDFF (c), where the fat fraction measured 12.8% US, ultrasound.
NATURAL HISTORY OF INCIDENTAL HEPATIC STEATOSIS
Although hepatic steatosis may be of clinical significance for a variety of reasons, many times, it is discovered incidentally on cross-sectional imaging examinations performed for another indication. As such, the finding of unsuspected, asymptomatic incidental hepatic steatosis is of uncertain significance. However, given the ubiquity of this benign condition relative to symptomatic NAFLD, one might suspect that in an individual patient, this finding may be of dubious clinical significance. Compared with symptomatic NAFLD, incidental steatosis has a very high prevalence.18,40,66 In a study published in 2014, Pickhardt et al67 attempted to understand the long-term natural history of incidental steatosis in a large cohort of adults who had undergone non-contrast CT. After these patients were followed for up to a decade (mean follow-up interval was greater than 7 years), not a single patient with moderate or severe steatosis progressed to any symptomatic NAFLD state. It was concluded that having the asymptomatic form of NAFLD (i.e. incidental steatosis) may have a negligible risk of progressing to NASH and fibrosis in an individual patient, at least in the 5–10-year time horizon. This study also investigated the incidence of cardiovascular events. Although steatosis was also found to be a biomarker for future cardiovascular events, it was determined not to be an independent risk factor when diabetes, hypertension and other known conditions were factored in. The study concluded that incidental hepatic steatosis is a very common and benign condition and, in isolation, may not need to be reported or acted upon. Asymptomatic steatosis is clearly related to metabolic syndrome and other obesity-related diseases, but its relationship with NASH is unclear. As such, while incidental steatosis can be easily detected with cross-sectional imaging, the more clinically relevant biomarkers that might predict progression to NASH and hepatic fibrosis remain an elusive diagnostic challenge.
IMAGING AND RELEVANCE TO SYMPTOMATIC NAFLD
NASH falls under the broad umbrella of NAFLD, representing a small but important subset with an inflammatory state of likely progressive disease. As mentioned, the risk factors or triggers that might transform simple hepatic steatosis to NASH are poorly understood. NAFLD can be divided into two primary branches: asymptomatic NAFLD (discussed above) and symptomatic NAFLD, with the latter including NASH, fibrosis and cirrhosis (Figure 2). Asymptomatic steatosis can be easily detected through the imaging techniques discussed previously. Symptomatic NAFLD, however, is significantly more difficult to investigate, since there are currently no imaging tools that can effectively detect the presence of early inflammation and distinguish this from early fibrosis. CT or MR findings of marked, heterogeneous steatosis with hepatomegaly may suggest the presence of NASH in a patient who is symptomatic, but liver biopsy is generally necessary to confirm this suspected clinical and imaging diagnosis. Once NAFLD has progressed to frank cirrhosis, the morphologic changes in the liver and associated findings of portal hypertension are readily detectable at cross-sectional imaging, particularly CT and MR.
One potential method for the non-invasive detection of pre-cirrhotic symptomatic NAFLD (i.e. NASH and early fibrosis) is elastography, whether by ultrasound or MR techniques.45 Elastography can demonstrate increased stiffness of the liver parenchyma resulting from inflammation and/or fibrosis. Although ultrasound techniques for elastography (e.g. transient elastography) are widely available and easy to employ, both the overall accuracy for fibrosis stage and the technical success rate are lower than that of MR elastography, especially in patients who are obese.19,68 Nonetheless, even MR elastography cannot reliably distinguish between inflammation related to NASH and early fibrosis, both of which lead to increased liver stiffness.19 Further research is needed to identify the associated biomarkers that may help detect and distinguish these two forms of symptomatic NAFLD and more fully understand the potential link that exists between asymptomatic and symptomatic NAFLD. We are currently investigating changes in liver morphology at CT that may predict earlier stages of fibrosis and may be complementary to elastography. One such approach is comparing the volumes of Couinaud Segments I–III, which tend to show compensatory hypertrophy, with Segments IV–VIII, which tend to shrink with advancing fibrosis. By comparing the volume ratio of these segments (i.e. I–III/IV–VIII), which we term the “liver segmental volume ratio” or LSVR, we found that one can distinguish between normal and cirrhosis better than established linear measures.69 A more recent unpublished work shows that this LSVR can also distinguish between lesser degrees of hepatic fibrosis, similar to elastography. Additional research focuses on the nodularity of the liver surface. By applying a validated objective tool for measuring the liver surface nodularity score, one can also distinguish between some stages of hepatic fibrosis. A third area of active investigation involves texture analysis of the hepatic parenchyma at CT. Unlike elastography, which must be prospectively acquired, all of these novel investigations have the advantage of being applied both prospectively and retrospectively. Furthermore, the issue of stiffness overlap between NASH and early fibrosis with elastography is not a problem with these CT-based techniques.
VISCERAL ADIPOSITY
Aside from liver fat, visceral adiposity is another example of fat aggregation that cannot be measured accurately with non-imaging methods, yet is highly correlated with other obesity-related diseases. In particular, visceral fat directly contributes to the “apple” vs “pear” body habitus, where high visceral fat results in an apple-shaped habitus, compared with the pear-shaped habitus formed by the higher concentration of subcutaneous fat (Figure 4). One of the main techniques to measure obesity is BMI, but this widely used method fails to differentiate visceral vs subcutaneous fat. Patients can have the same overall BMI but have markedly different relative amounts of visceral vs subcutaneous fat. In general, a higher percentage of visceral fat typically correlates with a higher risk of cardiovascular event, metabolic syndrome and NASH,70–72 although some have found conflicting results.73 As with hepatic adiposity, non-contrast CT and MR can effectively quantify visceral fat and distinguish it from subcutaneous and hepatic fat.74 Although the relationship has not been completely elucidated between visceral fat, hepatic fat, cardiovascular disease, liver disease and metabolic syndrome, it seems clear that there is some level of association between all of them and that there needs to be consideration for routinely obtaining and incorporating this measure to help reveal the true clinical picture.
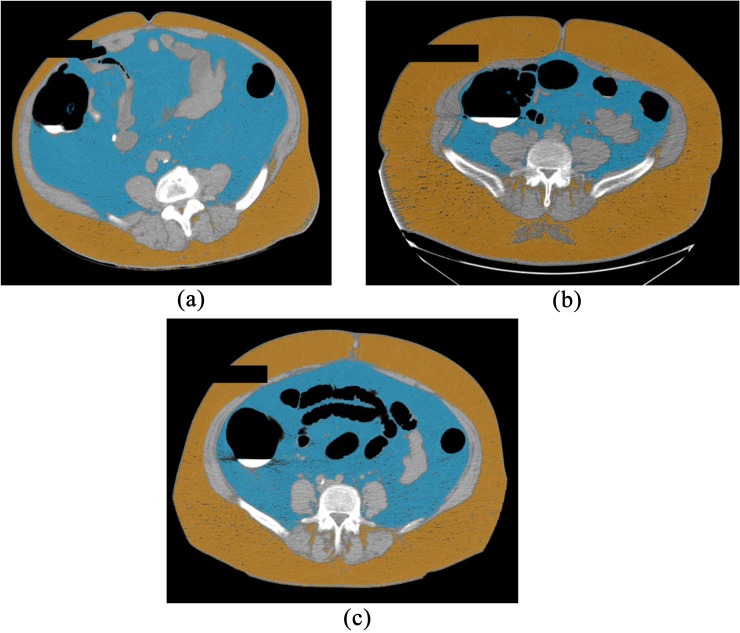
Figure 4.
Three examples of visceral vs subcutaneous fat localization and quantification at CT using a simple semi-automated software tool. The first patient (a) demonstrates high visceral fat content (blue) relative to subcutaneous fat (orange), compatible with an “apple-shaped” body habitus. The second patient (b) demonstrates the “pear-shaped” body habitus, with relative increase in subcutaneous fat over visceral fat. The third patient (c) demonstrates a more balanced state of obesity, with large amounts of both visceral and subcutaneous fat. These same measures can be obtained with MR (not shown). For colour image see online.
CORRELATING METABOLIC SYNDROME WITH COMBINED VISCERAL AND HEPATIC FAT MEASUREMENTS
Since both visceral and hepatic adiposity were found to be strongly associated with the components of metabolic syndrome, but are not yet included in the formal definition, Pickhardt and colleagues74 set out to determine this correlation in adults who were asymptomatic using unenhanced CT. The study examined cross-sectional CT imaging of visceral, hepatic and subcutaneous fat distributions in 474 adults who were asymptomatic, where the prevalence of metabolic syndrome was 35% (according to the standard definition). Interestingly, there were noticeable differences between males and females. Visceral fat accumulation was shown to be a strong predictor of metabolic syndrome for both females and males, but was significantly better for females. Meanwhile, subcutaneous fat accumulation was a poor predictor in females, but it was the best predictor for males. Hepatic steatosis was highly specific for metabolic syndrome for both genders, but was an insensitive measure. Importantly, visceral fat was elevated in over half of the patients who did not qualify for metabolic syndrome but had a documented cardiovascular event and was also elevated in over 30% of individuals who were non-obese according to BMI (<30 kg m−2). This suggests that visceral fat may be an important separate consideration among individuals at risk that are not included in the standard definitions of metabolic syndrome and BMI.
Case study: metabolic syndrome and NAFLD from the radiologist's perspective
A 45-year-old female presented with right flank pain in 2007 and underwent non-contrast CT evaluation (Figure 5a). Liver attenuation at this time measured 58 HU, a normal level without significant steatosis.18,75 The patient did not appear obese at this point and did not demonstrate excessive visceral fat. Furthermore, she did not carry a diagnosis of hypertension, hyperlipidaemia, liver disease, diabetes or metabolic syndrome.
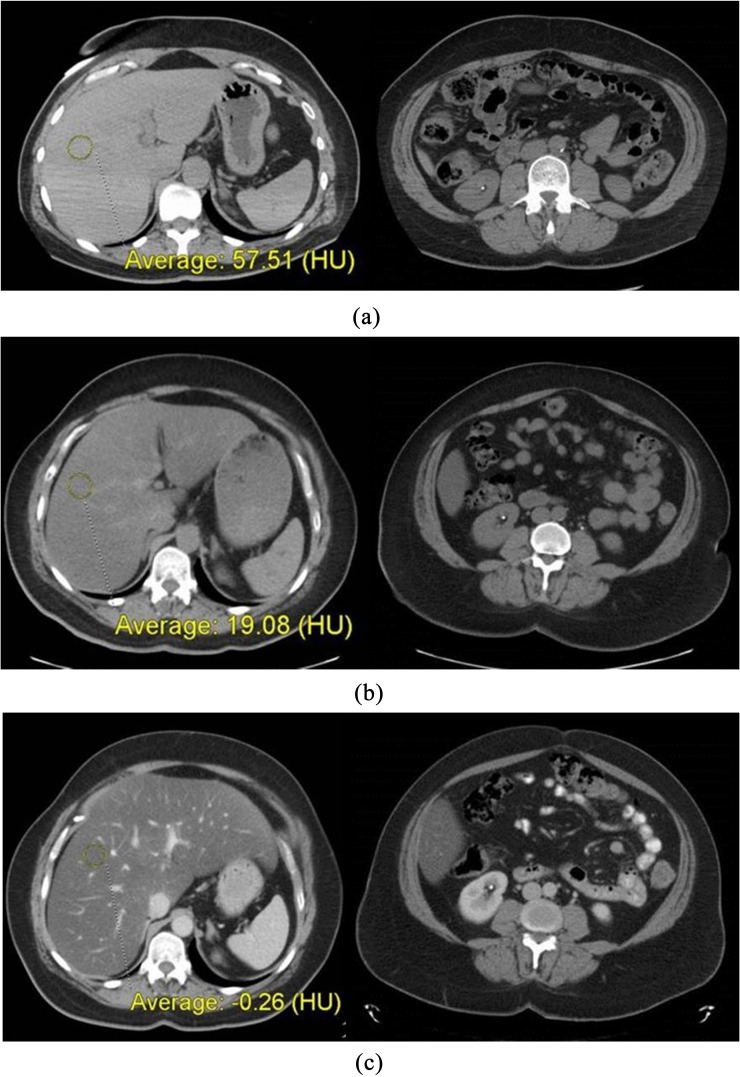
Figure 5.
Case study: (a) shows two CT images from 2007, when liver attenuation was within normal limits. Note the non-obstructing calculus in the right lower pole kidney, which serves to mark the level for comparison of visceral fat with subsequent CT scans. (b) shows images from follow-up non-contrast CT in 2012, which demonstrate interval development of both advanced hepatic steatosis and increased visceral (and subcutaneous) fat. (c) shows images from contrast-enhanced CT in 2014, which demonstrate marked progression of hepatic steatosis, as well as further increase in visceral fat. The associated development of hepatomegaly and right upper quadrant pain suggest symptomatic non-alcoholic fatty liver disease (possibly non-alcoholic steatohepatitis) in the absence of other identifiable causes. Clinical work-up at this time confirmed metabolic syndrome.
In 2009, she was diagnosed with hypertension. In 2012, she again presented with right flank pain and underwent repeat non-contrast CT for suspected urolithiasis (Figure 5b). Her liver attenuation had dropped to 19 HU, compatible with advanced steatosis (moderate or severe) and likely representing NAFLD. In addition, both her visceral and subcutaneous fat content had increased compared with 2007. Still, there was no mention of metabolic work-up or consideration of liver disease at this point.
In 2014, she presented with right upper quadrant pain (different from her previous episodes of flank pain) and this time underwent contrast-enhanced abdominal CT (Figure 5c). At this point, there had been a clear incremental increase in abdominal girth, including both visceral and subcutaneous compartments. Her liver attenuation was now <0 HU, signifying severe steatosis, especially given that this was a post-contrast portal venous-phase scan. In addition to the markedly decreased hepatic attenuation, the liver now also appeared diffusely enlarged. Given these CT findings in the presence of right upper quadrant pain, the radiologist raised concern for symptomatic NAFLD, particularly the development of NASH. In addition to suggesting the possibility of NASH, for which consultation with a gastroenterologist was recommended, the radiologist also recommended a nutritional consultation and work-up for metabolic syndrome. At this point, the patient had still not been diagnosed clinically with either metabolic syndrome or NAFLD. Based on the CT report, further work-up revealed a BMI of 46.8 kg m−2 (morbidly obese), blood glucose level of 219 mg dl−1 (normal, 70–99 mg dl−1), glycated haemoglobin (HbA1c) of 8.7% (normal, 4.6–6.0%), triglyceride level of 322 mg dl−1 (normal, 3–149 mg dl−1) and high-density lipoprotein (HDL) level of 35 mg dl−1 (normal, 40–125). She received a new diagnosis of Type 2 diabetes mellitus and was referred for a hepatology consultation for her progressive liver disease. She was formally diagnosed with metabolic syndrome and began a regimen of dietary modification and exercise to help control her symptoms. Liver biopsy was recommended, but the patient had refused to this point. Nonetheless, the GI service considers her to have NASH.
This case appears to represent the rare occurrence where asymptomatic NAFLD (simple steatosis) has progressed to symptomatic NAFLD (NASH). This case also demonstrates how hepatic steatosis and visceral fat are key biomarkers for predicting certain obesity-related outcomes. Although one might assume that the conditions of metabolic syndrome and symptomatic NAFLD would be identified through clinical evaluation, the radiologist should nonetheless be prepared to be the first to suggest their presence when interpreting CT and MR examinations. This case is in stark contrast to the daily observation of incidental asymptomatic steatosis at CT or MR, which is usually of doubtful clinical relevance in the population at large.
CONCLUSION
In summary, current non-contrast CT and MRI techniques provide an excellent, objective non-invasive means for quantifying visceral and hepatic fat. The various studies and reviews which have been published over the past few years show progressive adoption of some of these techniques into practice for localizing and quantifying these types of adiposity as important biomarkers. These modalities have shown to be useful in both research capacities and some clinical applications, although their precise role is unclear when it comes to investigating NASH and symptomatic NAFLD. While it is apparent that the quantity and distribution of body fat matters in metabolic syndrome and fatty liver disease, the relevance of incidental steatosis to symptomatic forms of NAFLD remains unclear owing to the ubiquitous nature of the former compared with the relative infrequency of the latter. Furthermore, although we are well equipped to quantify types of fat using cross-sectional imaging, the current modalities are not yet capable of reliably detecting and distinguishing inflammation from early fibrosis characteristic of the symptomatic NAFLD pathway (NASH and cirrhosis). With continued investigation of emerging imaging techniques, we may better understand the role that certain imaging biomarkers play in the progression of NASH and NAFLD. In addition, further study may help elucidate the complex interaction between enigmatic diseases like NAFLD and metabolic syndrome.
Disclosures
Dr Pickhardt is co-founder of Virtuo CTC and shareholder in Cellectar Biosciences
Contributor Information
Peter M Graffy, Email: PGraffy@uwhealth.org.
Perry J Pickhardt, Email: ppickhardt2@uwhealth.org.
REFERENCES
- 1.Haslam DW, James WP. Obesity. Lancet 2005; 366: 1197–209. doi: 10.1016/S0140-6736(05)67483-1 [DOI] [PubMed] [Google Scholar]
- 2.James PT, Leach R, Kalamara E, Shayeghi M. The worldwide obesity epidemic. Obes Res 2001; 9 (Suppl. 4): 228S–33S. doi: 10.1038/oby.2001.123 [DOI] [PubMed] [Google Scholar]
- 3.Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA 2001; 286: 1195–200. doi: 10.1001/jama.286.10.1195 [DOI] [PubMed] [Google Scholar]
- 4.Wyatt SB, Winters KP, Dubbert PM. Overweight and obesity: prevalence, consequences, and causes of a growing public health problem. Am J Med Sci 2006; 331: 166–74. doi: 10.1097/00000441-200604000-00002 [DOI] [PubMed] [Google Scholar]
- 5.Cha E, Akazawa MK, Kim KH, Dawkins CR, Lerner HM, Umpierrez G, et al. Lifestyle habits and obesity progression in overweight and obese American young adults: lessons for promoting cardiometabolic health. Nurs Health Sci 2015; 17: 467–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen HL. The scope and impact of obesity in Vermont - strategies for change. Prev Med 2015; 80: 44–6. doi: 10.1016/j.ypmed.2015.03.016 [DOI] [PubMed] [Google Scholar]
- 7.Parikh NI, Pencina MJ, Wang TJ, Lanier KJ, Fox CS, D'Agostino RB, et al. Increasing trends in incidence of overweight and obesity over 5 decades. Am J Med 2007; 120: 242–50. doi: 10.1016/j.amjmed.2006.06.004 [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Beydoun MA. The obesity epidemic in the United States—gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev 2007; 29: 6–28. doi: 10.1093/epirev/mxm007 [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Beydoun MA, Liang L, Caballero B, Kumanyika SK. Will all Americans become overweight or obese? Estimating the progression and cost of the US obesity epidemic. Obesity 2008; 16: 2323–30. doi: 10.1038/oby.2008.351 [DOI] [PubMed] [Google Scholar]
- 10.Strandheim A, Halland H, Saeed S, Cramariuc D, Hetland T, Lønnebakken MT, et al. Obesity-associated metabolic changes influence resting and peak heart rate in women and men. Scand Cardiovasc J 2015; 49: 337–43. doi: 10.3109/14017431.2015.1081273 [DOI] [PubMed] [Google Scholar]
- 11.Chaput JP, Saunders TJ, Tremblay MS, Katzmarzyk PT, Tremblay A, Bouchard C. Workplace standing time and the incidence of obesity and type 2 diabetes: a longitudinal study in adults. BMC Public Health 2015; 15: 111. doi: 10.1186/s12889-015-1353-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.French SA, Story M, Jeffery RW. Environmental influences on eating and physical activity. Annu Rev Public Health 2001; 22: 309–35. doi: 10.1146/annurev.publhealth.22.1.309 [DOI] [PubMed] [Google Scholar]
- 13.Lycett D. The Association of religious affiliation and body mass index (BMI): an analysis from the health survey for England. J Relig Health 2015; 54: 2249–67. doi: 10.1007/s10943-014-9975-3 [DOI] [PubMed] [Google Scholar]
- 14.Birnbaum BA, Hindman N, Lee J, Babb JS. Multi-detector row CT attenuation measurements: assessment of intra- and interscanner variability with an anthropomorphic body CT phantom. Radiology 2007; 242: 109–19. doi: 10.1148/radiol.2421052066 [DOI] [PubMed] [Google Scholar]
- 15.Bydder GM, Chapman RW, Harry D, Bassan L, Sherlock S, Kreel L, et al. Computed tomography attenuation values in fatty liver. J Comput Tomogr 1981; 5: 33–5. doi: 10.1016/0149-936X(81)90054-0 [DOI] [PubMed] [Google Scholar]
- 16.Bydder GM, Kreel L, Chapman RW, Harry D, Sherlock S, Bassan L. Accuracy of computed tomography in diagnosis of fatty liver. Br Med J 1980; 281: 1042. doi: 10.1136/bmj.281.6247.1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duman DG, Celikel C, Tuney D, Imeryuz N, Avsar E, Tozun N. Computed tomography in nonalcoholic fatty liver disease: a useful tool for hepatosteatosis assessment?. Dig Dis Sci 2006; 51: 346–51. doi: 10.1007/s10620-006-3136-9 [DOI] [PubMed] [Google Scholar]
- 18.Boyce CJ, Pickhardt PJ, Kim DH, Taylor AJ, Winter TC, Bruce RJ, et al. Hepatic steatosis (Fatty Liver Disease) in asymptomatic adults identified by unenhanced low-dose CT. AJR Am J Roentgenol 2010; 194: 623–8. doi: 10.2214/AJR.09.2590 [DOI] [PubMed] [Google Scholar]
- 19.Chen J, Talwalkar JA, Yin M, Glaser KJ, Sanderson SO, Ehman RL. Early detection of nonalcoholic steatohepatitis in patients with nonalcoholic fatty liver disease by using MR elastography. Radiology 2011; 259: 749–56. doi: 10.1148/radiol.11101942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fan JG, Zhu J, Li XJ, Chen L, Lu YS, Li L, et al. Fatty liver and the metabolic syndrome among Shanghai adults. J Gastroenterol Hepatol 2005; 20: 1825–32. doi: 10.1111/j.1440-1746.2005.04058.x [DOI] [PubMed] [Google Scholar]
- 21.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA 2012; 307: 491–7. doi: 10.1001/jama.2012.39 [DOI] [PubMed] [Google Scholar]
- 22.Hamaguchi M, Kojima T, Takeda N, Nakagawa T, Taniguchi H, Fujii K, et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med 2005; 143: 722–8. doi: 10.7326/0003-4819-143-10-200511150-00009 [DOI] [PubMed] [Google Scholar]
- 23.Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology 2003; 37: 917–23. doi: 10.1053/jhep.2003.50161 [DOI] [PubMed] [Google Scholar]
- 24.McKimmie RL, Daniel KR, Carr JJ, Bowden DW, Freedman BI, Register TC, et al. Hepatic steatosis and subclinical cardiovascular disease in a cohort enriched for type 2 diabetes: the Diabetes Heart Study. Am J Gastroenterol 2008; 103: 3029–35. doi: 10.1111/j.1572-0241.2008.02188.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alberti KG, Zimmet P, Shaw J; IDF Epidemiology Task Force Consensus Group. The metabolic syndrome—a new worldwide definition. Lancet 2005; 366: 1059–62. doi: 10.1016/S0140-6736(05)67402-8 [DOI] [PubMed] [Google Scholar]
- 26.Alberti K, Zimmet P, Shaw J. Metabolic syndrome—a new world-wide definition. A consensus statement from the International Diabetes Federation. Diabetic Med 2006; 23: 469–80. doi: 10.1111/j.1464-5491.2006.01858.x [DOI] [PubMed] [Google Scholar]
- 27.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet 2005; 365: 1415–28. doi: 10.1016/S0140-6736(05)66378-7 [DOI] [PubMed] [Google Scholar]
- 28.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005; 112: 2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404 [DOI] [PubMed] [Google Scholar]
- 29.Stepanova M, Afendy M, Vernon GC, Nader F, Younossi ZM. Components of metabolic syndrome and the rising prevalence of non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH) in the United States. Gastroenterology 2011; 140: S988–S. [Google Scholar]
- 30.Adams LA, Waters OR, Knuiman MW, Elliott RR, Olynyk JK. NAFLD as a risk factor for the development of diabetes and the metabolic syndrome: an eleven-year follow-up Study. Am J Gastroenterol 2009; 104: 861–7. doi: 10.1038/ajg.2009.67 [DOI] [PubMed] [Google Scholar]
- 31.Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology 2005; 129: 113–21. doi: 10.1053/j.gastro.2005.04.014 [DOI] [PubMed] [Google Scholar]
- 32.Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, Bellentani S. Prevalence of and risk factors for nonalcoholic fatty liver disease: the dionysos nutrition and liver Study. Hepatology 2005; 42: 44–52. doi: 10.1002/hep.20734 [DOI] [PubMed] [Google Scholar]
- 33.Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology 2011; 140: 124–31. doi: 10.1053/j.gastro.2010.09.038 [DOI] [PubMed] [Google Scholar]
- 34.Younossi Z, Stepanova M, Afendy M, Fang Y, YounossiYounossi Y, Mir H, et al. The changing face of chronic liver disease (cld) in the United States: the rising epidemic of non-alcoholic fatty liver disease (NAFLD). J Hepatol 2011; 54: S8–S. [Google Scholar]
- 35.Andrés-Blasco I, Herrero-Cervera A, Vinué Á, Martínez-Hervás S, Piqueras L, Sanz MJ, et al. Hepatic lipase deficiency produces glucose intolerance, inflammation and hepatic steatosis. J Endocrinol 2015; 227: 179–91. doi: 10.1530/JOE-15-0219 [DOI] [PubMed] [Google Scholar]
- 36.Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science 2011; 332: 1519–23. doi: 10.1126/science.1204265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cosma A, Cecchet D, Gaiani S, Coracina A, Pellizzari P, Pizzi C, et al. Clinical and biochemical determinants of the extent of liver steatosis in type 2 diabetes mellitus. Eur J Gastroenterol Hepatol 2015; 27: 1386–91. doi: 10.1097/MEG.0000000000000462 [DOI] [PubMed] [Google Scholar]
- 38.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest 2011; 121: 2111–7. doi: 10.1172/JCI57132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med 2010; 363: 1341–50. doi: 10.1056/NEJMra0912063 [DOI] [PubMed] [Google Scholar]
- 40.Pickhardt PJ, Park SH, Hahn L, Lee SG, Bae KT, Yu ES. Specificity of unenhanced CT for non-invasive diagnosis of hepatic steatosis: implications for the investigation of the natural history of incidental steatosis. Eur Radiol 2012; 22: 1075–82. doi: 10.1007/s00330-011-2349-2 [DOI] [PubMed] [Google Scholar]
- 41.Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology 2006; 43 (2 Suppl. 1): S99–S112. doi: 10.1002/hep.20973 [DOI] [PubMed] [Google Scholar]
- 42.Qayyum A, Chen DM, Breiman RS, Westphalen AC, Yeh BM, Jones KD, et al. Evaluation of diffuse liver steatosis by ultrasound, computed tomography, and magnetic resonance imaging: which modality is best?. Clin Imaging 2009; 33: 110–5. doi: 10.1016/j.clinimag.2008.06.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sande EP, Martinsen AC, Hole EO, Olerud HM. Interphantom and interscanner variations for Hounsfield units–establishment of reference values for HU in a commercial QA phantom. Phys Med Biol 2010; 55: 5123–35. doi: 10.1088/0031-9155/55/17/015 [DOI] [PubMed] [Google Scholar]
- 44.Tobari M, Hashimoto E, Yatsuji S, Torii N, Shiratori K. Imaging of nonalcoholic steatohepatitis: advantages and pitfalls of ultrasonography and computed tomography. Intern Med 2009; 48: 739–46. doi: 10.2169/internalmedicine.48.1869 [DOI] [PubMed] [Google Scholar]
- 45.Tang A, Cloutier G, Szeverenyi NM, Sirlin CB. Ultrasound elastography and MR elastography for assessing liver fibrosis: part 2, diagnostic performance, confounders, and future directions. AJR Am J Roentgenol 2015; 205: 33–40. doi: 10.2214/AJR.15.14553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Friedman LS. Controversies in liver biopsy: who, where, when, how, why?. Curr Gastroenterol Rep 2004; 6: 30–6. doi: 10.1007/s11894-004-0023-4 [DOI] [PubMed] [Google Scholar]
- 47.Garcia-Tsao G, Boyer JL. Outpatient liver biopsy: how safe is it?. Ann Intern Med 1993; 118: 150–3. doi: 10.7326/0003-4819-118-2-199301150-00013 [DOI] [PubMed] [Google Scholar]
- 48.Joy D, Thava VR, Scott BB. Diagnosis of fatty liver disease: is biopsy necessary?. Eur J Gastroenterol Hepatol 2003; 15: 539–43. [DOI] [PubMed] [Google Scholar]
- 49.Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology 2005; 128: 1898–906. doi: 10.1053/j.gastro.2005.03.084 [DOI] [PubMed] [Google Scholar]
- 50.Neuschwander-Tetri BA, Clark JM, Bass NM, Van Natta ML, Unalp-Arida A, Tonascia J, et al. ; NASH Clinical Research Network. Clinical, laboratory and histological associations in adults with nonalcoholic fatty liver disease. Hepatology 2010; 52: 913–24. doi: 10.1002/hep.23784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guha IN, Parkes J, Roderick P, Chattopadhyay D, Cross R, Harris S, et al. Noninvasive markers of fibrosis in nonalcoholic fatty liver disease: validating the European liver fibrosis panel and exploring simple markers. Hepatology 2008; 47: 455–60. doi: 10.1002/hep.21984 [DOI] [PubMed] [Google Scholar]
- 52.Mishra P, Younossi ZM. Abdominal ultrasound for diagnosis of nonalcoholic fatty liver disease (NAFLD). Am J Gastroenterol 2007; 102: 2716–7. doi: 10.1111/j.1572-0241.2007.01520.x [DOI] [PubMed] [Google Scholar]
- 53.Kodama Y, Ng CS, Wu TT, Ayers GD, Curley SA, Abdalla EK, et al. Comparison of CT methods for determining the fat content of the liver. AJR Am J Roentgenol 2007; 188: 1307–12. doi: 10.2214/AJR.06.0992 [DOI] [PubMed] [Google Scholar]
- 54.Lee SW, Park SH, Kim KW, Choi EK, Shin YM, Kim PN, et al. Unenhanced CT for assessment of macrovesicular hepatic steatosis in living liver donors: comparison of visual grading with liver attenuation index. Radiology 2007; 244: 479–85. doi: 10.1148/radiol.2442061177 [DOI] [PubMed] [Google Scholar]
- 55.Kim DY, Park SH, Lee SS, Kim HJ, Kim SY, Kim MY, et al. Contrast-enhanced computed tomography for the diagnosis of fatty liver: prospective study with same-day biopsy used as the reference standard. Eur Radiol 2010; 20: 359–66. doi: 10.1007/s00330-009-1560-x [DOI] [PubMed] [Google Scholar]
- 56.Artz NS, Hines CD, Brunner ST, Agni RM, Kühn JP, Roldan-Alzate A, et al. Quantification of hepatic steatosis with dual-energy computed tomography comparison with tissue reference standards and quantitative magnetic resonance imaging in the ob/ob Mouse. Invest Radiol 2012; 47: 603–10. doi: 10.1097/RLI.0b013e318261fad0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guiu B, Loffroy R, Cercueil JP, Krause D. Multiecho MR imaging and proton MR spectroscopy for liver fat quantification. Radiology 2008; 249: 1081. doi: 10.1148/radiol.2493081034 [DOI] [PubMed] [Google Scholar]
- 58.Longo R, Pollesello P, Ricci C, Masutti F, Kvam BJ, Bercich L, et al. Proton MR spectroscopy in quantitative in vivo determination of fat content in human liver steatosis. J Magn Reson Imaging 1995; 5: 281–5. doi: 10.1002/jmri.1880050311 [DOI] [PubMed] [Google Scholar]
- 59.Meisamy S, Hines CD, Hamilton G, Sirlin CB, McKenzie CA, Yu H, et al. Quantification of hepatic steatosis with T1-independent, T2-corrected MR imaging with spectral modeling of fat: blinded comparison with MR spectroscopy. Radiology 2011; 258: 767–75. doi: 10.1148/radiol.10100708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhong L, Chen JJ, Chen J, Li L, Lin ZQ, Wang WJ, et al. Nonalcoholic fatty liver disease: quantitative assessment of liver fat content by computed tomography, magnetic resonance imaging and proton magnetic resonance spectroscopy. J Dig Dis 2009; 10: 315–20. doi: 10.1111/j.1751-2980.2009.00402.x [DOI] [PubMed] [Google Scholar]
- 61.Reeder SB, Hu HH, Sirlin CB. Proton density fat-fraction: a standardized mr-based biomarker of tissue fat concentration. J Magn Reson Imaging 2012; 36: 1011–4. doi: 10.1002/jmri.23741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bashir MR, Zhong X, Nickel MD, Fananapazir G, Kannengiesser SA, Kiefer B, et al. Quantification of hepatic steatosis with a multistep adaptive fitting MRI approach: prospective validation against MR spectroscopy. AJR Am J Roentgenol 2015; 204: 297–306. doi: 10.2214/AJR.14.12457 [DOI] [PubMed] [Google Scholar]
- 63.Reeder SB, McKenzie CA, Pineda AR, Yu H, Shimakawa A, Brau AC, et al. Water–fat separation with IDEAL gradient-echo imaging. J Magn Reson Imaging 2007; 25: 644–52. doi: 10.1002/jmri.20831 [DOI] [PubMed] [Google Scholar]
- 64.Kramer HP, Kliewer MA, Pickhardt PJ, Hernando D, Chen GH, Reeder SB. Accuracy of liver fat quantification by CT, MR, and US: a prospective comparison with MR spectroscopy. Presented at the 2014 RSNA Scientific Assembly. Chicago, IL: RSNA.
- 65.Longo R, Ricci C, Masutti F, Vidimari R, Crocé LS, Bercich L, et al. Fatty infiltration of the liver. Quantification by 1H localized magnetic resonance spectroscopy and comparison with computed tomography. Invest Radiol 1993; 28: 297–302. doi: 10.1097/00004424-199304000-00006 [DOI] [PubMed] [Google Scholar]
- 66.Tominaga K, Kurata JH, Chen YK, Fujimoto E, Miyagawa S, Abe I, et al. Prevalence of fatty liver in Japanese children and relationship to obesity. An epidemiological ultrasonographic survey. Dig Dis Sci 1995; 40: 2002–9. doi: 10.1007/BF02208670 [DOI] [PubMed] [Google Scholar]
- 67.Pickhardt PJ, Hahn L, del Rio AM, Park SH, Reeder SB, Said A. Natural history of hepatic steatosis: observed outcomes for subsequent liver and cardiovascular complications. Am J Roentgenology 2014; 202: 752–8. doi: 10.2214/AJR.13.11367 [DOI] [PubMed] [Google Scholar]
- 68.Reeder SB, Ranallo F, Taylor AJ. CT and MRI for determining hepatic fat content. AJR Am J Roentgenol 2008; 190: W167. doi: 10.2214/AJR.07.2942 [DOI] [PubMed] [Google Scholar]
- 69.Hunt OF, Lubner MG, del Rio AM, Pickhardt PJ. The liver segmental volume ratio (LSVR) for non-invasive detection of cirrhosis: comparison with splenic volume and established linear measures. J Comput Assist Tomogr; in press. [DOI] [PMC free article] [PubMed]
- 70.Fan JG, Farrell GC. VAT fat is bad for the liver, SAT fat is not! J Gastroenterol Hepatol 2008; 23: 829–32. doi: 10.1111/j.1440-1746.2008.05474.x [DOI] [PubMed] [Google Scholar]
- 71.Park BJ, Kim YJ, Kim DH, Jung YJ, Yoon JH, Kim CY, et al. Visceral adipose tissue area is an independent risk factor for hepatic steatosis. J Gastroenterol Hepatol 2008; 23: 900–7. doi: 10.1111/j.1440-1746.2007.05212.x [DOI] [PubMed] [Google Scholar]
- 72.Sobhonslidsuk A, Jongjirasiri S, Thakkinstian A, Wisedopas N, Bunnag P, Puavilai G. Visceral fat and insulin resistance as predictors of non-alcoholic steatohepatitis. World J Gastroenterol 2007; 13: 3614–8. doi: 10.3748/wjg.v13.i26.3614 [DOI] [PubMed] [Google Scholar]
- 73.Ryckman EM, Summers RM, Liu J, del Rio AM, Pickhardt PJ. Visceral fat quantification in asymptomatic adults using abdominal CT: is it predictive of future cardiac events? Abdom Imaging 2015; 40: 222–6. doi: 10.1007/s00261-014-0192-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pickhardt PJ, Jee Y, O'Connor SD, del Rio AM. Visceral adiposity and hepatic steatosis at abdominal CT: association with the metabolic syndrome. AJR Am J Roentgenol 2012; 198: 1100–7. doi: 10.2214/AJR.11.7361 [DOI] [PubMed] [Google Scholar]
- 75.Hahn L, Reeder SB, Del Rio AM, Pickhardt PJ. Longitudinal changes in liver fat content in asymptomatic adults: hepatic attenuation on unenhanced CT as an imaging biomarker for steatosis. AJR Am J Roentgenol 2015; 205: 1167–72. doi: 10.2214/AJR.15.14724 [DOI] [PMC free article] [PubMed] [Google Scholar]