Abstract
Objective:
The study was conducted to describe ultrasound-guided high-intensity focused ultrasound (USgHIFU) ablation in the treatment of intra-abdominal aggressive fibromatosis in seven patients who had failed surgery.
Methods:
We retrospectively investigated seven patients with pathologically proven intra-abdominal aggressive fibromatosis and surgical failure, who were treated with USgHIFU between June 2013 and February 2015. The main causes for surgical failure were a large tumour size or adjacent tissue invasion by the tumour. All of the patients were treated with palliative intent, to reduce symptoms of the diseases. The medical records were reviewed during the follow-up period, and the patients were asked to compare the symptoms of their disease as improved, unchanged or worsened, based on their levels before treatment. In addition, contrast-enhanced MRI was conducted to follow the size of the tumours before and after therapy.
Results:
The procedure was successfully accomplished in all of the patients without severe side effects. The median diameter of the tumours was 10.3 cm (range, 7.6–13.6 cm) and the mean ablation rate (the percentage rate of the non-perfused volume compared with the tumour volume on enhanced MRI after treatment) was 92.5 ± 3.7% (range, 86.5–96.8%). One patient underwent two treatments for a large tumour size, and other patients received single-visit therapy. All of the patient clinical symptoms remitted significantly after 6 months. The regression rates of the tumours were 34.8 ± 8.2% (range, 22.4–46.1%) and 58.2 ± 12.7% (range, 43.8–70.3%), respectively, at 6 and 12 months after treatment.
Conclusion:
USgHIFU ablation could be an effective alternative minimally invasive therapy for the achievement of local control of intra-abdominal aggressive fibromatosis.
Advances in knowledge:
The conclusions indicate that USgHIFU ablation could be a promising alternative treatment for the achievement of local control of intra-abdominal aggressive fibromatosis.
INTRODUCTION
Aggressive fibromatosis is a benign tumour formed by fibroblastic proliferation. There are two types of aggressive fibromatosis, the intra- and extra-abdominal types. The former type mainly occurs in the trunk and extremities and is often encountered in infancy or childhood; the latter occurs frequently in the mesentery and retroperitoneum and has a tendency to infiltrate and encase surrounding tissues such as major blood vessels, skeletal muscle and intestines. Clinically, there are usually no symptoms, if the tumours in these locations are not excessively large or cause compression of adjacent organs; however, these tumours can become very large.1 Complete surgical resection is the basic treatment method for intra-abdominal aggressive fibromatosis; however, it is difficult to be resected completely because of the large size of the tumour and complicated anatomic relationship inside the abdomen, making it extremely easy to relapse.2,3 Therefore, the current therapies are unsatisfactory, and more effective treatments need to be developed.
Previous research has suggested that high-intensity focused ultrasound (HIFU) is a safe and effective method for uterine fibroids,4 liver cancer5 and pancreatic cancer.6 Compared with surgery, HIFU is non-invasive, shows faster post-operative recovery and is less expensive. However, to the best of our knowledge, there has been no report of HIFU ablation in the treatment of intra-abdominal aggressive fibromatosis. The objective of this article was to describe our initial experience with ultrasound-guided high-intensity focused ultrasound ablation in patients with intra-abdominal aggressive fibromatosis.
METHODS AND MATERIALS
Patients and tumours
Institutional ethics committee approval was obtained for this study. From August 2013 to February 2015, seven patients with intra-abdominal aggressive fibromatosis that had been confirmed by pathology were included. They all had failed surgery and had undergone ultrasound-guided high-intensity focused ultrasound to achieve local control. The main reason for surgical failure was the large tumour size or tumour invasion to surrounding tissues.
The median age of the patients was 23 years (range, 18–32 years), the median tumour diameter was 10.3 cm (range, 7.6–13.6 cm) and the patients included four males and three females. All of the patients underwent contrast-enhanced MRI before and after the procedure. During follow-up, the patients were asked to compare the symptoms of the diseases as improved, unchanged or worsened with their levels before treatment; in addition, serial contrast-enhanced MRI was used to follow the size of the tumours.
Equipment and procedures
All of the procedures were performed with the Model-JC HIFU tumour-treating system (Chongqing Haifu Technology, Chongqing, China). The energy was generated by a transducer integrated with a diagnostic ultrasound probe that provided real-time monitoring. The correlation parameters of therapy equipment were as follows: transducer diameter, 20 cm; focal length, 15 cm; frequency, 0.8 MHz; power, 0–400 W.
Before treatment, all of the patients underwent strict bowel preparation and skin preparation. Bowel preparation was performed using an oral polyethylene glycol-electrolyte powder, which is a high-performance and safe new bowel preparation medicine, and residue-free faeces as the criteria; skin preparation included shaving the hair and degreasing and degassing the skin of the abdominal wall using a vacuum device. An i.v. sedative (midazolam, 1–4 mg) and analgesic (fentanyl, 50–400 μg) were used to relieve discomfort and prevent voluntary or involuntary movement during the procedures.
The patients were treated in the prone position, the abdominal wall was submerged in degassed water and a water balloon was placed on the abdominal wall to push the intestinal canal in the HIFU beam path. The target area and adjacent structure could be detected by real-time ultrasonographic imaging. The treatment began from the maximum plane of the tumour, from the deep to shallow areas and slice by slice (thickness of section was 5 mm), and the focus was at least 1.5 cm away from the boundary of the tumour, to prevent damage to the surrounding normal tissue. The therapeutic energy and intensity could be regulated, according to the feedback from the patient and changes in the greyscale on ultrasonographic imaging during treatment. Once the hyperechoic area covered almost the entire lesion on ultrasonographic imaging, the treatment was terminated. After treatment, the prone position was recommended for 2 h in all patients, to protect the surrounding normal tissue from harm of the residual temperature of the lesion.
Post-treatment assessment and follow-up
After treatment, all of the patients were required to stay at the hospital for 1 week, to carefully observe possible complications such as skin burn, intestine and nerve damage, fever and hepatic and renal malfunction. All of the patients were contacted at 3, 6 and 12 months after the procedure, immediately after treatment and during the follow-up period. Contrast-enhanced MRI was used to assess the therapeutic response and tumour volume changes, and the patients were asked to compare the symptoms of the diseases as improved, unchanged or worsened with their levels before treatment. The tumour volume (V) and treated area were measured in three dimensions—longitudinal (D1), anteroposterior (D2) and transverse (D3)—and were calculated using the following equation:
RESULTS
The HIFU ablation procedure was successfully accomplished in all of the patients. Among them, six patients received one treatment and one patient received two treatments for a large tumour size (Table 1).
Table 1.
Baseline data of patients
| Patient number | Gender | Age (years) | Max diameter (cm) | Main symptoms | Duration of follow-up (months) | Treatment time (min) | Ablation ratio (%) | Side effects |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 23 | 21.8 | Pressure | 18 | 136.3 | 96.8 | Platelet decline, fever |
| 2 | M | 28 | 10.3 | Abdominal distension | 12 | 62.1 | 88.3 | Fever |
| 3 | M | 18 | 7.6 | Abdominal distension | 13 | 40.6 | 92.5 | None |
| 4 | M | 32 | 13.6 | Motor dysfunction | 15 | 88.5 | 91.2 | Acroparaesthesia, fever |
| 5 | F | 14 | 9.4 | Pressure | 14 | 55.4 | 86.5 | None |
| 6 | M | 76 | 6.7 | Abdominal distension | 13 | 58.2 | 93.7 | None |
| 7 | F | 20 | 12.1 | Backaches | 17 | 74.8 | 95.4 | Fever |
F, female; M, male; max, maximum.
During the treatment, all of the patients felt pain in the treated area, and the average visual analogue scale pain score was just 3.2 points (range, 2–4 points). Immediately after the treatment, the skin in the treated area became swollen, with no skin burn, pain, perforation or obvious haemorrhage, and medications used in clinical practice for decreasing swelling were used. One patient who had acroparaesthesia of the lower limb on the second day post treatment, without movement function handicap, was cured with neurotrophic drug treatment. Another patient's platelet count declined sharply, falling to 25 × 109 per litre from 148 × 109 per litre over 3 days post treatment; bone marrow aspiration excluded proliferative disease of the haematopoietic or lymphatic system, and the platelet count gradually returned to the normal state without treatment within 3 days. Four patients had afternoon low-grade fever (<38.0 °C) lasting 3 days. No other complications were encountered after HIFU ablation (Table 1). All of the patients were ambulatory after 2 h of treatment and were discharged within 1 week after treatment.
The mean ablation rate was 92.5%, according to contrast-enhanced MRI immediately after the treatment. All of the patient clinical symptoms, including abdominal distension and pressure, motor dysfunction of the lower extremities and backaches, remitted to different degrees immediately after treatment and significantly improved after 6 months. The mean follow-up was 14 months (range, 13–17 months), and the 6-month and 12-month regression rates of the tumour were 34.8% and 58.2%, respectively (Table 2). A persistent non-perfusion area was observed in the treated area in all of the patients, and the residual disease around the tumours grew slowly (Figure 1 and 2).
Table 2.
Treatment results of lesions
| Variable | Value |
|---|---|
| Treatment time (min) | 62.1 (55.4–88.5) |
| Ablation ratio (%) | 92.5 ± 3.7 (86.5–96.8) |
| Duration of follow-up (months) | 14 (13–17) |
| Regression rate at 6 months after treatment (%) | 34.8 ± 8.2 (22.4–46.1) |
| Regression rate at 12 months after treatment (%) | 58.2 ± 12.7 (43.8–70.3) |
| Symptom improvement rate (%) | 100 |
| Incidences of adverse event (SIRC) (%) | 28.6 (2/7) |
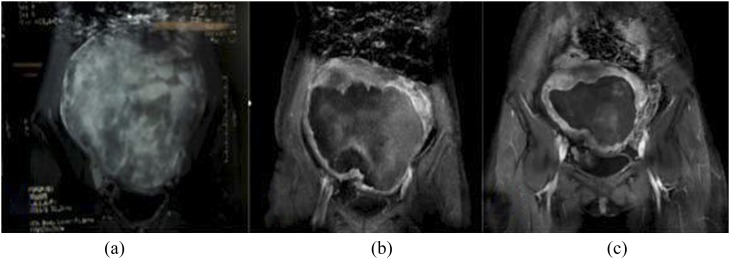
Figure 1.
Contrast-enhanced coronal MRI before and after ultrasound-guided high-intensity focused ultrasound treatment: (a) intra-abdominal aggressive fibromatosis showing significant enhancement before treatment; (b, c) intra-abdominal aggressive fibromatosis size has obviously shrunk and the area of necrosis is still visible on contrast-enhanced MRI at 6 and 12 months after treatment.
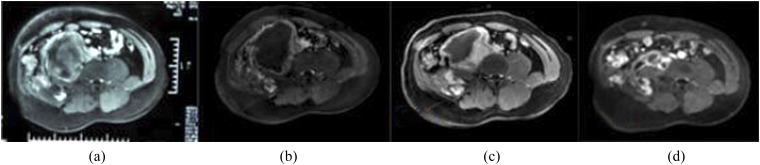
Figure 2.
Contrast-enhanced axial MRI before and after ultrasound-guided high-intensity focused ultrasound treatment: (a) intra-abdominal aggressive fibromatosis showing slight enhancement before treatment; (b) the negative-predictive value (NPV) is visible inside the intra-abdominal aggressive fibromatosis in contrast-enhanced MRI immediately after treatment; (c, d) intra-abdominal aggressive fibromatosis size has obviously shrunk at 6 and 12 months after treatment, although the NPV ratio has decreased.
DISCUSSION
Presently, surgery is the main method to cure intra-abdominal aggressive fibromatosis.7,8 However, large intra-abdominal aggressive fibromatoses usually infiltrate adjacent structures and result in high local failure rates.2,3,9 To our knowledge, there are no published examples of HIFU being used to treat intra-abdominal aggressive fibromatosis after surgical failure. In this study, we first investigated the role of HIFU ablation as a palliative treatment for patients with inoperable tumours.
Intra-abdominal aggressive fibromatoses, as deep fibromatoses, are larger in size, are more frequently locally infiltrative and relapse but do not metastasize compared with superficial fibromatoses.1 Complete surgical resection is the traditional treatment for aggressive fibromatosis; however, local recurrence rates have been reported to be as high as 33%.2,3,9,10 Meanwhile, many lesions might be unresectable; usually, such a situation most commonly occurs in patients with a large-sized tumour and a complicated anatomic relationship inside the abdomen.1,11,12 This situation is very difficult to solve. The evolving role of alternative methods of local control is currently being investigated.
Investigators are currently exploring local treatments as variable as local irradiation, cryoablation, radiofrequency and HIFU. Radiation can be used as therapy individually or in combination with surgery. Patients who are not surgical candidates or who refuse surgery are indicated for radiation therapy alone, and several studies have shown that radiation therapy alone results in durable local control rates of 70–80%.2,13–15 However, studies on radiotherapy have focused on extra-abdominal aggressive fibromatosis, and most of the tumour sizes are significantly <10 cm; therefore, the median total dose of 56 Gy has a better therapeutic effect on local control. In this study, all of the tumours were diagnosed as intra-abdominal aggressive fibromatosis with sizes >10 cm. A larger dose radiotherapy may improve therapeutic efficacy, but intestinal radiation injuries are a dose-limiting factor during the course of abdominal radiotherapy. If increasing the irradiation dose and exceeding bowel tolerance will increase the risk of bowel perforation and bleeding, these may result in a poor response to radiotherapy alone. HIFU can create a coagulative necrosis region by the high-temperature effect produced by the focused ultrasound beam, without harming tissues outside of the focus. The dimensions of the focal region are 1.5 × 1.5 × 10 mm. This process is repeated section by section, to achieve complete ablation of the tumour. HIFU would not share these limitations like radiotherapy, and the results suggest that HIFU achieves a satisfactory ablation rate and local control rates with no severe complications after treatment.
Cryoablation and radiofrequency are both local therapies for aggressive fibromatosis. The study by Kujak16 also revealed that cryoablation may be an effective alternative treatment for the local control of small- and moderate-sized extra-abdominal aggressive fibromatosis; however, the effect is poor in patients with larger tumours whose volume is >10 cm. Radiofrequency ablation has been reported recently in aggressive fibromatosis poorly suited for surgery. Ilaslan et al17 used radiofrequency ablation to treat five desmoid tumours in four patients; however, complications were seen in two patients: one patient had cellulitis and another had soft-tissue necrosis. Radiofrequency ablation causes tissue thermocoagulation necrosis via the heat generated by ion oscillation and collision generated by the radiofrequency current; therefore, active tissue heating was chiefly concentrated in the zone of a few millimetres surrounding the active electrode.7,18 Thus, radiofrequency ablation might not be a good candidate for larger tumours. Different therapy methods can potentially cause various injuries, but HIFU ablations did not seem to be subjected to this limitation, and the results of the present study confirmed this.
Wang et al once made a preliminary study on the treatment of extra-abdominal aggressive fibromatosis.19 The results indicated that HIFU ablation could be used as an effective minimally invasive therapy for local control of tumours. Although the study focused on intra-abdominal aggressive fibromatosis, its anatomic relationship with the adjacent structures, such as the intestines, nerves and blood vessels, was more complex than extra-abdominal aggressive fibromatosis. In addition, the size of intra-abdominal aggressive fibromatosis tended to be larger, making the treatment more challenging. Other topic therapies including irradiation, cryoablation and radiofrequency were limited. As seen in this study, HIFU might be a safe and effective method and might bring new hope for patients who show failure in routine surgery.
In our subjects, one patient had acroparaesthesia of the lower limb after treatment, and we believed it might have been because the tumours were close to the sensory nerve, which was stimulated by hyperthermia during treatment. Because the damage was not fatal, the acroparaesthesia was cured by neurotrophic drug treatment. Better communication is needed between patients and operators during treatment; once acroparaesthesia occurs, the operator should change the treatment plan, such as adjusting the power, focus position and treatment intensity. Such measures could help reduce cases of acroparaesthesia. In another patient, the platelet count declined sharply; through analysis, we believed that it was non-specific haemolysis caused by heating of the tumour over long periods of time. Owing to the huge size, fractional therapy for a giant tumour might be the effective means for reducing this complication. The low-grade fever was caused by necrosis of the tumour tissue, a phenomenon that is common in thermal ablation therapy without pathological significance.
Although promising, there are some limitations in the study. The small sample size, short follow-up and lack of supporting randomized controlled trials determined that the study could not provide the strongest evidence. Further research with randomized controlled trials with higher quality, multiple centres and large samples is needed, to validate the effectiveness of HIFU for the treatment of intra-abdominal aggressive fibromatosis.
CONCLUSION
Our preliminary results indicated that HIFU might be an effective, minimally invasive, promising alternative treatment for the local control of intra-abdominal aggressive fibromatosis after surgical failure. Further research is needed to validate our findings.
FUNDING
This article was supported by the National Key Technology Research and Development Programme of China (2013BAI01B01).
Contributor Information
Wen-Peng Zhao, Email: zwp215@163.com.
Zhi-Yu Han, Email: hanzhiyu301@hotmail.com.
Jing Zhang, Email: zjbch@sina.com.
Xiao-ling Yu, Email: dyuxl301@aliyun.com.
Zhi-Gang Cheng, Email: qlczg@hotmail.com.
Xiang Zhou, Email: zhou.xiang@yeah.net.
Ping Liang, Email: liangping301@hotmail.com.
REFERENCES
- 1.Fisher C, Thway K. Aggressive fibromatosis. Pathology 2014; 46: 135–40. doi: 10.1097/PAT.0000000000000045 [DOI] [PubMed] [Google Scholar]
- 2.Ramamurthy R, Arumugam B, Ramanandham B. Recurrence patterns and management options in aggressive fibromatosis. Indian J Surg Oncol 2012; 3: 222–7. doi: 10.1007/s13193-012-0146-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bertani E, Testori A, Chiappa A, Misitano P, Biffi R, Viale G, et al. Recurrence and prognostic factors in patients with aggressive fibromatosis. The role of radical surgery and its limitations. World J Surg Oncol 2012; 10: 184. doi: 10.1186/1477-7819-10-184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao WP, Chen JY, Zhang L, Li Q, Qin J, Peng S, et al. Feasibility of ultrasound-guided high intensity focused ultrasound ablating uterine fibroids with hyperintense on T2 weighted MR imaging. Eur J Radiol 2013; 82: e43–9. doi: 10.1016/j.ejrad.2012.08.020 [DOI] [PubMed] [Google Scholar]
- 5.Cheung TT, Fan ST, Chu FS, Jenkins CR, Chok KS, Tsang SH, et al. Survival analysis of high-intensity focused ultrasound ablation in patients with small hepatocellular carcinoma. HPB (Oxford) 2013; 15: 567–73. doi: 10.1111/hpb.12025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li CC, Wang YQ, Li YP, Li XL. High-intensity focused ultrasound for treatment of pancreatic cancer: a systematic review. J Evid Based Med 2014, 7(4): 270–281. [DOI] [PubMed] [Google Scholar]
- 7.Walczak BE, Rose PS. Desmoid: the role of local therapy in an era of systemic options. Curr Treat Options Oncol 2013; 14: 465–73. doi: 10.1007/s11864-013-0235-7 [DOI] [PubMed] [Google Scholar]
- 8.Eastley NC, Hennig IM, Esler CP, Ashford RU. Nationwide trends in the current management of desmoid (aggressive) fibromatosis. Clin Oncol (R Coll Radiol) 2015; 27: 362–8. doi: 10.1016/j.clon.2015.02.006 [DOI] [PubMed] [Google Scholar]
- 9.Shin SH, Ko KR, Cho SK, Choi YL, Seo SW. Surgical outcome of desmoid tumors: adjuvant radiotherapy delayed the recurrence, but did not affect long-term outcomes. J Surg Oncol 2013; 108: 28–33. doi: 10.1002/jso.23343 [DOI] [PubMed] [Google Scholar]
- 10.Cates JM, Stricker TP. Surgical resection margins in desmoid-type fibromatosis: a critical reassessment. Am J Surg Pathol 2014; 38: 1707–14. doi: 10.1097/PAS.0000000000000276 [DOI] [PubMed] [Google Scholar]
- 11.Küçük L, Keçeci B, Sabah D, Yücetürk G: Aggressive fibromatosis: evaluation of prognostic factors and outcomes of surgical treatment. Acta Orthop Traumatol Turc 2014; 48: 55–60. doi: 10.3944/AOTT.2014.3171 [DOI] [PubMed] [Google Scholar]
- 12.La Greca G, Santangelo A, Primo S, Sofia M, Latteri S, Russello D, et al. Clinical and diagnostic problems of desmoid-type fibromatosis of the mesentery: case report and review of the literature. Ann Ital Chir 2014; 85. ePub ahead of print. [PubMed] [Google Scholar]
- 13.Kamath SS, Parsons JT, Marcus RB, Zlotecki RA, Scarborough MT. Radiotherapy for local control of aggressive fibromatosis. Int J Radiat Oncol Biol Phys 1996; 36: 325–8. doi: 10.1016/S0360-3016(96)00321-5 [DOI] [PubMed] [Google Scholar]
- 14.Micke O, Seegenschmiedt MH; German Cooperative Group on Radiotherapy for Benign Diseases. Radiation therapy for aggressive fibromatosis (desmoid tumors): results of a national Patterns of Care Study. Int J Radiat Oncol Biol Phys 2005; 61: 882–91. doi: 10.1016/j.ijrobp.2004.07.705 [DOI] [PubMed] [Google Scholar]
- 15.Kriz J, Eich HT, Haverkamp U, Seegenschmiedt MH, Heide J, Bruns F, et al. Radiotherapy is effective for desmoid tumors (aggressive fibromatosis)—long-term results of a German multicenter study. Oncol Res Treat 2014; 37: 255–60. doi: 10.1159/000362398 [DOI] [PubMed] [Google Scholar]
- 16.Kujak JL, Liu PT, Johnson GB, Callstrom MR. Early experience with percutaneous cryoablation of extra-abdominal desmoid tumors. Skeletal Radiol 2010; 39: 175–82. doi: 10.1007/s00256-009-0801-z [DOI] [PubMed] [Google Scholar]
- 17.Ilaslan H, Schills J, Joyce M, Marks K, Sundaram M. Radiofrequency ablation: another treatment option for local control of desmoid tumors. Skeletal Radiol 2010; 39: 169–73. [DOI] [PubMed] [Google Scholar]
- 18.Nahum Goldberg S, Dupuy DE. Image-guided radiofrequency tumor ablation: challenges and opportunities—part I. J Vasc Interv Radiol 2001; 12: 1021–32. doi: 10.1016/S1051-0443(07)61587-5 [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Wang W, Tang J. Ultrasound-guided high intensity focused ultrasound treatment for extra-abdominal desmoid tumours: preliminary results. Int J Hyperthermia 2011; 27: 648–53. doi: 10.3109/02656736.2011.597047 [DOI] [PubMed] [Google Scholar]