Abstract
Objective:
While split-field intensity-modulated radiation therapy (SF-IMRT) decreases dose to low neck (LAN) structures such as the glottic larynx compared with full-neck intensity-modulated radiation therapy (IMRT), it is unknown whether SF-IMRT affords superior dose avoidance to organs than whole neck-field volumetric-modulated arc therapy (WF-VMAT).
Methods:
10 patients treated definitively with radiation for oropharyngeal, oral cavity or nasopharyngeal carcinoma were compared. Only patients ideally suited for SF-IMRT plans were included. The glottic larynx, supraglottic larynx, arytenoids, pharyngeal constrictors, oesophagus, brachial plexus and target volume coverage in the LAN were compared between WF-VMAT and SF-IMRT.
Results:
Volumetric-modulated arc therapy (VMAT) yielded statistically significant decreases in maximum dose to the arytenoids and mean dose to the oesophagus. There was no difference in dose to the glottic larynx, supraglottic larynx, pharyngeal constrictors and brachial plexus. WF-VMAT led to improved coverage to 50/2 Gy fraction equivalent in LAN compared with SF-IMRT using an anteroposterior (AP) LAN field but no difference to the 60/2 Gy fraction equivalent between SF-IMRT and WF-VMAT using AP/posterior–anterior LAN boost.
Conclusion:
WF-VMAT affords equivalent glottic and supraglottic larynx dose and lower dose to the arytenoids and oesophagus. WF-VMAT better covers most LAN target structures. Given these findings as well as concerns with matchline cold spots or hotspots with SF-IMRT, patients requiring comprehensive elective nodal irradiation should typically be treated with WF-VMAT.
Advances in Knowledge:
SF-IMRT for larynx sparing has better dosimetric results to normal structures than whole-neck IMRT, but with increased matchline recurrence risk. We show dosimetric equivalence or superiority of WF-VMAT compared with SF-IMRT.
INTRODUCTION
Intensity-modulated radiation therapy (IMRT) allows precise radiation delivery to volumes at risk of involvement with tumour, while sparing surrounding normal tissues and organs at risk (OARs). This has led to decreased toxicity, as demonstrated in a Phase III study.1 IMRT may be delivered to the whole neck or with a split-field intensity-modulated radiation therapy (SF-IMRT) arrangement combining conformal IMRT to the primary and upper neck with anterior and/or posterior fields treating the low neck (LAN). Compared with whole-neck IMRT field, SF-IMRT generally allows decreased dose to the glottic larynx.2 However, this creates uncertainties in dose at the matchline between the IMRT and LAN fields, as well as leads to increased patient treatment time on the table by adding additional fields.
Volumetric-modulated arc therapy (VMAT) is intensity-modulated arc therapy using a dynamic multileaf collimator (MLC). With this technique, the desired fluence is achieved by modulating the MLC with a variable dose rate and variable gantry speed. This technique also provides better dose distributions that conform more accurately to the three-dimensional (3D) targets, sparing the normal tissues and OARs. There are several studies that show that this technique delivers dose precisely with less monitor units and shorter treatment time.3,4
We therefore undertake this dosimetric study to determine whether it is possible to achieve equal or better dosimetric parameters to LAN structures for whole-neck VMAT plans than SF-IMRT plans.
METHODS AND MATERIALS
Only patients without disease involvement of the larynx, vallecula or pharyngoepiglottic folds, and without nodal disease or disease limited to the retropharyngeal, Level 1 and/or Level 2 nodal levels but requiring elective nodal treatment were included. We identified 10 such patients with oral cavity (1), oropharyngeal (6), or nasopharyngeal carcinoma (3). 9 patients were treated with definitive radiation or chemoradiation, with a total dose of 70 Gy in 35 fractions. 1 patient was treated after resection to the local site only without neck dissection and was treated with 60 Gy in 30 fractions to the primary site.
All clinical target volumes (CTV) as well as clinical target volumes in the low neck (CTV-LN), planning target volumes (PTV) as well as planning target volumes in the low neck (PTV-LN) and OARs were defined and contoured by a single experienced physician (PHA) to minimize contouring variability, with the LAN structures contoured in accordance with the National Radiation Group atlas,5 in order to standardize contouring in a manner as independent from physician contouring practices as possible. CTV-LN consisted of the caudal half of bilateral Level 3, the entirety of Level 4 and Level 5, if there was gross disease present in Level 2. An isometric 3-mm expansion from CTV was used to define PTV. All patients were planned at the time of presentation with whole-neck VMAT. The whole neck-field volumetric-modulated arc therapy (WF-VMAT) plans were generated with two 360° (full rotation) arcs, one clockwise and the other counterclockwise. To minimize the tongue and groove effect, collimator rotations not >45° in the opposite direction for both arcs were used during optimization. The VMAT plans were optimized for the whole-neck region including upper and lower neck targets in a single plan, sparing the normal tissues and OARs using simultaneous integrated boost. This study was approved by the Hospital of the University of Pennsylvania Institutional Review Board.
Separate single isocentric SF-IMRT plans with IMRT for the upper neck and a single or opposed arrangement for the lower neck were generated for the same patients in our comparison study. Both WF-VMAT and SF-IMRT plans were optimized with 6-MV beams and millennium 120 MLC using the Varian Eclipse™ treatment-planning system. SF-IMRT plans consisted of a single isocentric technique with IMRT plans for the upper neck, while the lower neck plan consisted of a single anteroposterior (AP) field or AP/posterior–anterior fields (AP/PA). The isocentre was placed at the midpoint between the cervical vertebrae and trachea, one slice above the level of arytenoids for all patients. The SF-IMRT plans were optimized for dose delivery with sliding window technique, using the standard nine fields and a fixed gantry angle. For the purpose of SF-IMRT optimization, separate dosimetric PTV were created by copying the physician-contoured PTV. These dosimetric PTV were split into upper and lower subvolumes by deleting the PTV contour at the isocentre slice. For optimization of the upper neck IMRT plan, one more contour above the isocentre slice was deleted. This was carried out to avoid any unwanted hotspots at the matchline region.
For the SF-IMRT plans, the lower anterior neck was planned as per standard practice, normalized to a depth of 3 cm. As per standard clinical practice, the low–intermediate-risk PTV in this region was targeted to receive a dose of 50 Gy (2 Gy × 25 fractions); this would typically encompass the ipsilateral and contralateral Levels 3 and 4. For patients requiring treatment to Level 3 to the high–intermediate dose (usually if a Level 2 nodal level was involved with tumour), a boost plan of 10 Gy (2 Gy × 5 fractions) was added to achieve a total dose of 60 Gy (2 Gy × 30 fractions). A standard 4 × 2-cm larynx block was added, in order to minimize the total larynx dose. After 22 fractions, a cord block was added for the remaining three fractions to limit the cord dose within the cord tolerance dose limit of 45 Gy. As per standard clinical practice, there were no dose constraints placed on the LAN structures in plans generated using SF-IMRT, and there were no minimal coverage requirements for the 50-Gy and 60-Gy targets.
In patients treated with WF-VMAT, the low–intermediate-risk PTV was treated to 59.5 Gy (1.7 Gy × 35 fractions), the dosimetric equivalent of 50 Gy in 25 fractions. For the one patient who was treated after resection to the primary site but not to the neck, the low–intermediate-risk PTV was treated to 54 Gy (1.8 Gy × 30 fractions), the dosimetric equivalent of 50 Gy in 25 fractions (PTV50). The high–intermediate-risk PTV was treated to 63 Gy (1.8 Gy × 35 fractions), the dosimetric equivalent of 60 Gy in 30 fractions (PTV60). In WF-VMAT plans, no constraints were placed for the supraglottic larynx. The glottic larynx was constrained to a mean dose of 20 Gy. Pharyngeal constrictors were constrained to a mean dose of 50 Gy. The brachial plexus was constrained to a maximum point dose of 66 Gy, with a V60 (volume receiving over 60 Gy) of 15%. The arytenoid point maximum dose was constrained to 25 Gy. The oesophagus was constrained to a mean dose of 30 Gy. There were no coverage constraints specified for the LAN CTV50-LN, PTV50-LN, CTV60-LN and PTV60-LN target volumes in patients treated with WF-VMAT. Instead, constraints for CTV and PTV coverage for the entire head and neck were specified, specifying >99% of the CTV volume for the entire head and neck receiving prescription dose and 95% of the PTV volume for the entire head and neck receiving prescription dose. Therefore, it would be possible to have undercoverage of the LAN CTV or PTV while still meeting the coverage constraints encompassing the entire head and neck.
Doses to the critical OARs in the LAN including the glottic larynx, supraglottic larynx, arytenoids, pharyngeal constrictors, oesophagus and brachial plexus and coverage to target volumes including CTV-LN and PTV-LN in the LAN were compared between the WF-VMAT and SF-IMRT plans.
Owing to the different fractionation schemes in the lower risk neck (59.5 Gy in 35 fractions for WF-VMAT and 50 Gy in 25 fractions for SF-IMRT) and in the higher risk neck (63 Gy in 35 fractions for WF-VMAT and 60 Gy in 30 fractions for SF-IMRT), biologically equivalent doses (BED) to LAN structures were also calculated for the equivalent of treatment at 2 Gy per fraction, utilizing α/β ratios based on a literature review of α/β ratios of LAN structures with a ratio of α/β = 4 for the glottic larynx (necrosis), α/β = 3.8 for the supraglottic larynx (oedema), α/β = 5.2 for the brachial plexus (nerve damage), α/β = 3 for the oesophagus (stricture) and α/β = 4 for the arytenoid.6 Target dose parameters included volume receiving 100% of prescription dose (V100) to the CTV and PTV structures receiving 50 and 60 Gy. Conformality index quantifying dose conformity to the target was calculated for the PTV50 and PTV60 structures [Conformality index = (PTV volume receiving 95% of prescription dose)/(PTV volume)], with results closest to 1 representing the ideal. Dose homogeneity index was calculated for the PTV50 and PTV60 structures [(D2 − D98) × 100/(prescription dose); D2 is dose to 2% of target volume, D98 is dose to 98% of target volume], with results closest to 0 representing ideal uniformity.
For dose calculation, the anisotropic analytical algorithm v. 11.0.30 in Eclipse (Varian Medical Systems, Palo Alto, CA) was used, with the dose–volume optimizer v. 11.0.30 used for IMRT optimization and progressive resolution optimizer v. 11.0.30 used for VMAT optimization. The statistical package within Excel® (Microsoft Corporation®, Redmond, WA) was used to generate two-sided paired t-tests for the relevant structures, with a p-value of <0.05 denoting a statistical significance.
Patients were routinely scoped before and 3–4 months after radiation therapy. Since patients were treated with WF-VMAT, the results of nasopharyngolaryngoscopy were available for only those using this technique. Patients were anaesthetized using a 4% lidocaine solution sprayed 5–15 min prior to scoping. A flexible nasopharyngoscope was then placed in the nose and images saved using an associated image capture system (Olympus Medical, Center Valley, PA).
RESULTS
Of the 10 patients, 6 patients had squamous cell carcinoma of the oropharynx, 2 patients had squamous cell carcinoma of the oral cavity and 3 patients had squamous cell carcinoma of the nasopharynx (Table 1).
Table 1.
Patient characteristics
| Site | Stage | Treatment intent |
|---|---|---|
| Base of tongue | Stage IVA (T2N2bM0) | Definitive |
| Base of tongue | Stage III (T1N1M0) | Definitive |
| Base of tongue | Stage IVA (T4aN2bM0) | Definitive |
| Base of tongue | Stage IVA (T4aN0M0) | Definitive |
| Uvula | Stage II (T2N0M0) | Definitive |
| Tonsil/soft palate | Stage II (T2N0M0) | Definitive |
| Floor of mouth | Stage IVA (T4aN0M0) | Definitive |
| Nasopharynx | Stage II (T2N1M0) | Definitive |
| Nasopharynx | Stage IVA (T4N0M0) | Definitive |
| Nasopharynx | Stage III (T3N0M0) | Adjuvant |
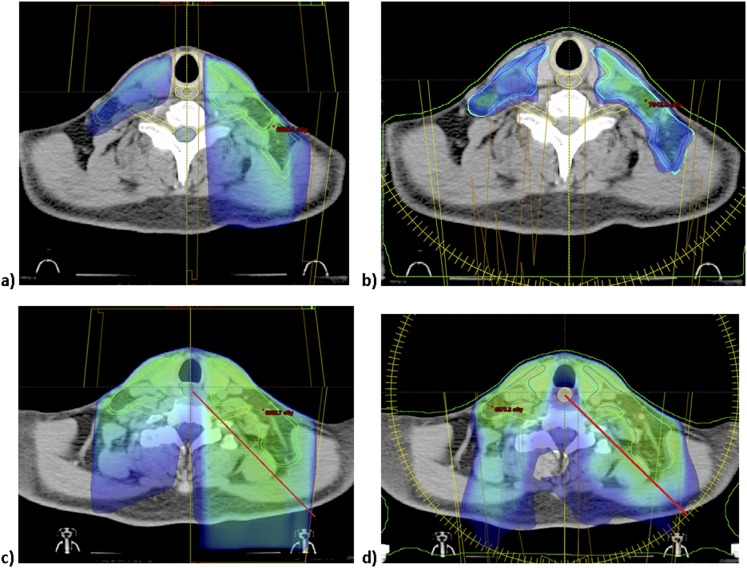
Representative WF-VMAT and SF-IMRT plans are shown in Figure 1. WF-VMAT plans had a lower maximum dose to the arytenoids (23.8-Gy VMAT vs 46.7-Gy SF-IMRT, p < 0.001; Table 2) and mean oesophagus dose (24.5 Gy vs 39.7 Gy, p < 0.001; Table 2) than SF-IMRT. Coverage of the LAN target volumes was superior for patients treated with WF-VMAT than for the AP neck to the low–intermediate-risk volumes targeted to a dose of 50 Gy in 25 fractions (CTV50 V100 of 99.3% WF-VMAT vs 91.5% for SF-IMRT, p = 0.01; PTV50 V100 of 95.6% vs 86%, p = 0.029; Table 3), with superior conformality of WF-VMAT to the PTV50 volume (0.99 WF-VMAT vs 0.969 SF-IMRT, p = 0.003, Table 3).
Figure 1.
Representation of coverage of lower neck target volumes using (a) split-field intensity-modulated radiation therapy (SF-IMRT) and (b) whole-neck volumetric-modulated arc therapy (VMAT). At the level of the oesophageal inlet, dose above 30 Gy is represented in the dose cloud, demonstrating higher dose to the oesophagus with (a, c) SF-IMRT plan to the lower neck (maximum dose to the oesophagus 42 Gy) than with (a, d) VMAT plan (maximum dose to the oesophagus of 26 Gy).
Table 2.
Dose to avoidance structures using volumetric-modulated arc therapy (VMAT) vs split-field intensity-modulated radiation therapy (SF-IMRT). There was a statistically significant decrease in maximum (max) dose to the arytenoids and mean dose to the oesophagus. The two techniques were otherwise equivalent
| Avoidance structure | VMAT | SF-IMRT | p-values |
|---|---|---|---|
| Glottic larynx | 20.1 Gy (mean) | 19.5 Gy (mean) | 0.65 |
| Supraglottic larynx | 39.6 Gy (mean) | 40.9 Gy (mean) | 0.67 |
| Pharyngeal constrictors | 46.6 Gy (mean) | 46.9 Gy (mean) | 0.50 |
| Brachial plexus | 64.2 Gy (max) | 67.2 Gy (max) | 0.37a |
| 15.2% (V60) | 12.8% (V60) | 0.64 | |
| Arytenoids | 23.8 Gy (max) | 46.7 Gy (max) | <0.001 |
| Oesophagus | 24.6 Gy (mean) | 39.7 Gy (mean) | <0.001 |
V60, volume receiving over 60 Gy.
p-values are in bold when p < 0.05.
Denotes statistically significant calculations at a biologically equivalent dose in 2 Gy fraction were performed.
Table 3.
Dose to elective nodal levels with volumetric-modulated arc therapy (VMAT) vs split-field intensity-modulated radiation therapy (SF-IMRT), specific to targets in the low neck. VMAT had improved coverage of CTV50 and PTV50, while PTV60 had better coverage of PTV60 but not CTV60
| Target structure | Parameter | VMAT (mean ± SEM) | SF-IMRT (mean ± SEM) | Number | p-values |
|---|---|---|---|---|---|
| CTV50 low–intermediate low neck (50 Gy/25 fraction equivalent) | V100 | 99.3 ± 0.2% | 91.5 ± 2.4% | 10 | 0.011 |
| PTV50 low–intermediate low neck (50 Gy/25 fraction equivalent) | V100 | 95.6 ± 0.8% | 86.0 ± 3.3% | 10 | 0.029 |
| PTV50 | CI | 0.990 ± 0.003 | 0.969 ± 0.011 | 10 | 0.003 |
| PTV50 | DHI | 21.9 ± 1.8 | 27.7 ± 2.7 | 10 | 0.148 |
| CTV60 high–intermediate low neck (60 Gy/30 fraction equivalent) | V100 | 98.5 ± 0.8% | 97.9 ± 1.3% | 3 | 0.304 |
| PTV60 high–intermediate low neck (60 Gy/30 fraction equivalent) | V100 | 94.6 ± 0.8% | 96.9 ± 1.4% | 3 | 0.029 |
| PTV60 | CI | 0.992 ± 0.002 | 0.995 ± 0.004 | 3 | 0.446 |
| PTV60 | DHI | 22.2 ± 1.5 | 19.4 ± 6.1 | 3 | 0.692 |
CI, conformality index; CTV, clinical target volumes; DHI, dose homogeneity index; PTV, planning target volumes; SEM, standard error of the mean; V100, volume receiving 100% of prescription dose.
(±) denotes standard error of the mean.
p-values are in bold when p < 0.05.
WF-VMAT plans had similar mean doses to the glottic larynx (20.1-Gy WF-VMAT vs 19.5-Gy SF-IMRT, p = 0.65), supraglottic larynx (39.6 Gy vs 40.9 Gy, p = 0.67), pharyngeal constrictors (46.6 Gy vs 46.9 Gy, p = 0.50) and maximum dose to the brachial plexus (64.2 Gy vs 67.3 Gy, p = 0.38; Table 1) compared with SF-IMRT. Coverage to the high–intermediate-risk region, targeted to a dose of 60 Gy in 30 fractions, was equivalent between VMAT and the AP/posterior–anterior fields to the LAN CTV60 (V100% 98.5% vs 97.9%, p = 0.30; Table 2), usually involving coverage of the lower portion of Level 3 when there was involvement of a Level 2 node.
OAR doses using a biologically equivalent dose of 2 Gy per fraction (Gy2) demonstrated results similar to the findings unadjusted for fraction size differences. Similar to the unadjusted results and using the α/β ratios based on Kehwar et al, there was no difference in the mean dose to the glottic larynx (15.3-Gy2 WF-VMAT vs 15.6-Gy2 SF-IMRT, p = 0.78) and supraglottic larynx (34.1-Gy2 WF-VMAT vs 35.4-Gy2 SF-IMRT, p = 0.70) between WF-VMAT and SF-IMRT, but there was a difference in the maximum arytenoid dose (18.7-Gy2 WF-VMAT vs 46.6-Gy2 SF-IMRT, p < 0.001) and mean oesophageal dose (18.3-Gy2 WF-VMAT vs 35.7-Gy2 SF-IMRT, p < 0.001), favouring whole-neck VMAT. In contrast to the raw dosimetric data in which there was no difference in the maximum brachial plexus dose between WF-VMAT and SF-IMRT, when BED was taken into account, there was a significantly lower maximum brachial plexus dose (62.1-Gy2 WF-VMAT vs 76.1-Gy2 SF-IMRT, p = 0.04), favouring whole-neck VMAT.
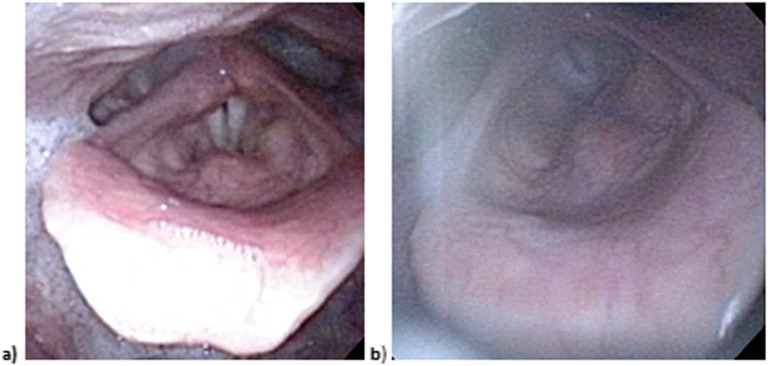
6 out of 10 patients treated with WF-VMAT had both pre-treatment and post-treatment pictures of the larynx taken. At the median 5-month follow-up, 1/6 patients had mild arytenoid oedema (maximum arytenoid dose 16 Gy) and 1/6 patients had mild epiglottic oedema (mean supraglottic larynx dose 48 Gy). Pictures from the laryngoscopy of a representative patient who underwent definitive WF-VMAT to the full neck show no discernible difference between the pre-treatment and post-treatment larynx (Figure 2).
Figure 2.
Photographs of the larynx (a) prior to treatment and (b) 4 months after treatment for a patient with Stage II nasopharyngeal cancer, demonstrating no significant oedema from treatment with a full-neck volumetric-modulated arc therapy field.
DISCUSSION
In our planning study, we find that the dose to most avoidance structures is equivalent between WF-VMAT and SF-IMRT. However, WF-VMAT has a significant advantage in terms of lower arytenoid dose as well as significantly lower dose to the oesophagus. At a biologically equivalent dose, we also find a lower maximum dose to the brachial plexus with WF-VMAT. We find that the nodal target at low–intermediate risk, typically treated to the equivalent dose of 50 Gy in 25 fractions, is insufficiently covered with standard SF-IMRT fields compared with WF-VMAT and that SF-IMRT has worse conformality in the LAN. For the nodal target at high–intermediate risk, which is typically treated to the equivalent dose of 60 Gy in 30 fractions, coverage to the target volumes in the LAN using SF-IMRT is superior for PTV60, but treatment with WF-VMAT still meets our planning objective and with equivalent CTV60 coverage.
The appropriate use of SF-IMRT vs whole-neck static-field IMRT treatment plans remains controversial. An advantage of SF-IMRT is that it is not as reliant on the discretion of the planner owing to the standardized technique. Advocates for whole-neck static-field IMRT have expressed concerns about dose inhomogeneities with SF-IMRT from matchline errors leading to potential underdosing or overdosing. There has been little clinical information on the risk of nodal recurrence with SF-IMRT. In a study from Fox Chase Cancer Center in which all patients with nodal disease >3 cm underwent planned neck dissection after definitive-intent radiation or chemoradiation, the subset of 37 patients receiving SF-IMRT did not show an increase in regional recurrence compared with the 54 patients who received WF-IMRT.7 An abstract from the Washington University in St. Louis containing 248 patients, 92 patients of whom were treated definitively and in which SF-IMRT was used in 97% of patients, demonstrates that 5/248 (2%) patients failed in the LAN and 2/248 (1%) patients failed at the matchline. This study concluded that a small but measureable risk of failure in the LAN merited the use of WF-IMRT.8 In a series of 69 patients from Stanford University Hospital with 46 patients treated with definitive-intent radiotherapy, 1 (1.4%) patient experienced failure at the junction of the LAN and upper neck IMRT fields. This patient was treated with an SF-IMRT field post-operatively for a tonsil cancer with N2b disease; none of the patients treated definitively experienced a junctional failure, although little information is given on the number of patients treated with WF-IMRT vs SF-IMRT. The authors concluded that they would not offer SF-IMRT in patients treated after neck dissection.9 Matchline issues when utilizing SF-IMRT have been further addressed with a dynamic match technique, which would decrease the chances of a hotspot or cold spot at the match between the upper IMRT and 3D lower neck fields.10
Prior studies have shown an advantage for SF-IMRT plans in allowing lower doses to the larynx than WF-IMRT plans. A planning study from MD Anderson Cancer Center demonstrated a mean glottic larynx dose of 18.7 Gy with SF-IMRT vs 47 Gy with WF-IMRT, with coverage to the prescription dose equivalent V100% of 76% with SF-IMRT vs 79% with WF-IMRT.11 Using a representative set of six patients, a planning study from Memorial Sloan Kettering Cancer Center showed that there was a significant decrease in the mean glottic larynx dose for oropharyngeal and nasopharyngeal cancers treated with SF-IMRT vs WF-IMRT. A planning study of 15 patients from the University of Alabama using a dynamic match showed improved dose to the larynx with SF-IMRT compared with WF-IMRT, with less dose to the constrictors but inferior elective PTV coverage for SF-IMRT.12 In a clinical study from the Peter McCallum Institute of 28 patients, 20 patients of whom were treated with WF-IMRT, the decreased pharyngo-oesophageal dose in the 8 patients treated with SF-IMRT (mean 27-Gy SF-IMRT vs 55-Gy WF-IMRT) correlated with a lower rate of long-term feeding tube dependence.13
When greater attention is given to reducing dose to the glottic larynx with WF-IMRT plans, there is less difference in larynx doses between WF-IMRT and SF-IMRT. A study from Christie Hospital showed a mean dose of 29 Gy with WF-IMRT vs 24 Gy with SF-IMRT.14 A study from the University of Florida demonstrated a mean larynx dose of 28 Gy with WF-IMRT vs 26 Gy with SF-IMRT.15 While the dose to the constrictors and glottic and supraglottic larynx has been correlated with the risk of aspiration as demonstrated on video fluoroscopy, dose to the oesophagus has not been correlated with the risk of aspiration but has been correlated with dysphagia16 and is not well reported in the literature. A study found that a higher oesophageal dose is highly correlated with worse scores on the eating domain on the 30-item Head and Neck Cancer Inventory (HNCI) quality of life survey.17 A lower mean dose to the oesophagus has also been correlated with lower rates of oesophageal stricture.18 Although we are not aware of studies that examined the effect of maximum dose to the arytenoids on swallowing and voice function, a cited planning objective to avoid swallowing dysfunction in oropharyngeal cancers has been avoidance of dose to the arytenoid cartilage and associated muscles owing to its role in glottic closure, supraglottic adduction and epiglottic inversion.19
This is the first study of which we are aware where a full-neck plan was overall largely equivalent to and in some ways dosimetrically superior to an SF-IMRT plan, while avoiding matchline uncertainties. The clinical significance of the decreased oesophageal and arytenoid dose with VMAT compared with SF-IMRT is unknown.
CONCLUSION
In this largely dosimetric study of patients considered ideal for SF-IMRT, we find that WF-VMAT affords equivalent dose to the glottic and supraglottic larynx and is superior in terms of dose to the arytenoids, oesophagus and possibly the brachial plexus. WF-VMAT also better covers most LAN targets. These findings suggest that for most patients with head and neck cancer requiring comprehensive elective nodal irradiation, whole-neck VMAT may be preferred in most cases over an SF-IMRT technique.
Contributor Information
Shibu J Anamalayil, Email: Shibu.Anamalayil@uphs.upenn.edu.
Boon-Keng K Teo, Email: Kevin.Teo@uphs.upenn.edu.
Alexander Lin, Email: Alexander.Lin2@uphs.upenn.edu.
Robert A Lustig, Email: Robert.Lustig@uphs.upenn.edu.
Peter H Ahn, Email: peter.ahn@uphs.upenn.edu.
REFERENCES
- 1.Nutting CM, Morden JP, Harrington KJ, Urbano TG, Bhide SA, Clark C, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol 2011; 12: 127–36. doi: 10.1016/S1470-2045(10)70290-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amdur RJ, Li JG, Liu C, Hinerman RW, Mendenhall WM. Unnecessary laryngeal irradiation in the IMRT era. Head Neck 2004; 26: 257–63. doi: 10.1002/hed.10379 [DOI] [PubMed] [Google Scholar]
- 3.Verbakel WF, Cuijpers JP, Hoffmans D, Bieker M, Slotman BJ, Senan S. Volumetric intensity-modulated arc therapy vs conventional IMRT in head-and-neck cancer: a comparative planning and dosimetric study. Int J Radiat Oncol Biol Phys 2009; 74: 252–9. doi: 10.1016/j.ijrobp.2008.12.033 [DOI] [PubMed] [Google Scholar]
- 4.Vanetti E, Clivio A, Nicolini G, Fogliata A, Ghosh-Laskar S, Agarwal JP, et al. Volumetric modulated arc radiotherapy for carcinomas of the oro-pharynx, hypo-pharynx and larynx: a treatment planning comparison with fixed field IMRT. Radiother Oncol 2009; 92: 111–7. doi: 10.1016/j.radonc.2008.12.008 [DOI] [PubMed] [Google Scholar]
- 5.Grégoire V, Ang K, Budach W, Grau C, Hamoir M, Langendijk JA, et al. Delineation of the neck node levels for head and neck tumors: a 2013 update. DAHANCA, EORTC, HKNPCSG, NCIC CTG, NCRI, RTOG, TROG consensus guidelines. Radiother Oncol 2014; 110: 172–81. doi: 10.1016/j.radonc.2013.10.010 [DOI] [PubMed] [Google Scholar]
- 6.Kehwar T, Sharma S. Use of normal tissue tolerance doses into linear quadratic equation to estimate normal tissue complication probability. Radiat Oncol Online J 2003. Available from: http://www.rooj.com/Radiation%20 Tissue%20 Tolerance.htm [DOI] [PubMed] [Google Scholar]
- 7.Turaka A, Li T, Nicolaou N, Lango MN, Burtness B, Horwitz EM, et al. Use of a conventional low neck field (LNF) and intensity-modulated radiotherapy (IMRT): No clinical detriment of IMRT to an anterior LNF during the treatment of head-and neck-cancer. Int J Radiat Oncol Biol Phys 2011; 79: 65–70. doi: 10.1016/j.ijrobp.2009.10.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thorstad W, Hong S, Hope A, Lindsay P, Haughey B, Deasey J, et al. Patterns of failure in patients receiving intensity modulated radiation therapy (IMRT) for head and neck cancer. Int J Radiat Oncol Biol Phys 2005; 63: S74. [Google Scholar]
- 9.Daly ME, Lieskovsky Y, Pawlicki T, Yau J, Pinto H, Kaplan M, et al. Evaluation of patterns of failure and subjective salivary function in patients treated with intensity modulated radiotherapy for head and neck squamous cell carcinoma. Head Neck 2007; 29: 211–20. doi: 10.1002/hed.20505 [DOI] [PubMed] [Google Scholar]
- 10.Duan J, Shen S, Spencer SA, Ahmed RS, Popple RA, Ye SJ, et al. A dynamic supraclavicular field-matching technique for head-and-neck cancer patients treated with IMRT. Int J Radiat Oncol Biol Phys 2004; 60: 959–72. doi: 10.1016/S0360-3016(04)01283-0 [DOI] [PubMed] [Google Scholar]
- 11.Dabaja B, Salehpour MR, Rosen I, Tung S, Morrison WH, Ang KK, et al. Intensity-modulated radiation therapy (IMRT) of cancers of the head and neck: comparison of split-field and whole-field techniques. Int J Radiat Oncol Biol Phys 2005; 63: 1000–5. doi: 10.1016/j.ijrobp.2005.03.069 [DOI] [PubMed] [Google Scholar]
- 12.Caudell JJ, Burnett OL, 3rd, Schaner PE, Bonner JA, Duan J. Comparison of methods to reduce dose to swallowing-related structures in head and neck cancer. Int J Radiat Oncol Biol Phys 2010; 77: 462–7. doi: 10.1016/j.ijrobp.2009.05.020 [DOI] [PubMed] [Google Scholar]
- 13.Fua TF, Corry J, Milner AD, Cramb J, Walsham SF, Peters LJ. Intensity-modulated radiotherapy for nasopharyngeal carcinoma: clinical correlation of dose to the pharyngo-esophageal axis and dysphagia. Int J Radiat Oncol Biol Phys 2007; 67: 976–81. doi: 10.1016/j.ijrobp.2006.10.028 [DOI] [PubMed] [Google Scholar]
- 14.Webster GJ, Rowbottom CG, Ho KF, Slevin NJ, Mackay RI. Evaluation of larynx-sparing techniques with IMRT when treating the head and neck. Int J Radiat Oncol Biol Phys 2008; 72: 617–22. doi: 10.1016/j.ijrobp.2008.06.1495 [DOI] [PubMed] [Google Scholar]
- 15.Galloway TJ, Amdur RJ, Liu C, Yeung AR, Mendenhall WM. Revisiting unnecessary larynx irradiation with whole-neck IMRT. Pract Radiat Oncol 2011; 1: 27–32. doi: 10.1016/j.prro.2010.09.002 [DOI] [PubMed] [Google Scholar]
- 16.Feng FY, Kim HM, Lyden TH, Haxer MJ, Feng M, Worden FP, et al. Intensity-modulated radiotherapy of head and neck cancer aiming to reduce dysphagia: early dose—effect relationships for the swallowing structures. Int J Radiat Oncol Biol Phys 2007; 68: 1289–98. doi: 10.1016/j.ijrobp.2007.02.049 [DOI] [PubMed] [Google Scholar]
- 17.Dornfeld K, Simmons JR, Karnell L, Karnell M, Funk G, Yao M, et al. Radiation doses to structures within and adjacent to the larynx are correlated with long-term diet- and speech-related quality of life. Int J Radiat Oncol Biol Phys 2007; 68: 750–7. doi: 10.1016/j.ijrobp.2007.01.047 [DOI] [PubMed] [Google Scholar]
- 18.Laurell G, Kraepelien T, Mavroidis P, Lind BK, Fernberg JO, Beckman M, et al. Stricture of the proximal esophagus in head and neck carcinoma patients after radiotherapy. Cancer 2003; 97: 1693–700. doi: 10.1002/cncr.11236 [DOI] [PubMed] [Google Scholar]
- 19.Eisbruch A, Schwartz M, Rasch C, Vineberg K, Damen E, Van As CJ, et al. Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer: which anatomic structures are affected and can they be spared by IMRT? Int J Radiat Oncol Biol Phys 2004; 60: 1425–39. doi: 10.1016/j.ijrobp.2004.05.050 [DOI] [PubMed] [Google Scholar]