Abstract
Objective:
We report the imaging outcomes of all pregnant patients referred for suspected thromboembolism over a 43-month period.
Methods:
We identified 168 patients who underwent ventilation/perfusion (VQ) single-photon emission CT (SPECT), CT pulmonary angiography (CTPA) or a Doppler ultrasound scan of the lower legs, as well as a control group of 89 non-pregnant age- and sex-matched patients who underwent VQ SPECT during the same period. Imaging outcomes were recorded, and radiation doses were calculated for individual patients.
Results:
VQ SPECT and CTPA were equally likely to diagnose pulmonary embolism (PE) in about one patient out of every seven patients investigated. One in three CTPA scans was of suboptimal quality. A Doppler ultrasound examination of the legs will find deep venous thrombosis much less often, in about 1 patient out of every 15 patients investigated. The prevalence of PE in pregnant patients (as diagnosed by VQ SPECT) was similar to that in the non-pregnant, age- and sex-matched control group. The effective dose and the absorbed radiation dose to the maternal breast were lower with VQ SPECT. The foetal dose is comparable for both VQ SPECT and CTPA.
Conclusion:
VQ SPECT and CTPA provide a similar diagnostic yield for diagnosing PE during pregnancy, but VQ SPECT does so with a lower radiation dose to the mother (effective dose and breast dose).
Advances in knowledge:
Ours is the first report of the diagnostic performance of VQ SPECT, rather than planar VQ scans, in pregnancy in a routine clinical setting.
INTRODUCTION
Pulmonary embolism (PE) is a potentially life-threatening condition that continues to pose a diagnostic challenge.1,2 The incidence of PE in pregnancy is about fivefold higher than in non-pregnant females of a similar age, and PE remains the leading non-obstetric cause of death during pregnancy in developed countries.3 There is approximately 1 PE per 1000 pregnancies and 3 times as many deep venous thromboses (DVTs),4 with the incidence being similar in all 3 trimesters.5 PE may be identified or excluded by several diagnostic and clinical tests. These include clinical scores (modified Wells' score6), plasma d-dimer measurement, CT pulmonary angiography (CTPA) and ventilation/perfusion (VQ) scanning,7–9 although the diagnostic performance of some of these, e.g. plasma d-dimer10 and CTPA,11–15 is impaired during pregnancy.
Imaging of the three-dimensional distribution of a radiopharmaceutical in myocardial, bone and oncological imaging by single-photon emission CT (SPECT) is now well established and has been used for lung imaging for some time.16 The European Association of Nuclear Medicine (EANM) have recently produced guidelines17,18 which have led to a more extensive interest in its use. Whilst there have been considerable studies on the diagnostic performance of “traditional” planar VQ scans, most commonly by the Prospective Investigation of Pulmonary Embolism Diagnosis (PIOPED) trials and their subsequent reanalyses,19–21 available data for VQ SPECT are inhomogeneous in terms of the ventilation tracer used and the reporting criteria. VQ SPECT was introduced into routine clinical practice in November 2009, and we now present a cohort of all pregnant patients who presented with suspected PE or DVT from November 2009 to May 2013.
METHODS AND MATERIALS
Clinical pathway
Pregnant patients with suspected venous thromboembolism (VTE) will have always been assessed by a physician before being referred for any imaging test. If imaging is considered necessary, a Doppler ultrasound examination of the lower legs is performed first, as this is not associated with a radiation exposure. If a DVT is found, treatment is commenced, and no further imaging is undertaken, on the assumption that any chest symptoms are related to PE. Otherwise, a history of significant lung or cardiac disease, a chest X-ray and renal function are taken into account, when deciding whether to refer the patient for a perfusion SPECT or CTPA to assess for PE. VQ referrals are accepted between 9 am and 5 pm on weekdays for a same-day scan; overnight and at weekends, the referrer can either request CTPA or anticoagulate the patients until their perfusion SPECT takes place. If perfusion defects are found, the patient is recalled for ventilation SPECT, to assess for mismatch the next day.
Patients
All patients who underwent perfusion SPECT, CTPA or Doppler ultrasound of the lower legs between November 2009 and May 2013 were identified from our radiology database. We then discarded reports not containing the words “pregnant” or “pregnancy”. Reports not corresponding to a first presentation with suspected VTE were also excluded. This left 168 patients available for analysis with a mean age of 28 ± 6 years (range 17–43 years). 16 (10%) patients were in the first trimester of pregnancy, 45 (27%) patients in the second and 99 (59%) patients in the third trimester. Gestational age was not recorded for eight patients. A control group consisted of 89 non-pregnant patients who underwent VQ SPECT during the same period, matched for age and sex to the subgroup of 89 pregnant patients who had perfusion SPECT. Ethics permission was not sought for this retrospective analysis.
Single-photon emission CT
For the perfusion SPECT, 100 MBq of 99mTc-macroaggregated albumin (MAA) (Covidien, Dublin, Ireland) was injected intravenously. 99mTc-diethylenetriamine-pentaacetic acid (Covidien, Dublin, Ireland) aerosol was used as a ventilation tracer for the first seven patients included in this report until mid-August 2010. The aerosol was produced using a SmartVent™ system (Diagnostic Imaging Ltd, Welford, UK) with a claimed mass median aerodynamic diameter of 1–1.5 µm. From mid-August 2010, the ventilation tracer 99mTc-Technegas (Cyclomedica Europe Ltd, Dublin, Ireland) was used. Both delivery systems were used according to manufacturer's instructions, with a typical administered activity of 30–40 MBq. The Millenium VG double-head gamma camera (GE Healthcare, Chalfont St Giles, UK) was most commonly used for scanning, but occasionally a Siemens Symbia® (Siemens Medical Systems, Erlangen, Germany) or GE Infinia camera (GE Healthcare) were used. All cameras were equipped with low-energy high-resolution collimators and were set up with an identical acquisition protocol, which was as follows: energy window 140 ± 10% keV, matrix 128 × 128, 360° rotation in 6° steps, acquisition time 24 s/step (100-MBq perfusion scan) or 12 s/step (200 MBq), 35 s/step (ventilation) and zoom 1.28×. Acquisition times were 13.5 min for the perfusion and 18.5 min for the ventilation SPECT, including the time needed for detector rotation in between steps. Image reconstruction was performed using ReSPECT 2.5 (Scivis GmbH, Göttingen, Germany). This uses an iterative algorithm with scatter correction, resolution recovery and six iterations with a varying number of subsets per iteration. Occasionally, realignment of reconstructed ventilation and perfusion studies was required. Corresponding sagittal, coronal and transverse slices were displayed using Hybrid Viewer™ (Hermes Medical Solutions AB, Stockholm, Sweden). Reporting criteria were those first proposed by Bajc et al22 and subsequently incorporated into EANM guidelines.17,18
CT pulmonary angiography
Images were acquired using a Lightspeed® VCT XTe CT scanner (GE Healthcare, Little Chalfont, Buckinghamshire, UK). For each scan, a pulmonary angiogram protocol was used, with automatic exposure-controlled milliampere (smart milliampere) varying in the head-to-foot plane. Scans were undertaken at either 120 kVp (until late 2012) or 100 kVp (from late 2012 onwards), with a helical pitch of 0.98 : 1 and 20-mm beam collimation. Comments on image quality by the original reporter (general radiologists) were extracted from the report. All studies were reanalysed by an experienced thoracic radiologist (RDR), including the measurement of pulmonary arterial opacification. A study was judged as having suboptimal contrast opacification, if <211 HU was achieved in the pulmonary trunk.23
Single-photon emission CT dosimetry
Effective doses from exposure to radiopharmaceuticals were calculated using OLINDA.24 The gestational age was taken into account for this. Biokinetic data were taken from the International Commission of Radiological Protection publication 80.25
CT dosimetry
Foetal doses were calculated utilizing the methodology presented by Winer-Muram et al,26 which requires knowledge of the xiphoid-to-foetus distance (Equation (1)). This was not known, as for CTPA examinations, the scan length falls short of the uterus. Instead, the xiphoid-to-foetus distance was estimated from the linear relationship found by Winer-Muram et al with gestational age. Whilst this introduces further uncertainty in our calculation, it better models foetal dose than a method that assumes the same volume and position as a non-pregnant model of the uterus (e.g. the ImPACT calculator). The data acquired were used alongside the following equation which links the mean foetal dose with the xiphoid-to-foetus distance per 100 mAs for transverse scans:
| (1) |
Winer-Muram et al modelled the mathematical phantom (mother) used in the Monte Carlo simulation as a water-equivalent ellipsoid with a mean eccentricity of 0.68 at the level of the xiphoid process. The foetus was modelled by a water-equivalent cylinder, dimensions and the xiphoid–foetus distance determined by ultrasound measurements for all of the 23 pregnant patients at ultrasound-determined gestational age.
For our estimation of the absorbed dose to the breast and maternal effective dose, peak kilovoltage, scan range, average tube current per rotation, rotation time, pitch and beam collimation were taken from the digital imaging and communications in medicine header for each patient. These factors were entered into the ImPACT Monte Carlo CT Dosimetry Calculator (v. 1.0 2009), to estimate the dose (milligray) to the breast as well as the maternal effective dose (millisievert). Lifetime risk estimates for breast cancer incidence and mortality resulting from an absorbed dose of 0.1 Gy to the female breast are available in biological effects of ionising radiation (BEIR) VII Phase 2 report.27 These factors were either directly used or linearly interpolated depending on patient age and scaled accordingly to the equivalent breast doses found using the ImPACT CT Dosimetry calculator. Results are given as a risk of breast cancer incidence per 100,000 persons.
Statistical analysis
This was performed with Prism® 6 (GraphPad Software Inc., La Jolla, CA).
RESULTS
Imaging pathway
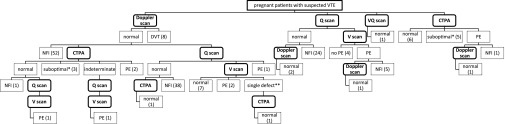
The imaging pathway actually taken by our 168 patients (as opposed to the agreed clinical pathway described in the first paragraph of the Methods and Materials section above) is shown in Figure 1. In summary, 60 (36%) patients had a Doppler scan as their only investigation (51 of those had leg symptoms), even though a DVT was identified in only 8 of those patients. In 62 (37%) patients (only 12 of whom had leg symptoms), a normal Doppler scan was followed by SPECT (n = 51), CTPA (n = 7) or both (n = 4). 46 (27%) patients did not have a Doppler scan prior to imaging with ionizing radiation, but had SPECT (n = 34) or CTPA (n = 12) as their only investigation. In the SPECT group, 67 (75%) patients had a perfusion-only scan using 100 MBq of 99mTc-MAA, 21 (24%) patients additionally required a ventilation scan on the next working day and 1 patient had VQ SPECT on the same day using 200 MBq of 99mTc-MAA. Findings on the chest X-ray were mostly in keeping with the intended imaging algorithm, with the following exceptions: in the SPECT group, there were three abnormal radiographs (showing consolidation) and one patient did not have a chest X-ray; in the CTPA group, three patients had a normal chest X-ray and presented within normal working hours; three patients did not have a chest X-ray.
Figure 1.
Imaging pathway taken by 168 patients investigated for suspected venous thromboembolism (VTE). CTPA, CT pulmonary angiography; DVT, deep venous thrombosis; NFI, no further imaging performed; PE, pulmonary embolism; Q scan, perfusion scan; V scan, ventilation scan; VQ scan, ventilation/perfusion scan; * Suboptimal contrast opacification, but no evidence of central PE; ** singular subsegmental mismatched perfusion defect.
Imaging outcomes
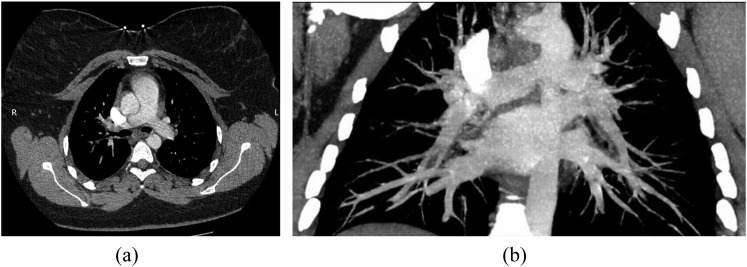
Abnormal findings were seen in 7% of Doppler scans, 12% of SPECT and 17% of CTPA (Table 1); the difference between SPECT and CTPA was not significant (Fisher's test). Imaging outcomes are summarized in Table 1. Comparison with an age- and sex-matched control group of 89 patients who were not pregnant shows no significant difference in the prevalence of PE between pregnant and non-pregnant patients (χ2 test). Eight (35%) CTPA scans were graded as suboptimal by the original reporter. On review, two patients clearly had suboptimal pulmonary arterial enhancement (165 and 181 HU, respectively) (Figure 2), and a further two scans were borderline (210 HU). Of note, all of these four scans were performed at 120 kVp, as were six of eight scans graded as suboptimal by the original reporter.
Table 1.
Imaging outcomes
| Diagnostic test | No PE or DVT | No central PEa | PE or DVT | 1 pointb | Non-diagnostic |
|---|---|---|---|---|---|
| SPECT (n = 89) | 77 | – | 11 | 1 | – |
| CTPA (n = 23) | 11 | 7 | 4 | 1 | |
| Doppler (n = 122) | 114 | – | 8 | – | |
| SPECT (control group, n = 89) | 75 | – | 11 | 3 | – |
CTPA, CT pulmonary angiography; DVT, deep venous thrombosis; PE, pulmonary embolism; SPECT, single-photon emission CT.
Suboptimal contrast enhancement of subsegmental pulmonary vessels.
Singular subsegmental mismatched perfusion defect.
Figure 2.
A 28-year-old female who was 11 weeks pregnant presented with a sudden onset of right-sided pleuritic chest pain and shortness of breath. She had a history of pulmonary embolism (PE) 6 years ago, but ventilation/perfusion scans 18 and 8 months ago were normal (not shown). She had a normal chest X-ray (not shown). Suboptimal contrast opacification was achieved at CT pulmonary angiography (165 HU in the main pulmonary artery), but no PE was seen. (a) Transverse view; (b) maximum intensity projection.
Comparison between VQ SPECT and CTPA
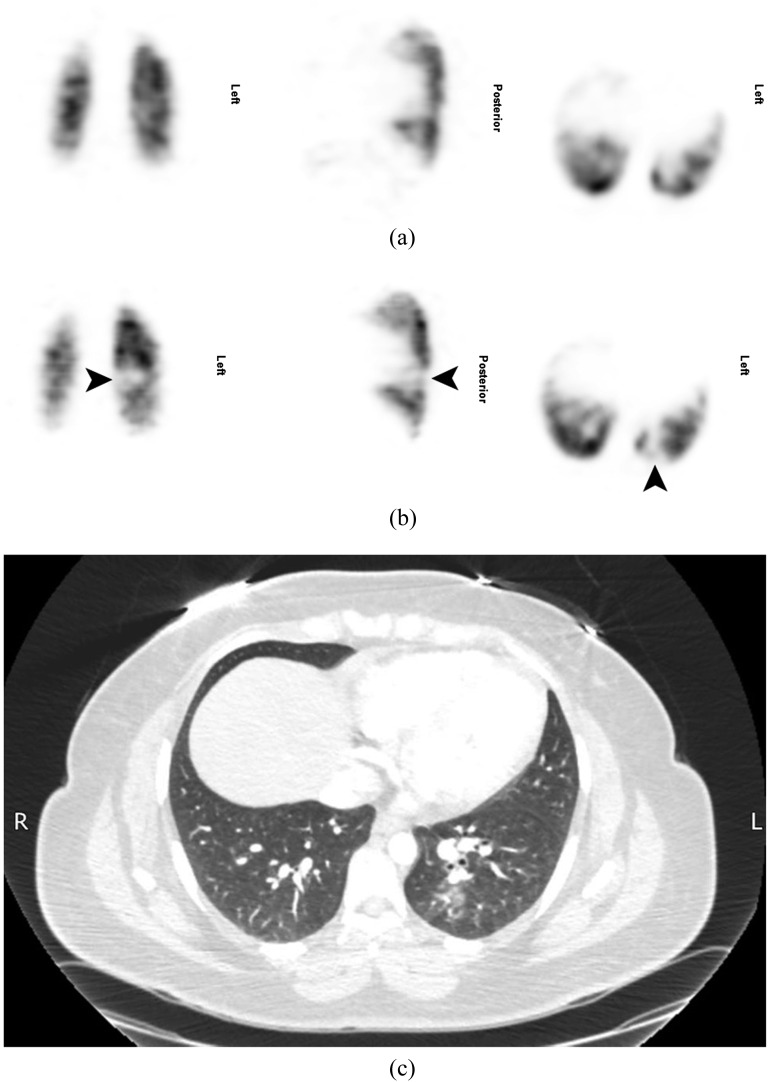
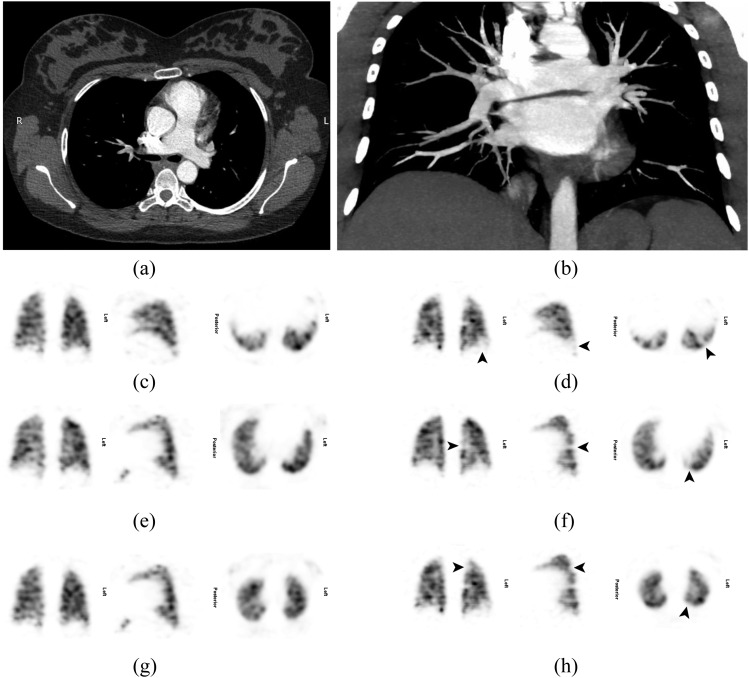
Only four patients had CTPA within 3 days of their SPECT. CTPA did not demonstrate PE in one patient with normal SPECT, one patient in whom SPECT had shown a singular subsegmental mismatched perfusion defect (Figure 3) and one patient in whom SPECT had shown PE (Figure 4). CTPA was indeterminate in one patient in whom SPECT had shown PE. Significant incidental findings (atelectasis, infection and axillary nodes in a patient with a history of breast cancer) not seen on the chest radiograph were noted in three (13%) CTPA scans. Dosimetric data for SPECT and CTPA are given in Table 2.
Figure 3.
A 30-year-old female who was 15 weeks pregnant presented with a sudden onset of pleuritic chest and back pain, a cough and small amounts of haemoptysis. She had a normal chest X-ray at presentation and a normal Doppler scan of the lower legs 3 days later (not shown). A lung perfusion single-photon emission CT (SPECT) was performed the same day (b), and the patient was recalled for a ventilation SPECT the following day (a). This showed a singular subsegmental mismatched perfusion defect at the left base (arrows) and the scan was reported as indeterminate. The same day, CT pulmonary angiography (c) achieved good contrast opacification (378 HU in the main pulmonary artery), but no pulmonary embolism was seen, although there was ill-defined nodularity and patchy ground-glass opacification in the superior and posterior segments of the left lower lobe in keeping with infection.
Figure 4.
A 26-year-old female who was 38 weeks pregnant presented with a 1-day history of right upper pleuritic back pain and some shortness of breath. She had a normal chest X-ray at presentation and a normal Doppler scan of the lower legs on the following day (not shown). The patient received anticoagulation with clexane; a lung perfusion single-photon emission CT (SPECT) was performed another day later (c, e, g, ventilation images; d, f, h, perfusion images), and she was recalled for a ventilation SPECT the following day. This showed two or possibly three subsegmental mismatched perfusion defects in the left lung (arrows) (a, transverse view; b, maximum intensity projection) in keeping with pulmonary embolism (PE). CT pulmonary angiography another 2 days later achieved good contrast opacification (375 HU in the main pulmonary artery), but no PE was seen.
Table 2.
Average (minimum–maximum) radiation doses associated with single-photon emission CT (SPECT) and CT pulmonary angiography (CTPA)
| Dose | SPECT (n = 89) |
CTPA (n = 23) | ||
|---|---|---|---|---|
| Q (n = 89) | V (n = 13) | Q + V (n = 89) | ||
| Maternal effective dose (mSv) | 1.4 (0.7–2.8) | 0.82 | 1.6 (0.70–3.6) | 7.8 (2–18) |
| Maternal breast dosea (mSv) | 0.49 (0.24–1.0) | 0.29 | 0.56 (0.24–1.3) | 20 (4–50) |
| Foetal dosea (µSv) | 71 (33–130) | 22 | 77 (33–150) | 110 (3.7–380) |
Q, perfusion; V, ventilation.
Equivalent dose.
DISCUSSION
Diagnostic performance
Only a small proportion of compression ultrasound studies are positive; so, most patients will have to undergo further testing.3 In recognition of this, the 2011 guidelines of the American Thoracic Society and the Society of Thoracic Radiology13 recommend bilateral venous compression ultrasound of the lower extremities only in females presenting with signs and symptoms of DVT, but recommend to proceed to imaging of the chest in all other females.
There are a number of obstacles relating to physiological changes during pregnancy which must be faced, in order to obtain a good-quality CTPA study. An increase in blood volume and cardiac output will shorten the arrival time of i.v. contrast in the pulmonary vessels, necessitating adjustments in triggered scan delays.14,28 Transient influx of unopacified blood from the inferior vena cava has also been identified as a cause for poor-quality CTPA scans during pregnancy.3,14 Consequently, the American Thoracic Society/Society of Thoracic Radiology guidelines recommend using CTPA only in females with no signs or symptoms of DVT and an abnormal chest X-ray.13
Results of previously published studies looking at imaging of VTE during pregnancy are summarized and compared with our study in Table 3. Shahir et al29 and Revel et al30 conclude equal diagnostic performance, although it has subsequently been questioned whether the methodology of the study by Shahir et al was sufficient to reach this conclusion.31 Ridge et al14 found perfusion scans more reliable.
Table 3.
Key characteristics of previous studies on imaging venous thromboembolism in pregnancy (in order of publication date)
| Study | CTPA |
VQ scan |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Diagnosis |
Alternative diagnosesa (%) | n | Diagnosis |
|||||
| PE (%) | No PE (%) | Indeterminate (%) | PE (%) | No PE (%) | Indeterminate (%) | ||||
| Ridge et al (2009)14 | 28 | 0 | 64 | 36 | 3.6 | 25 | 8 | 88 | 4 |
| Shahir et al (2010)29,b | 106 | 3.8 | 91 | 5.7 | 2.5 | 98 | 0 | 98 | 2 |
| Revel et al (2011)30,c | 43 | 16 | 65 | 19 | 12 | 91 | 11 | 70 | 19 |
| This studyd | 23 | 17 | 48 | 35 | 13 | 89 | 12 | 87 | 1 |
CTPA, CT pulmonary angiography; PE, pulmonary embolism; VQ, ventilation/perfusion.
Significant findings not seen on chest radiograph.
CT diagnostic quality: 76% good, 18% acceptable, 5.7% non-diagnostic.
CT enhancement: 50% optimal, 26% suboptimal, 24% poor; 4.7% respiratory artefacts.
35% suboptimal enhancement of subsegmental vessels.
Ridge et al14 found that 36% of CTPA scans in their pregnant group (n = 28) were non-diagnostic, which was significantly higher than 2.1% seen in a non-pregnant control group (n = 1420). U-King-Im et al15 report similar results, with 29% inadequate opacification in 264 CTPA scans during pregnancy, compared with 13% inadequate opacification in 122 scans in a non-pregnant control group. Similarly, Cahill et al12 found CTPA scans (17% of 108 scans) to be non-diagnostic significantly more often than VQ scans (13% of 196 scans). Bourjeily et al11 reported that 20% of 340 CTPA scans were of technically limited quality and 0.9% scans were non-diagnostic.
Dosimetry
The average maternal effective and breast doses were higher with CTPA than with VQ SPECT, whereas the foetal dose was comparable. Revel et al30 obtained a mean effective dose of 7.3 mSv with a 64-slice CT scanner. Ridge et al28 were able to decrease the mean effective dose from 5.6 to 4.8 mSv, by using a pregnancy-adapted imaging protocol. The use of effective dose for assessing the exposure of patients has severe limitations that must be considered when quantifying medical exposure. The use of the ImPACT CT Dosimetry calculator is based on a mathematical reference phantom and does not accurately model doses to individual patients. It is likely that organ and effective doses will be overestimated when applied to larger patients. Perisinakis et al32 describe the significance of body mass index and gestational age on effective dose. The relevant quantity for planning the exposure of patients and risk–benefit assessments is the equivalent dose or the absorbed dose to irradiated tissues.33 Readers may wish to refer to a general discussion on the typical errors associated with the application of effective dose to medical exposures,34 which reports a relative uncertainty of about ±40% for a reference patient and still higher for this study, where we have attempted to report the dose to individuals. Similar limitations apply to dose calculations for radiopharmaceuticals. With knowledge of such errors, we should be able to conclude that maternal effective doses are generally higher with CTPA, except for some smaller patients whose effective doses will be similar to VQ. When comparing the foetal dose for VQ and CTPA, with an understanding of the errors in each calculation, all we should conclude is that foetal doses are low and comparable (provided the primary CT radiation field does not directly irradiate the foetus). The use of a lead apron/shield to cover the abdomen during CTPA has been shown to moderately reduce the leakage and scattered radiation to the uterus from the CT collimators.33 We found that maternal breast dose is generally higher for CTPA than for VQ, but organ-based tube current modulation has been shown to reduce this.35 Further opportunities to reduce CTPA radiation doses include the use of statistical and model-based iterative reconstruction techniques,36 reducing the tube voltage, as we have already performed from 120 to 100 kVp,37 increasing the pitch to above 1 and decreasing the scan volume. Lowering the peak kilovoltage has the additional advantage of increasing the contrast attenuation within the pulmonary arteries, as the average X-ray energy approaches the k-edge of iodine (33 keV). This has the effect of maintaining the contrast-to-noise ratio, despite the increase in image noise consequent upon quantum mottle owing to reduced photon transmission.38,39 Scope for lowering the radiation dose from VQ SPECT is more limited, but options that could be explored include a more coarse matrix (64 × 64 as suggested in the EANM guidelines,18 as opposed to 128 × 128 as used in this study) and the use of medium-energy collimators with resolution-recovery software for SPECT reconstruction. Both of these options may allow a reduction in the administered activity.
KEY FINDINGS AND CONCLUSION
VQ SPECT and CTPA were equally likely to diagnose PE in about one patient out of every seven patients investigated.
A Doppler ultrasound examination of the legs will find DVT much less often, in about 1 patient out of every 15 investigated.
One in three CTPA scans was of suboptimal quality.
The likelihood of detecting PE by VQ SPECT in pregnant patients was similar to that in a non-pregnant, age- and sex-matched control group.
The effective dose and the radiation dose to the maternal breast were lower with VQ SPECT. The foetal dose is comparable for both VQ SPECT and CTPA.
We conclude that VQ SPECT and CTPA provide a similar diagnostic yield for diagnosing PE during pregnancy, but VQ SPECT does so in general with a lower radiation dose for the scanning protocols described.
FUNDING
This study was supported by the National Health Service.
Contributor Information
Thomas Grüning, Email: thomas.gruning@nhs.net.
Rebecca E Mingo, Email: rebecca.mingo@nhs.net.
Matthew G Gosling, Email: matthew.gosling@nhs.net.
Sally L Farrell, Email: sally.farrell@nhs.net.
Brent E Drake, Email: b.drake@nhs.net.
Robert J Loader, Email: robert.loader@nhs.net.
Richard D Riordan, Email: richardriordan@nhs.net.
REFERENCES
- 1.Goldhaber SZ. Pulmonary embolism. Lancet 2004; 363: 1295–305. doi: 10.1016/S0140-6736(04)16004-2 [DOI] [PubMed] [Google Scholar]
- 2.Thromboembolic disease in pregnancy and the puerperium: acute management. Green-top guideline no. 37b. London, UK: Royal College of Gynaecologists; 2015. [Google Scholar]
- 3.Cogley JR, Ghobrial PM, Chandrasekaran B, Allen SB. Pulmonary embolism evaluation in the pregnant patient: a review of current imaging approaches. Semin Ultrasound CT MR 2012; 33: 11–7. doi: 10.1053/j.sult.2011.09.001 [DOI] [PubMed] [Google Scholar]
- 4.Gray G, Nelson-Piercy C. Thromboembolic disorders in obstetrics. Best Pract Res Clin Obstet Gynaecol 2012; 26: 53–64. doi: 10.1016/j.bpobgyn.2011.10.003 [DOI] [PubMed] [Google Scholar]
- 5.Bourjeily G, Paidas M, Khalil H, Rosene-Montella K, Rodger M. Pulmonary embolism in pregnancy. Lancet 2010; 375: 500–12. doi: 10.1016/S0140-6736(09)60996-X [DOI] [PubMed] [Google Scholar]
- 6.Wells PS, Anderson DR, Rodger M, Ginsberg JS, Kearon C, Gent M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemost 2000; 83: 416–20. [PubMed] [Google Scholar]
- 7.Zöphel K, Bacher-Stier C, Pinkert J, Kropp J. Ventilation/perfusion lung scintigraphy: what is still needed? A review considering technetium-99m-labeled macro-aggregates of albumin. Ann Nucl Med 2009; 23: 1–16. doi: 10.1007/s12149-008-0187-3 [DOI] [PubMed] [Google Scholar]
- 8.Grüning T, Drake BE, Farrell SL, Nokes T. Three-year clinical experience with VQ SPECT for diagnosing pulmonary embolism: diagnostic performance. Clin Imaging 2014; 38: 831–5. doi: 10.1016/j.clinimag.2014.04.003 [DOI] [PubMed] [Google Scholar]
- 9.Grüning T, Khonsari M, Vivian GC, Nokes T. Can plasma D-dimer predict the result of a ventilation-perfusion scan? Clin Imaging 2010; 34: 179–84. doi: 10.1016/j.clinimag.2009.05.002 [DOI] [PubMed] [Google Scholar]
- 10.Sivandarajah S. Towards evidence-based emergency medicine: best BETs from the Manchester Royal Infirmary. BET 4: current evidence does not support the use of a negative D-dimer to rule out suspected pulmonary embolism in pregnancy. Emerg Med J 2011; 28: 245–6. doi: 10.1136/emj.2011.111617 [DOI] [PubMed] [Google Scholar]
- 11.Bourjeily G, Khalil H, Raker C, Martin S, Auger P, Chalhoub M, et al. Outcomes of negative multidetector computed tomography with pulmonary angiography in pregnant women suspected of pulmonary embolism. Lung 2012; 190: 105–11. doi: 10.1007/s00408-011-9329-9 [DOI] [PubMed] [Google Scholar]
- 12.Cahill AG, Stout MJ, Macones GA, Bhalla S. Diagnosing pulmonary embolism in pregnancy using computed-tomographic angiography or ventilation-perfusion. Obstet Gynecol 2009; 114: 124–9. doi: 10.1097/AOG.0b013e3181a99def [DOI] [PubMed] [Google Scholar]
- 13.Leung AN, Bull TM, Jaeschke R, Lockwood CJ, Boiselle PM, Hurwitz LM, et al. An official American Thoracic Society/Society of Thoracic Radiology clinical practice guideline: evaluation of suspected pulmonary embolism in pregnancy. Am J Respir Crit Care Med 2011; 184: 1200–8. doi: 10.1164/rccm.201108-1575ST [DOI] [PubMed] [Google Scholar]
- 14.Ridge CA, McDermott S, Freyne BJ, Brennan DJ, Collins CD, Skehan SJ. Pulmonary embolism in pregnancy: comparison of pulmonary CT angiography and lung scintigraphy. AJR Am J Roentgenol 2009; 193: 1223–7. doi: 10.2214/AJR.09.2360 [DOI] [PubMed] [Google Scholar]
- 15.U-King-Im JM, Freeman SJ, Boylan T, Cheow HK. Quality of CT pulmonary angiography for suspected pulmonary embolus in pregnancy. Eur Radiol 2008; 18: 2709–15. doi: 10.1007/s00330-008-1100-0 [DOI] [PubMed] [Google Scholar]
- 16.Corbus HF, Seitz JP, Larson RK, Stobbe DE, Wooten W, Sayre JW, et al. Diagnostic usefulness of lung SPET in pulmonary thromboembolism: an outcome study. Nucl Med Commun 1997; 18: 897–906. doi: 10.1097/00006231-199710000-00002 [DOI] [PubMed] [Google Scholar]
- 17.Bajc M, Neilly JB, Miniati M, Schuemichen C, Meignan M, Jonson B. EANM guidelines for ventilation/perfusion scintigraphy: Part 2. Algorithms and clinical considerations for diagnosis of pulmonary emboli with V/P(SPECT) and MDCT. Eur J Nucl Med Mol Imaging 2009; 36: 1528–38. doi: 10.1007/s00259-009-1169-y [DOI] [PubMed] [Google Scholar]
- 18.Bajc M, Neilly JB, Miniati M, Schuemichen C, Meignan M, Jonson B, et al. EANM guidelines for ventilation/perfusion scintigraphy : Part 1. Pulmonary imaging with ventilation/perfusion single photon emission tomography. Eur J Nucl Med Mol Imaging 2009; 36: 1356–70. doi: 10.1007/s00259-009-1170-5 [DOI] [PubMed] [Google Scholar]
- 19.Gottschalk A, Sostman HD, Coleman RE, Juni JE, Thrall J, McKusick KA, et al. Ventilation-perfusion scintigraphy in the PIOPED study. Part II. Evaluation of the scintigraphic criteria and interpretations. J Nucl Med 1993; 34: 1119–26. [PubMed] [Google Scholar]
- 20.Gray HW, Bessent RG. Pulmonary embolism exclusion: a practical approach to low probability using the PIOPED data. Prospective Investigation of Pulmonary Embolism Diagnosis. Eur J Nucl Med 1998; 25: 271–6. doi: 10.1007/s002590050228 [DOI] [PubMed] [Google Scholar]
- 21.Stein PD, Relyea B, Gottschalk A. Evaluation of individual criteria for low probability interpretation of ventilation-perfusion lung scans. J Nucl Med 1996; 37: 577–81. [PubMed] [Google Scholar]
- 22.Bajc M, Olsson CG, Olsson B, Palmer J, Jonson B. Diagnostic evaluation of planar and tomographic ventilation/perfusion lung images in patients with suspected pulmonary emboli. Clin Physiol Funct Imaging 2004; 24: 249–56. doi: 10.1111/j.1475-097X.2004.00546.x [DOI] [PubMed] [Google Scholar]
- 23.Wittram C. How I do it: CT pulmonary angiography. AJR Am J Roentgenol 2007; 188: 1255–61. doi: 10.2214/AJR.06.1104 [DOI] [PubMed] [Google Scholar]
- 24.Stabin MG, Sparks RB, Crowe E. OLINDA/EXM: the second-generation personal computer software for internal dose assessment in nuclear medicine. J Nucl Med 2005; 46: 1023–7. [PubMed] [Google Scholar]
- 25.Radiation dose to patients from radiopharmaceuticals (addendum 2 to ICRP publication 53). Ann ICRP 1998; 28: 1–126. [DOI] [PubMed] [Google Scholar]
- 26.Winer-Muram HT, Boone JM, Brown HL, Jennings SG, Mabie WC, Lombardo GT. Pulmonary embolism in pregnant patients: fetal radiation dose with helical CT. Radiology 2002; 224: 487–92. doi: 10.1148/radiol.2242011581 [DOI] [PubMed] [Google Scholar]
- 27.National Research Council (U.S.). Committee to assess health risks from exposure to low level of ionizing radiation. Health risks from exposure to low levels of ionizing radiation: BEIR VII Phase 2. Washington, D.C.: National Academies Press; 2006. [PubMed] [Google Scholar]
- 28.Ridge CA, Mhuircheartaigh JN, Dodd JD, Skehan SJ. Pulmonary CT angiography protocol adapted to the hemodynamic effects of pregnancy. AJR Am J Roentgenol 2011; 197: 1058–63. doi: 10.2214/AJR.10.5385 [DOI] [PubMed] [Google Scholar]
- 29.Shahir K, Goodman LR, Tali A, Thorsen KM, Hellman RS. Pulmonary embolism in pregnancy: CT pulmonary angiography versus perfusion scanning. AJR Am J Roentgenol 2010; 195: W214–20. doi: 10.2214/AJR.09.3506 [DOI] [PubMed] [Google Scholar]
- 30.Revel MP, Cohen S, Sanchez O, Collignon MA, Thiam R, Redheuil A, et al. Pulmonary embolism during pregnancy: diagnosis with lung scintigraphy or CT angiography? Radiology 2011; 258: 590–8. doi: 10.1148/radiol.10100986 [DOI] [PubMed] [Google Scholar]
- 31.Topatan B, Basaran A. Pulmonary CT angiography versus ventilation-perfusion scintigraphy during pregnancy: enigma continues? AJR Am J Roentgenol 2011; 196: W666.; author reply W667. doi: 10.2214/AJR.10.5832 [DOI] [PubMed] [Google Scholar]
- 32.Perisinakis K, Seimenis I, Tzedakis A, Damilakis J. Perfusion scintigraphy versus 256-slice CT angiography in pregnant patients suspected of pulmonary embolism: comparison of radiation risks. J Nucl Med 2014; 55: 1273–80. doi: 10.2967/jnumed.114.137968 [DOI] [PubMed] [Google Scholar]
- 33.Kennedy EV, Iball GR, Brettle DS. Investigation into the effects of lead shielding for fetal dose reduction in CT pulmonary angiography. Br J Radiol 2007; 80: 631–8. doi: 10.1259/bjr/31771954 [DOI] [PubMed] [Google Scholar]
- 34.Martin CJ. Effective dose: how should it be applied to medical exposures? Br J Radiol 2007; 80: 639–47. doi: 10.1259/bjr/25922439 [DOI] [PubMed] [Google Scholar]
- 35.Lungren MP, Yoshizumi TT, Brady SM, Toncheva G, Anderson-Evans C, Lowry C, et al. Radiation dose estimations to the thorax using organ-based dose modulation. AJR Am J Roentgenol 2012; 199: W65–73. doi: 10.2214/AJR.11.7798 [DOI] [PubMed] [Google Scholar]
- 36.Montet X, Hachulla AL, Neroladaki A, Lador F, Rochat T, Botsikas D, et al. Image quality of low mA CT pulmonary angiography reconstructed with model based iterative reconstruction versus standard CT pulmonary angiography reconstructed with filtered back projection: an equivalency trial. Eur Radiol 2015; 25: 1665–71. doi: 10.1007/s00330-014-3563-5 [DOI] [PubMed] [Google Scholar]
- 37.Matsuoka S, Hunsaker AR, Gill RR, Oliva IB, Trotman-Dickenson B, Jacobson FL, et al. Vascular enhancement and image quality of MDCT pulmonary angiography in 400 cases: comparison of standard and low kilovoltage settings. AJR Am J Roentgenol 2009; 192: 1651–6. doi: 10.2214/AJR.08.1730 [DOI] [PubMed] [Google Scholar]
- 38.Heyer CM, Mohr PS, Lemburg SP, Peters SA, Nicolas V. Image quality and radiation exposure at pulmonary CT angiography with 100- or 120-kVp protocol: prospective randomized study. Radiology 2007; 245: 577–83. doi: 10.1148/radiol.2452061919 [DOI] [PubMed] [Google Scholar]
- 39.Szucs-Farkas Z, Schibler F, Cullmann J, Torrente JC, Patak MA, Raible S, et al. Diagnostic accuracy of pulmonary CT angiography at low tube voltage: intraindividual comparison of a normal-dose protocol at 120 kVp and a low-dose protocol at 80 kVp using reduced amount of contrast medium in a simulation study. AJR Am J Roentgenol 2011; 197: W852–9. doi: 10.2214/AJR.11.6750 [DOI] [PubMed] [Google Scholar]