Abstract
Objective:
The present study evaluated the efficacy and toxicity of adaptive radiotherapy (RT) among patients with head and neck cancer.
Methods:
36 patients eligible for radical RT underwent RT planning scans and were planned for 54-Gy dose to both high-risk and low-risk target volumes in Phase I. All patients underwent a second (adaptive) scan during the fifth week of RT. Phase II plans for 16 Gy to high-risk planning target volume were developed on these mid-treatment scans. The primary end point was local response. Disease-free survival (DFS), overall survival (OS) and treatment-related morbidity were secondary end points.
Results:
Median reductions in gross primary and nodal disease volumes on mid-treatment scans were 34% and 43.2%, respectively. 16 patients experienced grade 3 acute mucositis. No patient had grade 3 or above haematologic toxicity. Four patients developed local recurrences, all within the RT field. Median DFS and OS were 17.5 and 23.5 months, respectively.
Conclusion:
Adaptation to changes in the anatomic and tumour volume or shape may help tilt the balance towards more efficient dose delivery as well as better normal tissue sparing.
Advances in knowledge:
This study supports the need for adaptive replanning for minimizing normal tissue toxicity without compromising local control and adds to the existing body of literature.
INTRODUCTION
Head and neck cancers (HNC) constitute one of the most common cancers in the developing world. In a recent study from India, of approximately 556,400 cancer deaths in the year 2010, the most fatal cancers were HNC, including malignancies of the oral cavity, lip and pharynx.1,2 They constitute 5.1% of the total cancer incidence in both genders and 14% of total cancer cases in males. Over 60% patients present with locally advanced disease. Locoregional failure constitutes the predominant recurrence pattern, and most fatalities result from uncontrolled local and/or regional disease.2,3
Definitive radiotherapy (RT) plays an important role in the management of locally advanced squamous-cell carcinomas. RT planning and treatment delivery for HNC has come a long way from being two-dimensional to three-dimensional. Use of highly conformal techniques such as intensity-modulated radiotherapy (IMRT) and image-guided radiotherapy (IGRT) have allowed radiation oncologists to deliver curative radiation doses to the tumour with higher accuracy, thereby restricting the dose to organs at risk and consequently reducing treatment-related morbidity. However, the sharp dose gradients imply that there should be no or minimal changes in the patient, tumour and organs at risk position.4 Although superior to conventional RT, IMRT or volumetric modulated arc therapy still causes significant toxicity. This may be explained, in part, by the fact that IMRT does not compensate for changes in the location of the disease and normal anatomy during the treatment course. However, the location, geometry and size of the tumour and normal tissues can change during the course of treatment. Such changes occur owing to multiple factors like shrinkage of primary tumour and nodal disease as a result of treatment response, alterations in the normal tissue bulk and position with respect to the target, weight loss and resolution of post-operative soft tissue changes.5–10 It is recognized that primary tumours can shrink volumetrically by up to 90% and parotid glands can involute and shift medially by up to 1 cm during treatment course.5 Applying the original plan to the altered patient anatomy can lead to higher than intended dose to the surrounding normal structures such as the parotid glands and spinal cord.
The alterations occurring during the course of RT can be compensated by adaptively modifying the treatment plan during the treatment course. This novel approach is known as adaptive radiotherapy (ART). ART utilizes repeat CT scans during the treatment course for replanning according to the altered location and shape of target volumes and normal tissue anatomy.11 ART has been discussed on a conceptual level for many years, but technical limitations have hampered its implementation in routine clinical practice. Adopting this practice in routine clinical use is challenging owing to the necessity of strict guidelines, stronger quality assurance, experienced manpower and additional cost for repeated scans and treatment planning.
An earlier publication from our group12 has shown the feasibility of a single mid-therapy rescan to adapt anatomic and tumour volume changes to help reduce the treated volumes and doses received by normal structures, especially in locally advanced HNC. In our study, we found that mean volume reduction rates (VRRs) for gross tumour volume (GTV) for primary, node and planning target volume (PTV) were 32.44%, 41.48% and 62.95% and mean daily VRRs were 1.05%, 1.38% and 2.01%, respectively.12 The mean reduction in maximum point dose (Dmax) for the spine was 260 cGy (range, 257–658 cGy). Similarly, there was a significant reduction in the volume receiving 10 and 15 Gy for bilateral parotids when ART was applied. We designed the present study to evaluate the efficacy and toxicity of ART among patients with HNC. The primary end point was local response. Disease-free survival (DFS), overall survival (OS) and treatment-related morbidity were secondary end points.
METHODS AND MATERIALS
Patients with histologically proven HNC and Karnofsky performance status More than 70, treated with adaptive protocol at our centre between April 2011 and December 2013, were included for this study. This study was in continuation with the institutional review board-approved dosimetric study of ART in HNC.12 3 patients had Stage III disease, while 33 patients had Stage IV disease. 33 patients received concurrent chemotherapy. Patient characteristics are shown in Table 1.
Table 1.
Demographics
| Patient characteristics | Details |
|---|---|
| Age (years) | n = 36 |
| 59 (median) | |
| Range 37–78 | |
| Male : female | 31 : 5 |
| KPS | |
| 90 | 20 (55.5%) |
| 80 | 10 (27.8%) |
| 70 | 2 (16.7%) |
| Smoker/tobacco chewer | 31 (86.1%) |
| Non-smoker/tobacco chewer | 5 (13.9%) |
| Comorbidity | |
| Diabetes | 4 (11.1%) |
| Hypertension | 3 (8.3%) |
| Coronary artery disease | 1 (2.8%) |
| Stages | |
| III | 3 (8.3%) |
| IV | 33 (91.7%) |
| Site | |
| Oropharynx | 21 (58.3%) |
| Larynx | 5 (13.9%) |
| Hypopharynx | 10 (27.8%) |
| Dose (Gy) | 66–70 (range) |
| Concurrent chemotherapy | |
| Cisplatin | 25 (69.4%) |
| Carboplatin | 5 (13.9%) |
| Nimotuzumab/cetuximab | 3 (8.3%) |
| Chemotherapy cycle 5 or more | 29 (80.5%) |
| Mean weight loss (kg) | 4.5 (range: 2.3–8) |
| Median reduction of primary GTV in mid-scan | 34.0% |
| Median reduction of nodal GTV in mid-scan | 43.2% |
GTV, gross tumour volume; KPS, Karnofsky performance status.
All patients underwent pre-treatment evaluation including detailed history, general physical examination and baseline laboratory and radiological investigations. Positron emission tomography and endoscopic evaluation were carried out in selected cases when indicated. Patients were staged according to the TNM staging system (American Joint Committee on Cancer cancer staging manual 7th edition). After informed consent, patients underwent mould room procedure (immobilization in thermoplastic mask) and simulation with contrast-enhanced CT scan (initial scan) with 3-mm slice thickness. IMRT or volumetric modulated arc therapy without simultaneous integrated boost (i.e. with a sequential schedule) plans were generated on CMS MONACO® v. 3.0 (Elekta, Stockholm, Sweden). Target volume and normal structures were delineated as per departmental protocol. The GTV for primary and lymph nodes were delineated as per clinical and radiological findings on simulation CT. The primary clinical target volume was generated by expanding the GTV for primary tumour by 1–1.5 cm and additionally all high-risk regions. The high-risk nodal clinical target volume was contoured by expanding the GTV for lymph nodes by 1 cm, and the low-risk nodal CTV included the remaining nodal levels at risk.
PTV was generated by giving a 5-mm expansion in all directions to CTVs. The treatment plans were verified and authorized after cross-sectional and dose–volume histogram analysis of the PTV and organs at risk. RT was delivered by 6-MV photon beams on a linear accelerator (Elekta Synergy® S or Elekta Infinity™, Stockholm, Sweden). Patient alignment was checked online before treatment by using cone-beam CT on the first day of RT and then repeated once every week. Online corrections were applied if there was deviation beyond the threshold limit ±1 mm. All patients were treated up to a curative dose of 7000 cGy in 35 fractions and weekly concurrent chemotherapy. This dose was delivered in two phases:
Phase I (initial plan): 5400 cGy at 200 cGy/fraction
Phase II (adaptive plan): 1600 cGy at 200 cGy/fraction.
Adaptive planning
All patients underwent mid-treatment contrast-enhanced CT (rescan) after the 23rd fraction, which was used for generating an adaptive plan. GTV as seen on the rescan was contoured as GTVP1 (GTV of primary on rescan) and GTVN1 (GTV of node on rescan) along with the new shape/location of normal structures relating to any changes in tumour volumes and normal anatomy. After delineating GTVP1 and GTVN1, a 10-mm margin was added to generate the respective CTVs (CTVP1 for primary and CTVN1 for node), taking care to incorporate the initial GTV, and then a 5-mm margin was added in all directions to create respective PTVs. Phase II was executed immediately after completion of Phase I.
Dietary intake was monitored by a dietician during the course of treatment. A diet of 2500–3000 kcal, 2-g protein per kilogram body weight per day (maximum 100 g day−1) and 0.5-g fat per kilogram per day (maximum 100 g day−1) was prescribed from the beginning of the treatment. Patients were offered symptomatic and supportive care in the form of i.v. fluids, parenteral nutrition, nasogastric (NG) tube, percutaneous endoscopic gastrostomy (PEG) etc., as required during and following the treatment. The requirement of PEG or NG tube was discussed and applied if the patient agreed (especially for the base of the tongue and hypopharyngeal cancers).
Chemotherapy
30 (83.3%) patients received platinum-based chemotherapy. Of these, 25 patients received weekly cisplatin (35 mg m−2) and 5 patients received weekly carboplatin (area under curve 2) in view of borderline serum creatinine level or glomerular filtration rate <50 ml min−1. Either of these agents were administered concurrently with radiation therapy, starting on Day 1 (D1), D8, D15, D22, D29, D36, D43. Three patients received either concurrent cetuximab or nimotuzumab (200 mg i.v. weekly with a loading dose of 400 mg m−2 1 week prior to starting RT).
Response, toxicity evaluation and survival
All patients were reviewed weekly during treatment to assess treatment-related toxicities. Response was assessed 6–8 weeks after the completion of treatment using the response evaluation criteria in solid tumours (RECIST v.1.1). Complete response was denoted as complete resolution of all morphologic disease, partial response included over 50% reduction in size of gross disease, stable disease included under 50% reduction or under 25% increase in size and progressive disease included over 25% increase in size. Acute toxicities were graded according to the National Cancer Institute common toxicity criteria v. 4, especially highlighting oral pain, oral mucositis, dysphagia, dry mouth and haematological parameters accounting for changes in blood counts. DFS and OS were estimated using the Kaplan–Meier method.
RESULTS
All patients completed the planned RT dose. 29 (80.5%) patients received at least 5 cycles of concurrent chemotherapy. The total dose of RT ranged from 66 to 70 Gy. Acute toxicity was recorded as the maximum toxicity grade observed during the treatment. Haematological and non-haematological acute toxicities are summarized in Table 2. Grade 3 acute skin toxicity, mucositis and dysphagia was registered in 2 (6%), 16 (45%) and 10 (28%) patients and resolved within 2 weeks after completing the treatment. No patient developed grade 3 or above haematological toxicity. In four patients, feeding tube (PEG or NG) was placed before or during the treatment. Late toxicity results are reported in Table 3. None of the patients had grade 3 or above xerostomia or dysphagia.
Table 2.
Acute toxicity
| Site/toxicity | Grade of toxicity |
|||
|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Skin | 14 (38.3%) | 20 (55.5%) | 2 (5.5%) | 0 (0%) |
| Mucosal | 0 (0.0%) | 20 (55.5%) | 16 (44.4%) | 0 (0%) |
| Dysphagia | 0 (0.0%) | 26 (72.2%) | 10 (27.7%) | 0 (0%) |
| Anaemia | 8 (22.2%) | 6 (16.7%) | 0 (0%) | 0 (0%) |
| Neutropenia | 10 (27.8%) | 5 (13.9%) | 0 (0%) | 0 (0%) |
| Thrombocytopenia | 7 (19.4%) | 0 (0%) | 0 (0%) | 0 (0%) |
Table 3.
Late toxicity
| Site/toxicity | Grade of toxicity |
|||
|---|---|---|---|---|
| Grade I | Grade II | Grade III | Grade IV | |
| Skin | 3 (8.3%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Mucosal | 11 (30.5%) | 4 (11.1%) | 0 (0%) | 0 (0%) |
| Subcutaneous fibrosis | 4 (11.1%) | 5 (13.9%) | 0 (0%) | 0 (0%) |
| Xerostomia | 14 (38.9%) | 3 (8.3%) | 0 (0%) | 0 (0%) |
| Larynx | 8 (22.2%) | 1 (2.8%) | 0 (0%) | 0 (0%) |
Response and survival
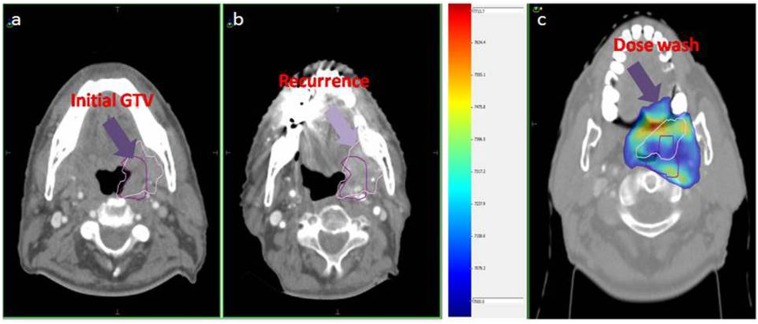
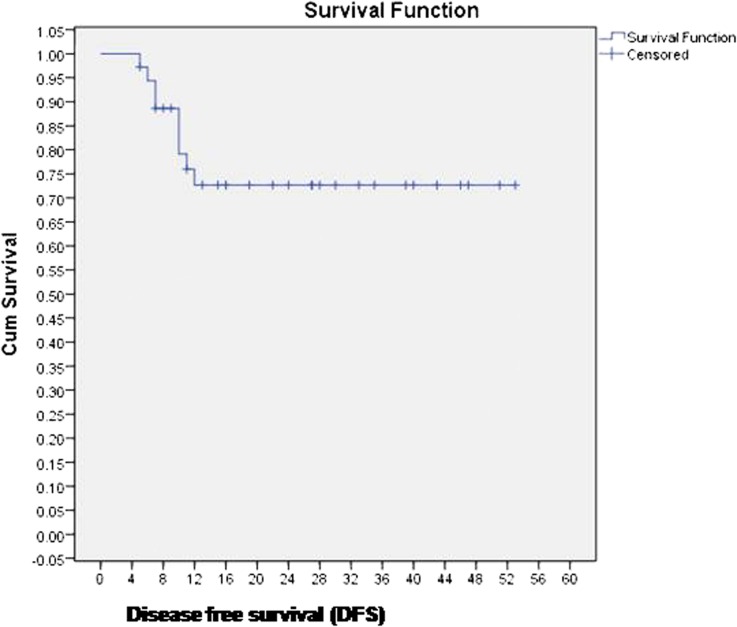
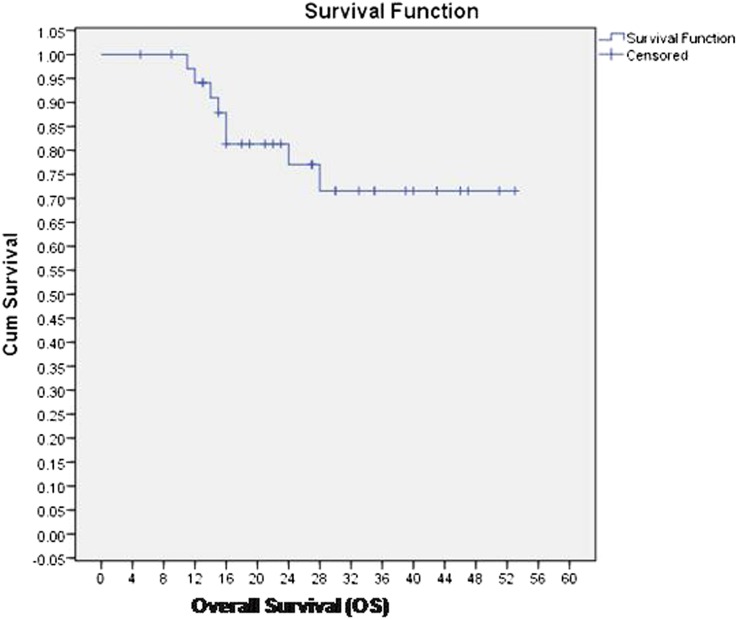
36 patients were evaluable at 6–8 weeks after completion of treatment. 29 (80.5%) patients achieved complete response at first follow-up. Five patients had residual locoregional disease and two patients had only nodal disease, for which they underwent salvage neck dissection. Four patients developed local recurrences on follow-up, and all four patients had in-field recurrences within the initial high dose volume (initial GTV) (Figure 1). Four patients had distant metastases; of these, two patients had local recurrence as well. Median DFS and OS were 17.5 months (range 11–53 months) and 25.5 months (range 11–53 months), and 2-year DFS and OS were 72% and 75%, respectively. Kaplan–Meier curves for DFS and OS are reported in Figures 2 and 3, respectively.
Figure 1.
(a) Initial gross tumour volume (GTV) (arrow), (b) GTV at recurrence (arrow) and (c) dose colour wash showing both initial and recurrent tumour volumes within the 70-Gy dose colour wash (arrow indicates the periphery of 70-Gy isodose).
Figure 2.
Disease-free survival (DFS) of the entire cohort. Cum, cumulative.
Figure 3.
Overall survival (OS) of the entire cohort. Cum, cumulative.
DISCUSSION
ART is conceptually an attractive approach to account for and correct tumour and normal tissue variations during treatment, but at present, there are limited data to guide its clinical application in day-to-day practice. Little practical evidence exists regarding issues like the timing of rescan, the dose at which adaptive planning should be executed, the basis of patient selection for adaptive planning and the volumes and margins to be considered. In literature, some studies have reported volumetric changes during adaptive planning. Barker et al5 have reported a median GTV reduction at a rate of 0.2 cm3 per day, corresponding to a 70% reduction on the last day of RT. In this study, both the primary tumour and involved nodes lost volume at approximately the same rate of 1.6% per day. Similarly, Yang et al13 reported a mean VRR of 0.43 in primary GTV for oropharyngeal cancer and 0.33 in primary GTV for hypopharyngeal cancer over a 4–5-week period. In our earlier publication, the mean VRR of primary GTV was 32% and mean daily VRR was 1.05% per day over a period of 4–5 weeks.12 The mean VRR and daily VRR of nodal GTV were 41.48% and 1.38%, respectively. Thus, the reduction rates in our study were comparable with those reported in literature, but the regression in nodal volume was more than that in primary tumour.
The study by Lee et al14 on megavoltage CT imaging showed a reduction in parotid volumes, with a median total loss of 21.3% volume or 0.7% per day. Parotids migrated medially with a median distance of −5.26 mm (0.00 to −16.35 mm) or −0.22 mm day−1.
O'Daniel et al15 studied the differences between planned and delivered parotid gland dose in patients with HNC receiving standard IMRT. IGRT provided modest but significant parotid dose reductions to the tune of 500–700 cGy in 45% of patients. Use of IGRT aligned to the C2 vertebral body provided modest but significant parotid dose reductions. Nonetheless, the actual parotid dose remained greater than planned doses (median 100 cGy) owing to parotid shrinkage and movement. In our dosimetric study, we also found significant reduction in the volume of parotids receiving a significant dose by adaptive planning using a rescan.12 By adaptive replanning, a portion of ipsilateral and contralateral parotid could be saved from being exposed to high radiation doses. This sparing of parotid volumes may translate into decreased incidence of xerostomia for such patients. Chunhui Han et al7 observed an average increase of 7.6% (range, 3.3–15.5%) in total Dmax to the spinal cord without daily setup corrections using megavoltage CT image registration.
In the past, there have been attempts to evaluate the optimum timing for adaptive replanning. Wu et al4 performed such a study, where 11 patients underwent weekly helical CT scans during routine IMRT. The authors reported that one adaptive replanning during mid-course improved the parotid mean dose sparing by 3%, two replanning by 5% and six replanning by 6%, assuming that adaptive replanning transpires 1 week prior to actual treatment delivery. If six weekly replans were used immediately, parotid dose sparing improved by 8%. Ahn et al16 reported that 65% of patients benefited from adaptive planning in terms of reduced dose to the normal structures by rescanning at 11, 22 and 33 fractions. But, in actual clinical situations, especially in the Indian scenario, repeating the scans and replanning on a weekly basis may not be a cost-effective strategy, as it requires additional cost and efforts for the patients along with additional requirement of manpower (radiation oncologists, medical physicists, technologists etc.). Schwartz et al,11 in their study, concluded that ART can provide dosimetric benefit with only one or two mid-treatment replanning events, and this appears to be a more practical and resource-efficient strategy.
The acute toxicity observed by Schwartz et al11 was comparable with that observed with IMRT. With a median follow-up of 31 months, there was 100% local and 95% regional disease control at 2 years.11 In our study, at 2 years, DFS and OS were 72% and 75%, respectively. This might be explained by the higher stage proportion of patients in our study, with most patients having Stage IV disease.
Use of contrast CT scans alone for remarking the GTV for Phase II plans is fraught with difficulties of discerning the tumour from the radiation-induced oedema/mucositis, and this was a major limitation of our study.
In our study, the acute and late toxicity results were comparable with those reported in the literature for locally advanced HNC treated with IMRT and concurrent chemotherapy. None of the patients developed grade 4 mucositis. A previous publication from our institute on chemoradiation for elderly patients with HNC had shown a 2-year locoregional control of 71.6% and 2-year OS of 88.9%.17
CONCLUSION
Treatment planning with adaptation to anatomic and tumour volume or shape changes may help tilt the balance towards better dose delivery as well as better normal tissue sparing. Large prospective studies may help stratify patient categories that are more likely to benefit from such approaches. Randomized Phase III studies would enable definitive conclusions on tolerability, efficacy and survival outcomes of such approaches.
Contributor Information
Tejinder Kataria, Email: tejinderkataria@medanta.org.
Deepak Gupta, Email: deepakonco@gmail.com.
Shikha Goyal, Email: shikha.goyal@medanta.org.
Shyam S Bisht, Email: shyam.bisht@medant.org.
Trinanjan Basu, Email: trinanjan.basu@medanta.org.
Ashu Abhishek, Email: ashu.abhishek@medanta.org.
Kushal Narang, Email: kushal.narang@medanta.org.
Susovan Banerjee, Email: susovan.banerjee@medant.org.
Shahida Nasreen, Email: shahida.nasreen@medanta.org.
Sasikumar Sambasivam, Email: S.Sasikumar@medanta.org.
Aruj Dhyani, Email: aruj.dharyni@medanta.org.
REFERENCES
- 1.Dikshit R, Gupta PC, Ramasundarahettige C, Gajalakshmi V, Aleksandrowicz L, Badwe R, et al. Cancer mortality in India: a nationally representative survey. Lancet 2012; 379: 1807–16. doi: 10.1016/S0140-6736(12)60358-4 [DOI] [PubMed] [Google Scholar]
- 2.Consolidated report of hospital based cancer registries: 2007–2011. [Cited 28 March 2016.] Available from: http://www.ncrpindia.org/ALL_NCRP_REPORTS/HBCR_REPORT_2007_2011/ALL_CONTENT/Main.htm [Google Scholar]
- 3.Perez CA, Brizel DM. The role of combined radiotherapy and chemotherapy in the management of locally advanced squamous cell carcinoma of the head and neck. In: Perez CA, Brady LW, Halperin EC, eds. Principles and practice of radiation oncology. 5th edn. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. pp. 807–19. [Google Scholar]
- 4.Wu Q, Chi Y, Chen PY, Krauss DJ, Yan D, Martinez A. Adaptive replanning strategies accounting for shrinkage in head and neck IMRT. Int J Radiat Oncol Biol Phys 2009; 75: 924–32. doi: 10.1016/j.ijrobp.2009.04.047 [DOI] [PubMed] [Google Scholar]
- 5.Barker JL, Jr, Garden AS, Ang KK, O'Daniel JC, Wang H, Court LE, et al. Quantification of volumetric and geometric changes occurring during fractionated radiotherapy for head-and-neck cancer using an integrated CT/linear accelerator system. Int J Radiat Oncol Biol Phys 2004; 59: 960–70. doi: 10.1016/j.ijrobp.2003.12.024 [DOI] [PubMed] [Google Scholar]
- 6.Castadot P, Lee JA, Geets X, Grégoire V. Adaptive radiotherapy of head and neck cancer. Semin Radiat Oncol 2010; 20: 84–93. doi: 10.1016/j.semradonc.2009.11.002 [DOI] [PubMed] [Google Scholar]
- 7.Han C, Chen YJ, Liu A, Schultheiss TE, Wong JY. Actual dose variation of parotid glands and spinal cord for nasopharyngeal cancer patients during radiotherapy. Int J Radiat Oncol Biol Phys 2008; 70: 1256–62. doi: 10.1016/j.ijrobp.2007.10.067 [DOI] [PubMed] [Google Scholar]
- 8.Schwartz DL, Dong L. Adaptive radiation therapy for head and neck cancer—Can an old goal evolve into a new standard? J Oncol 2011; 2011: 690595. doi: 10.1155/2011/690595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nath SK, Simpson DR, Rose BS, Sandhu AP. Recent advances in image-guided radiotherapy for head and neck carcinoma. J Oncol. 2009; 2009: 752135. doi: 10.1155/2009/752135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chao M, Xie Y, Moros EG, Le QT, Xing L. Image-based modeling of tumor shrinkage in head and neck radiation therapy. Med Phys 2010; 37: 2351–8. doi: 10.1118/1.3399872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartz DL, Garden AS, Thomas J, Chen Y, Zhang Y, Lewin J, et al. Adaptive radiotherapy for head-and-neck cancer: initial clinical outcomes from a prospective trial. Int J Radiat Oncol Biol Phys 2012; 83: 986–93. doi: 10.1016/j.ijrobp.2011.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma K, Kataria T, Lal M, Karthikeyan N, Gupta D, Jain S, et al. Adaptive radiotherapy in locally advanced head and neck cancers: a prospective study. Asian J Radiat Oncol 2013: 1: 1. [Google Scholar]
- 13.Yang SN, Liao CY, Chen SW, Liang JA, Tsai MH, Hua CH, et al. Clinical implications of the tumor volume reduction rate in head-and-neck cancer during definitive intensity-modulated radiotherapy for organ preservation. Int J Radiat Oncol Biol Phys 2011; 79: 1096–103. doi: 10.1016/j.ijrobp.2009.12.055 [DOI] [PubMed] [Google Scholar]
- 14.Lee C, Langen KM, Lu W, Haimerl J, Schnarr E, Ruchala KJ, et al. Evaluation of geometric changes of parotid glands during head and neck cancer radiotherapy using daily MVCT and automatic deformable registration. Radiother Oncol. 2008; 89: 81–8. doi: 10.1016/j.radonc.2008.07.006 [DOI] [PubMed] [Google Scholar]
- 15.O'Daniel JC, Garden AS, Schwartz DL, Wang H, Ang KK, Ahamad A, et al. Parotid gland dose in intensity-modulated radiotherapy for head and neck cancer: is what you plan what you get? Int J Radiat Oncol Biol Phys 2007; 69: 1290–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahn PH, Chen CC, Ahn AI, Hong L, Scripes PG, Shen J, et al. Adaptive planning in intensity-modulated radiation therapy for head and neck cancers: single-institution experience and clinical implications. Int J Radiat Oncol Biol Phys 2011; 80: 677–85. doi: 10.1016/j.ijrobp.2010.03.014 [DOI] [PubMed] [Google Scholar]
- 17.Kataria T, Gupta D, Bisht SS, Goyal S, Basu T, Srivastava A, et al. Chemoradiation in elderly patients with head and neck cancers: a single institution experience. Am J Otolaryngol. 2015; 36: 117–21. doi: 10.1016/j.amjoto.2014.07.015 [DOI] [PubMed] [Google Scholar]