Abstract
Objective:
To assess the colour Doppler and ultrasound features of testicular Leydig cell tumours (LCTs) in a population of 38 surgically proven lesions.
Methods:
From August 2008 to March 2015, we retrospectively included 38 surgically proven LCTs in 36 patients. Clinical data, scrotal colour Doppler, B-mode ultrasound and videos images were reviewed for each patient. The volume, echotexture of the testis, size, shape, echogenicity and the vascularization pattern of the lesion were evaluated. The tumour margins were categorized as either smooth or lobulated. The vascularization was classified as intense, moderate or without any hypervascularization. We defined the vascularization pattern groups as central, peripheral and mixed (the latter meaning both central and peripheral).
Results:
26 patients were referred for infertility [5 patients were subsequently diagnosed with Klinefelter syndrome (KS) and 5 patients with cryptorchidism]. 28 patients underwent testis-sparing surgery, while 8 patients underwent a radical orchiectomy. The LCTs were mostly infracentimetric (68.4%), with a median size of 7.0 mm (ranging from 4.0 to 11 mm). 50% of the lesions had lobulated margins, and these were significantly larger than the smooth lesions (p < 0.05). The content of the lesions was markedly homogeneous and hypoechoic. All lesions had sharp demarcations from the adjacent pulp. 36/38 lesions exhibited moderate-to-intense hypervascularization, with a mixed intrinsic and peripheral rim pattern. Larger lesions were more hypervascularized (p < 0.05). LCTs in patients with KS had atypical features.
Conclusion:
Typical sporadic LCTs appeared as isolated hypoechoic, infracentimetric masses, with a clear demarcation from the adjacent pulp. They presented intrinsic and peripheral rim hypervascularization.
Advances in knowledge:
By undertaking the largest imaging series of LCT to date (to our knowledge), we reassessed the typical sonographical aspects of LCTs, so as to provide guidance in regard to opting for testis-sparing surgery and for follow-up. LCTs present both intrinsic and rim vascularization detectable by colour Doppler ultrasound. Intrinsic vascularization and lobulated margins are common findings in testicular LCTs.
INTRODUCTION
Ultrasonography is the gold standard for the exploration of the testis. Increasing ultrasonography indication, along with progress in andrology, have made the discovery of non-palpable testicular lesions more common, either incidentally or during the assessment of infertility. Historically, the way to manage such masses was to proceed with a radical orchiectomy, followed by a pathology analysis. This would generally leave the patient with an impaired or lower level of fertility. It has been shown, however, that up to 80% of these lesions are benign,1,2 with most of them being Leydig cell tumours (LCTs).1,3,4 LCTs are sex cord–stromal tumours that develop from the testis stroma, mostly in the third and sixth decade of life.5 In pre-pubertal males, an LCT can lead to a precocious puberty, whereas in adults, it is mostly asymptomatic. In rare cases, it leads to gynaecomastia, loss of libido and infertility. There is no proven risk factor for developing LCT, although there are reports of a higher incidence in patients with Klinefelter syndrome (KS).6 Recent data have shown that LCTs can be regarded as benign lesions.7 Most LCTs are detected at an unpalpable stage,8 and numerous studies have proven that testis-sparing surgery is a suitable treatment option for these lesions, thus allowing for longer preservation of fertility.8–10 In ultrasound imaging, LCTs are detected as hypoechoic masses with peripheral vascularization. Most studies regarding the sonographic aspects of LCTs are based on a small number of lesions.11,12 In this study, we analyzed the colour Doppler ultrasound (CDUS) characteristics of 38 surgically proven LCTs. The main goal of this study was to reassess the CDUS features of testicular LCTs, thereby providing relatively straightforward assessment tools to the radiologists and urologists involved in the management of such testicular lesions.
METHODS AND MATERIALS
The local ethics committee approved the study, and written patient consent was not required.
In this monocentric study, we retrospectively analyzed the pathology database for all surgically proven LCTs between August 2008 and March 2015.
A total of 36 patients were included in this study. 1 patient had a bilateral LCT and 1 patient had 2 lesions on the same testis; therefore, 38 tumours were included. A senior surgeon (VI) with expertise in scrotal surgery performed the procedures. No pre-operative biopsy was performed. All patients benefited from intraoperative echo guidance. Intraoperative testicular biopsies were also performed to track associated germ-cell neoplasia in situ, and to evaluate spermatogenesis.
Follow-up consisted of a physical examination, scrotal sonography and tumour assays at 3 and 6 months, 1 year after surgery and then yearly.13,14
Analysis of the surgical samples was performed by a senior pathologist (SF). All lesions were finally categorized as Leydig cell tumours with no signs of malignancy according to Kim's criteria: tumour size, necrosis, presence of nuclear atypia, angiolymphatic invasion, infiltrating margins and number of mitotic features.3
All patients underwent a pre-operative standardized bilateral testicular ultrasonography performed successively on a Toshiba Ultrasound system, Aplio™ 80, Aplio™ XG and Aplio™ 500 (Toshiba Medical Systems Co. Ltd, Nasu, Japan). The investigators were three radiologists (LR, GC, LG) with expertise in scrotal ultrasound, and all lesions were reviewed prior to the surgery by the last author, who had the longest experience in scrotal imaging.
For all patients, both testicles were examined in B-mode with a 5–12 MHz linear array (width 5 cm) probe and in B-mode, colour Doppler or power Doppler with a high-frequency 8–18 MHz linear array (width of 4 cm) probe. The high-frequency probe was used for the precise analysis of the parenchyma, epididymis and vas deferens.
The results of every testicular ultrasound performed at our centre were compiled in a standardized report (including data such as testis size, the presence of nodules or masses and their sonographic features, aspects of testis pulp, testicular vascularization, varicocele and aspects of epididymitis and the deferens duct).
The B-mode was used to assess the volume of both testicles (calculated digitally by applying the formula: length × width × depth × 0.523); the general echotexture of the testicular pulp; the number, size, shape, localization and echogenicity of the lesions; the content of the lesions; the distance between the lesion and the tunica albuginea on transverse scans; and the presence (defined as >5 microliths per testis), distribution and grading of testicular microlithiasis (TM) (Grade 1: 5–10; Grade 2: 11–20; Grade 3: 21–30; and Grade 4: >30).15,16
The colour-coded Doppler and power mode parameters were adjusted to their most sensitive settings without introducing artefacts, and they were used to assess the intensity and the distribution of the detectable blood flow in the lesions.
Two radiologists (LR, FM), including one with over 15 years' experience in scrotal imaging, reviewed the standardized reports, images and videos in our picture archiving and communication system. All ultrasound findings were categorized by consensus. The radiologist who performed the ultrasonography could not be identified during the reviewing process. The reviewers were blinded to the written reports, which were analyzed secondarily. Any discrepancy between the reviewers was solved by consensus. The only discrepancy between the reviewers and the written reports concerned the grading of TM, which was not accurate on the basis of still images only.
The general testis echotexture was categorized as follows: normal, coarse or multinodular (defined by the presence of >two nodules). The shape of the lesions was categorized as round, oval or irregular.
The margins of the lesions were categorized as either smooth or lobulated. The echogenicity of each lesion was compared with the adjacent testicular pulp and categorized into three groups: plainly hypoechoic, faintly hypoechoic and hyperechoic. The content of the lesions was categorized as either homogeneous or heterogeneous. Vascularity was subjectively rated as hypovascularization, moderate hypervascularization or intense hypervascularization. The pattern of vascularization was categorized as peripheral, central and mixed (i.e. central and peripheral).
Statistical analysis
The relationship between the lesion sizes, shapes and vascularities was explored using the Mann–Whitney U test.
RESULTS
Clinical and surgical data
We included 36 patients aged 17–69 years, with a median age of 34 years (30–41 years). 26 (70.6%) patients were referred for infertility, including 5 patients who presented with KS, and 5 patients with undescended testicles (UDT). One patient had a history of Hodgkin's disease which had been treated by chemotherapy, and one patient had prostate cancer which had been treated by surgery and radiotherapy.
Four patients were referred for testicular pain, three patients for a palpable testicular mass and one patient for gynaecomastia. The radiologist involved did not know the patient's karyotype at the time of the examination. 28 (73.7%) patients underwent conservative surgery. 8 (26.3%) patients underwent a radical orchiectomy. Among the 38 lesions of our series, 7 intraoperative frozen section analyses (FSA) were required.
No patients presented metastases or local recurrence (the mean follow-up was 5.8 years).
Ultrasound findings
The ultrasound findings for all of the patients, for LCT associated with KS (KS-LCT) and sporadic LCT (excluding KS-LCT) are summarized in Table 1.
Table 1.
Clinical and sonographic characteristics of 38 Leydig cell tumours
| All lesions | KS-LCT | Total |
Klinefelter |
Sporadica |
|---|---|---|---|---|
| n = 38 | n = 6 | n = 32 | ||
| Number of nodules | 1 | 27 (71.1) | 3 (50.0) | 24 (75.0) |
| 2 | 5 (13.2) | 0 (0.0) | 5 (15.6) | |
| >2 | 6 (15.8) | 3 (50.0) | 3 (9.4) | |
| Echogenicity | Hypoechoic | 23 (60.5) | 3 (50.0) | 20 (62.5) |
| Faintly hypoechoic | 12 (31.6) | 1 (16.7) | 11 (34.4) | |
| Hyperechoic | 3 (7.9) | 2 (33.3) | 1 (3.1) | |
| Homogeneity | Homogeneous | 30 (78.9) | 6 (100.0) | 24 (75.0) |
| Heterogeneous | 8 (21.1) | 0 (0.0) | 8 (25.0) | |
| Size (mm) | Median (IQR) | 7.0 (4.0–11.0) | 3.0 (2.0–8.0) | 7.0 (5.0–11.5) |
| Margins | Lobulated | 19 (50.0) | 2 (33.3) | 17 (53.1) |
| Smooth | 19 (50.0) | 4 (66.7) | 15 (46.9) | |
| Vascularization | None | 2 (5.3) | 1 (16.7) | 1 (3.1) |
| Peripheral rim | 17 (44.7) | 4 (66.6) | 13 (40.7) | |
| Central | 2 (5.3) | 0 (0.0) | 2 (6.3) | |
| Mixed | 17 (44.7) | 1 (16.7) | 16 (50.0) | |
| Vascularization intensity | None | 2 (5.3) | 1 (16.7) | 1 (3.1) |
| Moderate | 18 (47.4) | 3 (50.0) | 15 (46.9) | |
| Intense | 18 (47.4) | 2 (33.3) | 16 (50.0) | |
| Adjacent pulp | Homogeneous | 29 (76.3) | 1 (16.7) | 28 (87.5) |
| Coarse | 6 (15.8) | 4 (66.7) | 2 (6.3) | |
| Multinodular | 3 (7.9) | 1 (16.7) | 2 (6.3) | |
| Microlithiasis | 4 (10.5) | 1 (16.7) | 3 (9.4) | |
| Ipsilateral testicular volume (mm3) | Median (IQR) | 8.4 (3.8–15.5) | 2.2 (1.8–3.2) | 10.1 (6.0–16.0) |
| Contralateral testicular volume (mm3) | Median (IQR) | 8.9 (4.1–11.4) | 1.4 (1.2–2.1) | 9.8 (6.1–13.0) |
| Distance from the tunica albuginea (mm) | Median (IQR) | 1.0 (1.0–3.0) | 1.3 (1.0–2.5) | 1.0 (1.0–3.3) |
KS-LCT, Leydig cell tumour found in patients with Klinfelter's syndrome; IQR, interquartile range.
All results are number of tumours (%) unless otherwise mentioned.
Lesions found in patients without Klinefelter syndrome.
The median volume of the ispilateral testicle was 8.4 mm3 (3.8–15.5 mm3).
The LCT had a median size of 7.0 mm in 26 (68.4%) infracentimetric lesions. 10 (26.3%) lesions were between 10 and 20 mm in size and 2 lesions were >20 mm in size (27 and 30 mm, respectively).
21 (55.3%) lesions were categorized as round, 11 (28.9%) lesions as oval and 6 (15.8%) lesions as irregular.
19 (50.0%) lesions had smooth margins and 19 (50.0%) lesions had lobulated margins (Figure 1).
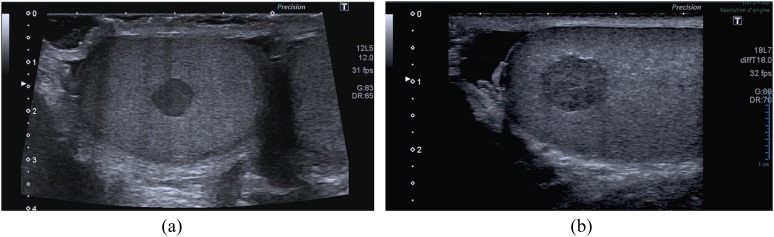
Figure 1.
Typical appearance of a Leydig cell tumour (LCT) using B-mode sonography. (a) A small hypoechoic homogeneous round-shaped and well-defined lesion in a 28-year-old male patient addressed for infertility. (b) A typical LCT can also appear with lobulated edges; this aspect is more often seen in larger lesions.
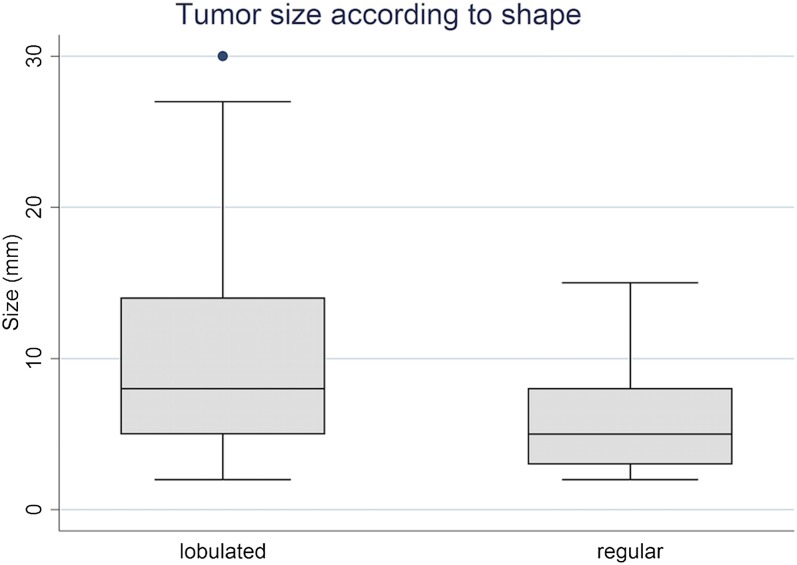
The mean size of the lobulated LCTs was 10.8 mm whereas the mean size of lesions with smooth margins was 6 mm (p < 0.05) (Figure 2).
Figure 2.
Correlation between the lesion size and general shape in 38 Leydig cell tumours. Boxes indicate interquartile range and error bars indicate 5th and 95th percentiles.
The lesion echogenicity was categorized as plainly hypoechoic for 23 lesions in comparison with the adjacent pulp.
The ultrasound echotexture of the nodule was rated as homogeneous for 30 (78.9%) lesions. The remaining eight lesions that were categorized as heterogeneous exhibited a patchy aspect (Figure 1).
Every lesion presented a sharp demarcation with the adjacent pulp, which was normal in 29 (76.3%) cases. The testis pulp was considered coarse or multinodular in six and three patients, respectively. 4 (10.5%) lesions presented TM rated as Grade 1. We did not encounter any grouped microliths or macrocalcification near the lesion.
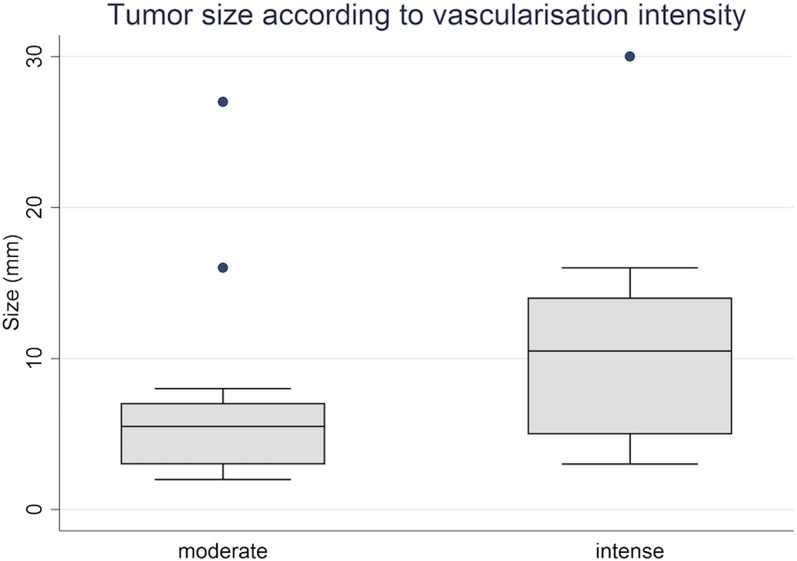
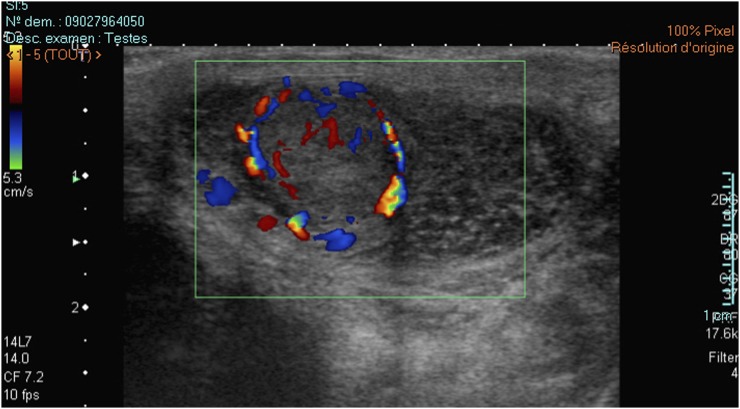
Colour Doppler, power Doppler or both was available for all patients. Hypervascularization (compared with the adjacent testis pulp) occurred in 36/38 lesions, of which 18 (47.4%) lesions were rated as intense hypervascularization and a further 18 (47.4%) lesions were rated as moderate hypervascularization. The mean size of the intensely hypervascularized lesions was 10.7 mm vs 6.8 mm for the moderately hypervascularized lesions (p < 0.05) (Figure 3). The vascularization pattern was categorized as central, peripheral or mixed for 2 (5.3%) lesions, 17 (44.7%) lesions and 17 (44.7%) lesions, respectively (Figure 4). The 17 lesions with a peripheral pattern presented a rim vascularization.
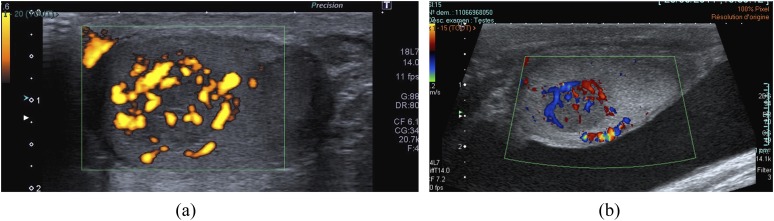
Figure 3.
Typical vascularization pattern of a Leydig cell tumour: a hypervascularized mass showing a corbelling vascularization pattern as seen with power Doppler (a) and (b) colour-coded Doppler.
Figure 4.
Correlation between the lesions size and vascularization intensity. Boxes indicate interquartile range and error bars indicate 5th and 95th percentiles.
In regard to KS-LCT, the median size of the testis was 2.2 mm3 (1.8–3.2 mm3), which was smaller than that for the sporadic LCTs. Likewise, lesions in the KS group had a median size of 3.0 mm (2.0–8.0 mm) vs 7.0 mm (5.0–11.5 mm) for the sporadic LCTs. Two of the KS-LCTs were rated as hyperechoic, whereas this was the case for only 1 (11.1%) sporadic LCT (Figure 5). TM was found in the testis pulp of one patient with KS-LCT.
Figure 5.
A typical Leydig cell tumour (LCT) as seen in a patient presenting with Klinefelter syndrome (KS). Note the small testis volume and coarse hypoechoic echotexture typically seen in patients with KS. The LCT appears as a large hyperechoic heterogeneous mass in B-mode sonography and presents marked hypervascularization with a central corbelling pattern.
DISCUSSION
Ultrasound remains the gold standard examination procedure for assessing the testis. It is also becoming more common, as indicated by the fact that it is now mandatory for male infertility work-ups, according to the European Association of Urology guidelines.17 Therefore, the identification of a non-palpable testicular lesion that can benefit from a conservative approach is a challenge. To the best of our knowledge, our study has compiled the largest imaging series of LCTs and has reassessed their CDUS aspects. The typical LCT is a round-shaped infracentimetric hypoechoic mass, with a clear delineation from the adjacent pulp. We have found that there is a correlation between the lesion size and its margins, with lobulated lesions being significantly larger than lesions with smooth margins.
Lobulated margins are often seen as a sign of malignancy, but they were found in 50% of our cases. This is a higher rate than what was found in the study by Maizlin et al,11 in which all lesions appeared to be round shaped. Our hypothesis is that the typical growth pattern in nests or clusters separated by the fibroid stroma described by Al-Agha et al18 is responsible for the lobulated aspect in larger lesions.
TM was found in only four patients rated as Grade 1, including one patient with KS and one patient with a history of UDT. Both KS and UDT are associated with a higher risk of TM.19,20 TM was randomly scattered in the testis pulp with no specific pattern around the lesion, as is seen with some malignant testicular tumours.1,19–21
The typical hypervascularized aspect of LCTs seen by using other imaging techniques has been reported recently in the literature. The studies by Lock et al and Isidori et al12,22 with contrast-enhanced ultrasonography and Manganaro et al23 with MRI report peculiar enhancement patterns for LCTs. While these techniques are promising, they are still undergoing development. Furthermore, based on their initial results, an overlap persists between seminomatous germ-cell tumours and LCTs in particular. To date, there is no technique that allows an accurate assessment of these small lesions. In our experience, CDUS therefore remains the basic examination procedure for LCT characterization.
Intrinsic vascularization has rarely been described in the literature. It was not found in any of the cases examined in the study by Maizlin et al, while in the study by Lock et al, the lesions were too small to be clearly assigned a Doppler signal.11,12 The mean size of the LCTs in our series was similar to that found by Lock et al, although the use of a higher frequency probe (18L7 MHz) with the power Doppler imaging increased the sensitivity for detecting microvascularization. We did not obtain pulsed Doppler data for LCT.
As 15.7% of our population was comprised of patients with KS, this group was overrepresented compared with the 3.1% occurrence of KS in the infertile male population.24,25 This difference can be explained by the higher risk of Leydig cell anomalies (e.g. hyperplasia, tumour) in patients with KS.2,26
The KS-LCTs were smaller and more hyperechoic than the sporadic lesions. The testis pulp in patients with KS is hypoechoic, with a coarse or micronodular echotexture.27 When encountered during a testicular ultrasound, anomalies of this nature should alert the radiologist to the possibility of KS associated with an LCT, and they should hence be a reason to undertake a karyotype exploration.
Four (11.8%) patients presented with UDT in our population, which is significantly more than the 1–3% prevalence in the general population of newborn males. This can be explained by the large recruitment of males who are infertile, with cryptorchidism being the main cause of azoospermia.28–32 There is no evidence in the literature of any association between UDT and LCT formation; and UDT is, of course, the main risk factor for developing malignant testicular tumours.3
Reports regarding the malignancy of LCTs are discordant. An accepted malignancy rate of 10% has been revised downwards. Most LCTs are diagnosed at an early stage and are of smaller size, thus leading to a favourable outcome.4,7,8,33 The indication for a testis-sparing surgery with carefully selected patients is widely accepted for non-palpable testicular lesions.8–10,34–38 According to Palermo et al,39 in order to qualify for a testis-sparing surgery, the lesion size must be <25 mm and represent <30% of the testis volume. A clear demarcation from the testis pulp and a safe distance from the rete testis are mandatory (the latter to preserve testicular vascularization). These characteristics matched in 79% of the lesions in our series. The eight patients who underwent radical orchiectomy comprised two patients with voluminous lesions (ranging from 27 to 30 mm), one patient with an ongoing history of prostate cancer, one patient who presented two different lesions on the same testis, three patients with cryptorchidism and one patient with KS for whom the size of the nodule did not allow for testicular preservation.
The relatively low number of FSA (7/36) is a sign that the sonographic evaluation associated with peculiar macroscopic features with a golden brown appearance and clear delineation within the testicle was sufficiently suggestive of an LCT for the surgeon to proceed without FSA.13 With concordant sonographical results and peroperative findings, the surgical team can reduce the time of peroperative ischaemia by avoiding FSA. The patient is warned of the possibility of a two-stage surgery, if the final pathology results are discordant. There were no misleading CDUS results during the period of the study.
In our centre, CDUS is the cornerstone for the management of non-palpable testicular nodules. If the CDUS findings are compatible with a benign lesion such as LCT, in case of a nodule <5 mm that matches the criteria of focal surgery, with negative tumour assays, we propose either active surveillance (at 3, 6, 12 months and then yearly over the next 3 years) or a testis-sparing surgery using an inguinal approach, depending on the degree of patient motivation.36 In case of a larger lesion (i.e. >5 mm), or if the findings of the CDUS are unclear, we usually propose a testis-sparing surgery with an inguinal approach and extemporaneous FSA in specialized centres with trained pathologists, preceded by sperm cryoconservation.
The goal of this therapeutic approach is to maximize fertility preservation and to allow more time to apply fertility-assistance procedures.
The main limitation of our study is its retrospective design. Our standardized report limited this bias by boosting the reproducibility of the procedure and by organizing pre-defined data sets. The results correlate with similar studies of the ultrasound characteristics of LCT. Even though ultrasound remains the best and most common examination to assess the testis, it is an operator-dependent procedure with subjective grading (e.g. the lesion's echogenicity and vascularization intensity). There is currently no quantitative tool, however, to rate echogenicity or vascularization intensity.
We did not compare the vascularization of germ-cell tumours (and especially seminomas) and LCT, and we therefore cannot propose an ultrasound algorithm. The goal of this study was primarily to assess the colour Doppler findings of a large series of LCT.
CONCLUSION
Typical sporadic LCTs that are found incidentally appear as an isolated hypoechoic infracentimetric mass with a clear demarcation from the adjacent pulp. They exhibit intrinsic hypervascularization associated with a typical peripheral rim pattern. Larger lesions more often also exhibit a lobulated shape and intense hypervascularization. Although these characteristics cannot determinate the nature of the lesion with certainty, they can assist the surgeon in deciding whether to opt for testis-sparing surgery or perhaps even active surveillance for the smallest lesions in a population that often presents with impairment of fertility.
Contributor Information
Florian Maxwell, Email: florianmaxwell@gmail.com.
Vincent Izard, Email: vincent.izard@aphp.fr.
Sophie Ferlicot, Email: sophie.ferlicot@aphp.fr.
Antoine Rachas, Email: antoine.rachas@aphp.fr.
Jean-Michel Correas, Email: jean-michel.correas@aphp.fr.
Gérard Benoit, Email: gerard.benoit@aphp.fr.
Marie-France Bellin, Email: marie-france.bellin@aphp.fr.
Laurence Rocher, Email: laurence.rocher@aphp.fr.
REFERENCES
- 1.Rocher L, Ramchandani P, Belfield J, Bertolotto M, Derchi LE, Correas JM, et al. Incidentally detected non-palpable testicular tumours in adults at scrotal ultrasound: impact of radiological findings on management radiologic review and recommendations of the ESUR scrotal imaging subcommittee. Eur Radiol 2015. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 2.Butruille C, Marcelli F, Ghoneim T, Lemaitre L, Puech P, Leroy X, et al. Prise en charge des nodules testiculaires dans une population de patients infertiles. Prog Urol 2012; 22: 45–52. doi: 10.1016/j.purol.2011.08.030 [DOI] [PubMed] [Google Scholar]
- 3.Kim I, Young RH, Scully RE. Leydig cell tumors of the testis. A clinicopathological analysis of 40 cases and review of the literature. Am J Surg Pathol 1985; 9: 177–92. doi: 10.1097/00000478-198503000-00002 [DOI] [PubMed] [Google Scholar]
- 4.Leonhartsberger N, Ramoner R, Aigner F, Stoehr B, Pichler R, Zangerl F, et al. Increased incidence of Leydig cell tumours of the testis in the era of improved imaging techniques. BJU Int 2011; 108: 1603–7. doi: 10.1111/j.1464-410X.2011.10177.x [DOI] [PubMed] [Google Scholar]
- 5.Farkas LM, Székely JG, Pusztai C, Baki M. High frequency of metastatic Leydig cell testicular tumours. Oncology 2000; 59: 118–21. doi: 10.1159/000012147 [DOI] [PubMed] [Google Scholar]
- 6.Rock A, Marcelli F, Robin G, Mitchell V, Leroy C, Rigot JM. Clinical and paraclinical features of Klinefelter syndrome consulting for male infertility. [In French]. Prog Urol 2014; 24: 757–63. doi: 10.1016/j.purol.2014.08.003 [DOI] [PubMed] [Google Scholar]
- 7.Heer R, Jackson MJ, El-Sherif A, Thomas DJ. Twenty-nine Leydig cell tumors: histological features, outcomes and implications for management. Int J Urol 2010; 17: 886–9. doi: 10.1111/j.1442-2042.2010.02616.x [DOI] [PubMed] [Google Scholar]
- 8.Nicolai N, Necchi A, Raggi D, Biasoni D, Catanzaro M, Piva L, et al. Clinical outcome in testicular sex cord stromal tumors: testis sparing vs. radical orchiectomy and management of advanced disease. Urology 2015; 85: 402–6. doi: 10.1016/j.urology.2014.10.021 [DOI] [PubMed] [Google Scholar]
- 9.Lopez-Fontana G, Lopez-Fontana R, Valdemoros P, Passardi F, Lopez Laur JD, Maurin C. Tumeurs testiculaires non palpables. Série rétrospective. Prog Urol 2014; 24: 46–50. doi: 10.1016/j.purol.2013.06.001 [DOI] [PubMed] [Google Scholar]
- 10.Powell TM, Tarter TH. Management of nonpalpable incidental testicular masses. J Urol 2006; 176: 96–8; discussion 99. doi: 10.1016/S0022-5347(06)00496-4 [DOI] [PubMed] [Google Scholar]
- 11.Maizlin ZV, Belenky A, Kunichezky M, Sandbank J, Strauss S. Leydig cell tumors of the testis gray scale and color Doppler sonographic appearance. J Ultrasound Med 2004; 23: 959–64. [DOI] [PubMed] [Google Scholar]
- 12.Lock G, Schröder C, Schmidt C, Anheuser P, Loening T, Dieckmann KP. Contrast-enhanced ultrasound and real-time elastography for the diagnosis of benign Leydig cell tumors of the testis—a single center report on 13 cases. Ultraschall Med 2014; 35: 534–9. doi: 10.1055/s-0034-1385038 [DOI] [PubMed] [Google Scholar]
- 13.Bozzini G, Picozzi S, Gadda F, Colombo R, DeCobelli O, Palou J, et al. Long-term follow-up using testicle-sparing surgery for Leydig cell tumor. Clin Genitourin Cancer 2013; 11: 321–4. doi: 10.1016/j.clgc.2012.12.008 [DOI] [PubMed] [Google Scholar]
- 14.Suardi N, Strada E, Colombo R, Freschi M, Salonia A, Lania C, et al. Leydig cell tumour of the testis: presentation, therapy, long-term follow-up and the role of organ-sparing surgery in a single-institution experience. BJU Int 2009; 103: 197–200. doi: 10.1111/j.1464-410X.2008.08016.x [DOI] [PubMed] [Google Scholar]
- 15.Richenberg J, Belfield J, Ramchandani P, Rocher L, Freeman S, Tsili AC, et al. Testicular microlithiasis imaging and follow-up: guidelines of the ESUR scrotal imaging subcommittee. Eur Radiol 2015; 25: 323–30. doi: 10.1007/s00330-014-3437-x [DOI] [PubMed] [Google Scholar]
- 16.Backus ML, Mack LA, Middleton WD, King BF, Winter TC, 3rd, True LD. Testicular microlithiasis: imaging appearances and pathologic correlation. Radiology 1994; 192: 781–5. doi: 10.1148/radiology.192.3.8058947 [DOI] [PubMed] [Google Scholar]
- 17.Practice Committee of the American Society for Reproductive Medicine. Diagnostic evaluation of the infertile male: a committee opinion. Fertil Steril 2015; 103: e18–25. Available from: http://ASRM@asrm.org. doi: 10.1016/j.fertnstert.2014.12.103 [DOI] [PubMed] [Google Scholar]
- 18.Al-Agha OM, Axiotis CA. An in-depth look at Leydig cell tumor of the testis. Arch Pathol Lab Med 2007; 131: 311–7. doi: 10.1043/1543-2165(2007)131[311:AILALC]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 19.Aizenstein RI, Hibbeln JF, Sagireddy B, Wilbur AC, O'Neil HK. Klinefelter’s syndrome associated with testicular microlithiasis and mediastinal germ-cell neoplasm. J Clin Ultrasound 1997; 25: 508–10. doi: [DOI] [PubMed] [Google Scholar]
- 20.Yee WS, Kim YS, Kim SJ, Choi JB, Kim SI, Ahn HS. Testicular microlithiasis: prevalence and clinical significance in a population referred for scrotal ultrasonography. Korean J Urol 2011; 52: 172–7. doi: 10.4111/kju.2011.52.3.172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heller HT, Oliff MC, Doubilet PM, O'Leary MP, Benson CB. Testicular microlithiasis: prevalence and association with primary testicular neoplasm. J Clin Ultrasound 2014; 42: 423–6. doi: 10.1002/jcu.22144 [DOI] [PubMed] [Google Scholar]
- 22.Isidori AM, Pozza C, Gianfrilli D, Giannetta E, Lemma A, Pofi R, et al. Differential diagnosis of nonpalpable testicular lesions: qualitative and quantitative contrast-enhanced US of benign and malignant testicular tumors. Radiology 2014; 273: 606–18. doi: 10.1148/radiol.14132718 [DOI] [PubMed] [Google Scholar]
- 23.Manganaro L, Vinci V, Pozza C, Saldari M, Gianfrilli D, Pofi R, et al. A prospective study on contrast-enhanced magnetic resonance imaging of testicular lesions: distinctive features of Leydig cell tumours. Eur Radiol 2015; 25: 3586–95. doi: 10.1007/s00330-015-3766-4 [DOI] [PubMed] [Google Scholar]
- 24.Ekerhovd E, Westlander G. Testicular sonography in men with Klinefelter syndrome shows irregular echogenicity and blood flow of high resistance. J Assist Reprod Genet 2002; 19: 517–22. doi: 10.1023/A:1020959818687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lanfranco F, Kamischke A, Zitzmann M, Nieschlag E. Klinefelter’s syndrome. Lancet 2004; 364: 273–83. doi: 10.1016/S0140-6736(04)16678-6 [DOI] [PubMed] [Google Scholar]
- 26.Forti G, Corona G, Vignozzi L, Krausz C, Maggi M. Klinefelter’s syndrome: a clinical and therapeutical update. Sex Dev 2010; 4: 249–58. doi: 10.1159/000316604 [DOI] [PubMed] [Google Scholar]
- 27.Accardo G, Vallone G, Esposito D, Barbato F, Renzullo A, Conzo G, et al. Testicular parenchymal abnormalities in Klinefelter syndrome: a question of cancer? Examination of 40 consecutive patients. Asian J Androl 2015; 17: 154–8. doi: 10.4103/1008-682X.128514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kolon TF, Herndon CD, Baker LA, Baskin LS, Baxter CG, Cheng EY, et al. Evaluation and treatment of cryptorchidism: AUA guideline. J Urol 2014; 192: 337–45. doi: 10.1016/j.juro.2014.05.005 [DOI] [PubMed] [Google Scholar]
- 29.Efthimiou I, Mamoulakis C, Papageorgiou G, Kazoulis S, Prevedorou D, Kontogiorgos G, et al. Unilateral malignant leydig cell tumor of testis in a patient with contralateral cryptorchidism. Urol J 2009; 6: 60–2. [PubMed] [Google Scholar]
- 30.Chung E, Brock GB. Cryptorchidism and its impact on male fertility: a state of art review of current literature. Can Urol Assoc J 2011; 5: 210–4. doi: 10.5489/cuaj.10106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hadziselimovic F, Hocht B, Herzog B, Buser MW. Infertility in cryptorchidism is linked to the stage of germ cell development at orchidopexy. Horm Res 2007; 68: 46–52. doi: 10.1159/000100874 [DOI] [PubMed] [Google Scholar]
- 32.Koni A, Ozseker HS, Arpali E, Kilinc E, Dogan HS, Akyol A, et al. Histopathological evaluation of orchiectomy specimens in 51 late postpubertal men with unilateral cryptorchidism. J Urol 2014; 192: 1183–8. doi: 10.1016/j.juro.2014.05.048 [DOI] [PubMed] [Google Scholar]
- 33.Carmignani L, Morabito A, Gadda F, Bozzini G, Rocco F, Colpi GM. Prognostic parameters in adult impalpable ultrasonographic lesions of the testicle. J Urol 2005; 174: 1035–8. doi: 10.1097/01.ju.0000170236.01129.d4 [DOI] [PubMed] [Google Scholar]
- 34.Giannarini G, Mogorovich A, Menchini Fabris F, Morelli G, De Maria M, Manassero F, et al. Long-term followup after elective testis sparing surgery for Leydig cell tumors: a single center experience. J Urol 2007; 178: 872–6; quiz 1129. [DOI] [PubMed] [Google Scholar]
- 35.Loeser A, Vergho DC, Katzenberger T, Brix D, Kocot A, Spahn M, et al. Testis-sparing surgery versus radical orchiectomy in patients with Leydig cell tumors. Urology 2009; 74: 370–2. doi: 10.1016/j.urology.2009.03.014 [DOI] [PubMed] [Google Scholar]
- 36.Silberstein JL, Bazzi WM, Vertosick E, Carver BS, Bosl GJ, Feldman DR, et al. Clinical outcomes of local and metastatic testicular sex cord-stromal tumors. J Urol 2014; 192: 415–9. doi: 10.1016/j.juro.2014.01.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eifler JB, Jr, King P, Schlegel PN. Incidental testicular lesions found during infertility evaluation are usually benign and may be managed conservatively. J Urol 2008; 180: 261–4. doi: 10.1016/j.juro.2008.03.021 [DOI] [PubMed] [Google Scholar]
- 38.Brunocilla E, Gentile G, Schiavina R, Borghesi M, Franceschelli A, Pultrone CV, et al. Testis-sparing surgery for the conservative management of small testicular masses: an update. Anticancer Res 2013; 33: 5205–10. [PubMed] [Google Scholar]
- 39.Palermo G, Antonucci M, Recupero SM, Fiorillo A, Vittori M, Bassi PF, et al. Focal surgery testis cancer: current state art. [In Italian]. Urologia 2013; 80: 290–6. doi: 10.5301/RU.2013.11687 [DOI] [PubMed] [Google Scholar]