Abstract
Background
CD4 T-cells expressing Foxp3 are expanded systemically during active tuberculosis (TB) regardless of HIV-1 co-infection. Foxp3+ CD4 T cells are targets of HIV-1 infection. However, expansion of HIV-1 infected Foxp3+ CD4 T cells at sites of HIV/TB co-infection, and whether they contribute to promotion of HIV-1 viral activity is not known.
Methods
Pleural fluid mononuclear cells (PFMC) from HIV/TB co-infected patients with pleural TB were characterized by immune-staining and FACS analysis for surface markers CD4, CD127, CCR5, CXCR4, HLA-DR and intracellular expression of Foxp3, HIVp24, IFN-γ and Bcl-2. Whole PFMC and bead separated CD4+CD25+CD127− T cells were assessed for HIV-1 LTR strong stop (SS) DNA by real-time PCR, which represents viral DNA post cell entry and initiation of reverse transcription.
Results
High numbers of HIV-1 p24 positive Foxp3+ and Foxp3+CD127− CD4 T cells were identified in PFMC from HIV/TB co-infected subjects. CD4+Foxp3+CD127− T cells displayed high expression of the cellular activation marker, HLA-DR. Further, expression of the HIV-1 co-receptors, CCR5 and CXCR4, were higher on CD4+Foxp3+T cells compared to CD4+Foxp3− T cells. Purified CD4+CD25+CD127− T cells isolated from PFMC of HIV/TB co-infected patients, were over 90% CD4+Foxp3+T cells, and exhibited higher HIV-1 SS DNA as compared to whole PFMC, and as compared to CD4+CD25+CD127− T cells from an HIV-infected subject with pleural mesothelioma. HIV-1 p24+ Foxp3+ CD4+T cells from HIV/TB patients higher in Bcl-2 expression as compared to both HIV-1 p24+ Foxp3− CD4 T cells, and Foxp3+ CD4+T cells without HIV-p24 expression.
Conclusion
Foxp3+ CD4 T cells in PFMC from HIV/TB co-infected subjects are predisposed to productive HIV-1 infection and have survival advantage as compared to Foxp3 negative CD4 T cells.
Keywords: Regulatory T-cell, Pleural TB, HIV/TB co-infection, HIV-1 p24, HIV-1 LTR, Apoptosis, Bcl-2, Foxp3
Introduction
During Human Immunodeficiency virus-1 and Tuberculosis (HIV/TB) co-infection, sites of active HIV/TB disease are the main source of viral activity as compared to virus generated systemically (1). High numbers of Foxp3+ CD4 T-cells characterize sites of Mycobacterium tuberculosis (MTB) infection (2–4). Foxp3+ CD4 T-cells have been found to be targets of HIV-1 infection among blood mononuclear cells (5, 6). Whether Foxp3+ CD4 T-cells are expanded and productively HIV-1 infected at sites of HIV/TB co-infection is not known.
Foxp3 gene expression is key in establishing the regulatory T-cell lineage both for thymus-derived natural (n) T-reg, and induced (i) T-reg derived from naïve CD4 T-cells outside of the thymus (Reviewed in (7)). Transforming growth factor beta (TGF-β) is critical to induction of expression of Foxp3 in T-cell receptor (TCR) activated naïve CD4 T-cells (8, 9). Whereas induction of iT-reg requires TGF- β, expansion of TGF- β induced iT-reg is dependent on the NFκb/c-Rel (10) and/or IL-2/Stat5 signaling (11) pathways. However, stability of Foxp3 gene expression in iT-reg has been found to be dependent on presence of TGF-β alone (12, 13). At sites of pleural HIV/TB co-infection, bio-active TGF-β and the pro-inflammatory cytokines (IL-8, IL-6), but not IL-2, are abundant (14). In this latter study, Foxp3 mRNA expression in PFMC correlated with pleural fluid levels of IL-6 and IL-8 and to a lesser extent with TGF-β, but not with levels of the T-cell cytokine, IFN-γ (14). These data suggest that intense TCR activation in the context of the inflammatory micro milieu that includes excess TGF-β at sites of pleural HIV/TB co-infection may be conducive to expansion of PFMC CD4 T-cells with stable Foxp3 expression.
Under Th1 polarizing conditions, a fraction of CD4+Foxp3+ T cells have been shown to express IFN-γ (15, 16). However, IFN regulatory factor-1 has been shown to promote inhibition of Foxp3 expression (17), and ultimately may lead to conversion of T-reg to Th1-like cells (18). Whether the Th1 cytokine milieu at pleural sites of HIV/TB co-infection (14, 19) also promotes IFN-γ reactive Foxp3+ CD4 T cells is not known.
Naïve T-cell precursors of Foxp3 positive T-reg are susceptible to HIV infection in vitro (20). Both heightened status of cellular activation (21) and increased expression of HIV-1 co-receptors (6) may underlie predisposition of T-reg to successful viral infection. Further, Foxp3 enhances NFκb occupancy at HIV-1 LTR in Jurkat T cell line (22), indicating the likely predisposition of Foxp3+ CD4 T-cells to productive HIV-1 infection. On the other hand, others have shown that transfection of Foxp3 gene in primary CD4 T cells represses HIV-1 transcription under conditions of immune activation (23, 24). Whether the intense immune activation at pleural sites of HIV/TB co-infection (Meng submitted) leads to repression or promotion of HIV-1 infection in Foxp3+ CD4 T cells is not known.
Based on our previous observations of enhanced HIV activity, increased TGF-β bioactivity, and high Foxp3 expression of PFMC at pleural sites of HIV/TB co-infection, we hypothesized that CD4+Foxp3+ T cells in PFMC may be HIV-1 infected and contribute to expansion of HIV-1 activity in situ. We found that among PFMC CD4+ T-cells, HIV-1 infection was enriched in the Foxp3+CD127− CD4 T-cell population. Co-expression of HIV-1 p24 and IFN-γ among Foxp3+ CD127− CD4 T-cells was higher than Foxp3−CD127+ CD4 T cells. Expression of both HIV-1 co-receptors (CXCR4 and CCR5) and HLA-DR was significantly higher among Foxp3+than Foxp3− CD4 T cells in PFMC. Also, HIV-1 p24+ Foxp3+ T cells were higher in Bcl2 expression as compared to both HIV-1 p24+Foxp3− and HIV-1 p24− Foxp3+ CD4 T cells.
Methods
Study Subjects
Patients hospitalized at Mulago Hospital at Makerere University in Kampala Uganda with symptoms of fever, cough, night sweats, dyspnea for at least 2 weeks who had moderate to large pleural effusions were identified and referred to the TB clinic of the TB Research Unit for evaluation of pleural TB. Informed consent (approved by the Institutional Review Board at CWRU and the Ugandan National AIDS Research Subcommittee) was obtained from all subjects. All patients underwent HIV-1 testing, and thoracocentesis and pleural biopsy as previously described (25). Diagnosis of pleural TB was based on positive culture of sputum and/or pleural fluid, and/or positive histology of pleural tissue for MTB infection. Only HIV+ patients were included in this study. HIV-1 viral load in plasma and pleural fluid was determined using the Roche Amplicor (1.2) System. All patients received standard short-course anti-TB chemotherapy and were referred to Makerere Joint AIDS Programme for anti-retroviral therapy.
A total of 16 HIV/TB co-infected patients were studied. Table 1 describes the characteristics of enrolled subjects.
Table 1.
| Age* | Female/male (%) | CD4 (/μl)* | Plasma VL (/ml)* | Pleural Fluid VL (/ml)* | TB diagnosis (%) Culture/Histology |
|---|---|---|---|---|---|
| 34 (25–50 yrs) | 18.7%/81.3% | 169 (59–445) | 2.8×105 (0.2–9.8×105) | 2.1×106(0.07–5.2×106) | 93.7%/6.3% |
Median (range)
Preparation of PBMC and PFMC
PFMC and PBMC were prepared by Ficoll Hypaque (Pharmacia Fine Chemicals, Piscataway, NJ) density gradient centrifugation as described (25). Viability was > 98% as assessed by trypan blue exclusion. By immune staining and FACS analysis PFMC were 40–60% CD4 T-cells and contained only 1–5% CD14+ macrophages, while autologous PBMC were 10–15% CD14+ monocytes.
Analysis of cell phenotype by flow cytometry
Antibody combination panels for four or five color immune-staining were as follows:
CD3PerCP, CD4 FITC, CD25APC, and rat Foxp3PE (all from eBioscience, San Diego, CA).
CD4 PercpCy5.5, HLA-DR APC, CD127 Pac Blue and rat Foxp3 PE (eBioscience, San Diego, CA).
CD4 PercpCy5.5, CCR5 FITC, CXCR4 APC (Biolegend, San Diego, CA) and Foxp3 PE.
CD4 PercpCy5.5, HIV-1 p24 FITC (Beckman Coulter, Brea, CA), Bcl-2 PE (BD Biosciences, San Jose, CA) and Foxp3 e-fluor660 (eBioscience).
CD4 PercpCy5.5, CD127 Pac Blue, FoxP3 PE, HIV-1 p24 FITC and IFN-γ Alexa Fluor 647 (Biolegend).
Isotype antibodies used included: IgG2a APC, IgG1 Pac Blue, rat IgG2a PE, IgG2a FITC, rat IgG2a AP, IgG1 FITC, IgG1 PE, IgG2a e-fluor660, and IgG1 Alexa Fluor 647.
The protocol and buffer set from eBioscience were used for all experiments involving intracellular staining. All samples were fixed and acquired within 1h of completion of staining, and analysis was by Miltenyi MACSQuant Flow cytometer.
Data were analyzed by FlowJo software (Treestar Inc, Ashland, OR) at the completion of study.
TUNEL assay of apoptosis
Terminal deoxynucleotidyl transferase (Tdt)-mediated nick end labeling (TUNEL) was used to detect apoptotic mononuclear cells as before (25). Briefly, mononuclear cells were surface stained with CD4 Percp and CD25 APC antibodies. Cells were fixed, permeabilized and incubated with Brd UTP (Sigma) in the presence or absence of TdT (Boehringer Indianapolis, IN). DNA breaks were identified by incubation with FITC antibody to Brd UTP (Becton Dickinson). Then, three color analysis was performed by FACScan flowcytometer (Becton Dickinson) and analyzed using WINDMD 2.8 software (Scripps Institute, La Jolla, CA).
Separation of PFMC using magnetic beads
The Human regulatory T-cell isolation kit II from Miltenyi Biotech (Auburn, CA) was used to isolate CD4+CD25+CD127− T cells as instructed by the manufacturer. Purity of isolated CD4 T cells for Foxp3+ expression exceeded 90%. Aliquots of whole PFMC and purified T cells were dissolved in Tri-reagent (Molecular Research Center, Cincinnati, OH) and stored at −70°C until use.
Measurement HIV-1 DNA and Foxp3 mRNA by real-time PCR
Cellular DNA was extracted from the lower phase of cell lysates in Tri-reagent according to the manufacturer’s instructions. Real time PCR using the Taqman methodology was by StepONEPlus thermo cycler (Applied Biosystems, Foster City, CA) for HIV-1 minus-strand strong stop (SS) DNA (26), and human beta globin DNA. In each sample DNA copies were normalized to the copy number of beta globin (0·5 × 1010 copies in 1×106 cells).
Total RNA was obtained from PFMC cell lysate. RT-PCR for Foxp3 mRNA was as before (14). Foxp3 mRNA levels in each sample were corrected to the copy numbers of ribosomal 18S (R18) (1×1010 copies of R18 in 1×106 cells).
Statistics
Normally distributed data sets were analyzed by student t-test. Wilcoxon or Kruskall Wallis tests were used for data sets that were not normally distributed. Correlation between variables was assessed using linear regression or spearman rank order correlation as appropriate. P ≤ 0.05 was considered significant.
Results
Increased frequencies of Foxp3+ CD25hi+ CD4 T cells at pleural sites of HIV/TB co-infection
First, PFMC and autologous PBMC from HIV-1/TB co-infected patients were examined for frequencies of CD4+CD25hi+ T-cells and co-expression of Foxp3. CD4+CD25hi+ T-cells were increased by 2.7-fold in PFMC as compared to autologous PBMC (p < 0.01) (Figure 1). Foxp3+ reactivity was enriched among CD4+CD25hi+ T-cells in PFMC and PBMC by 85% and 70% respectively. Foxp3+CD4+CD25hi+ T-cells were higher in PFMC as compared to PBMC (P < 0.05) (Figure 1).
Figure 1. Frequencies of T-reg are increased at sites of HIV/TB co- infection.
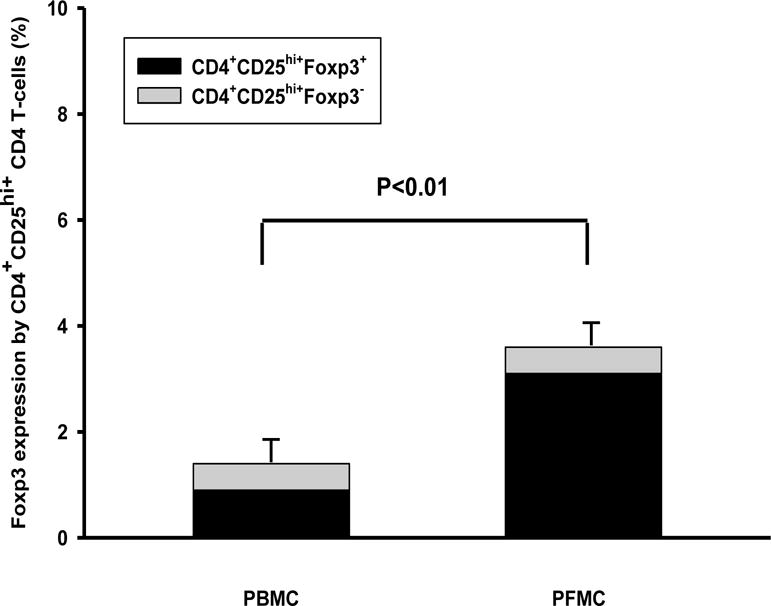
CD4+CD25hi+ in PFMC and autologous PBMC from HIV/TB co-infected patients with pleural TB were assessed for intra-cellular Foxp3 expression (n=16). As compared to autologous PBMC, PFMC contained higher CD4+CD25hi+ T cells (p<0.01) and higher proportion of Foxp3+CD25hi+ T cells (p < 0.05).
Next, Foxp3 mRNA expression in PFMC from HIV/TB co-infected subjects (Methods) was correlated to their pleural fluid viral loads. Foxp3 mRNA expression and HIV-1 viral load in pleural fluid were found to correlate to a moderate degree (r=0.780, p<0.002) (n=16).
Cumulatively, the data above indicate expansion of PFMC Foxp3+CD4+CD25hi+T-reg at sites of pleural HIV/TB co-infection and a positive association of Foxp3 expression with HIV-1 viral load at pleural sites of HIV/TB co-infection.
HIV-1 infection of Foxp3+ CD4 T cells in PFMC from HIV/TB co-infected patients
Differentiation of CD4+Foxp3+ CD127− from transitional CD4+Foxp3+ CD127+ T cell (27, 28), and from CD4+Foxp3−CD127+ was performed on PFMC from 6 HIV/TB co-infected subjects. For this purpose, protocols employing five color immunostaining and FACS analysis were employed (Methods). HIV-1 p24 and IFN-γ reactivity of CD4+ Foxp3positive or negative PFMC T-cells that were positive or negative for CD127 (IL7 receptor) expression were assessed. An algorithm for analysis of HIV-1 p24 and IFN-γ reactivity in CD4+Foxp3+ CD127−and CD4+Foxp3−CD127+ T-cell subsets is shown in Figure 2A. As noted, a relatively small % of transitional CD4+Foxp3+CD127 +T-cells were identified in PFMC (Figure 2A).
Figure 2. Frequencies of CD4+Foxp3+ CD127− and CD4+Foxp3−CD127+ PFMC co-expressing HIV-1 p24 alone or in combination with IFN-γ.
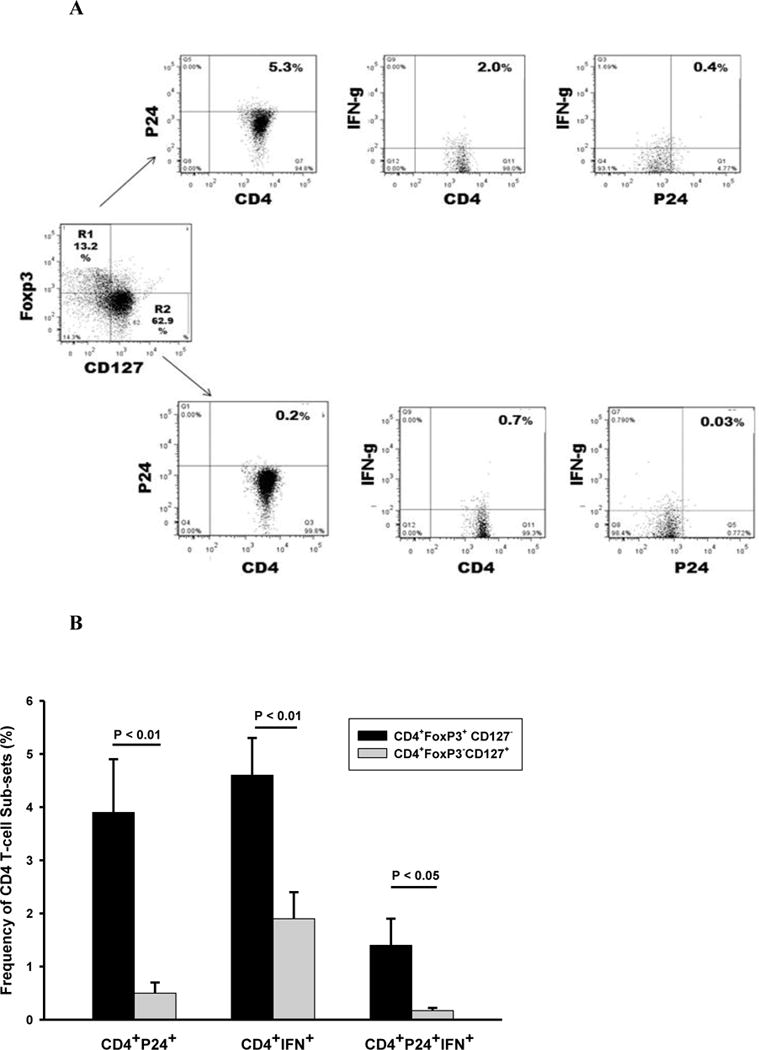
CD4+Foxp3+ CD127− and CD4+Foxp3−CD127+ in PFMC from HIV/TB patients (n=6) were characterized for their expression of HIV-1 p24 and IFN-γ. A. Algorithm for assessment of frequencies of CD4+Foxp3+ CD127− and CD4+Foxp3−CD127+ PFMC co-expressing HIV p24 and IFN-γ alone or in combination. B. Among CD4+Foxp3+ CD127− T cells, higher frequencies of HIV-1 p24+ (p<0.01), IFN-γ+ (p<0.01) and dual HIVp24+IFNγ+ (P<0.05) as compared to CD4+Foxp3−CD127+ T cells were found.
Cumulative data is shown in Figure 2B. Frequencies of CD4+FoxP3+CD127− T-cells positive for HIV-1 p24 were 7.8 fold higher when compared to CD4+FoxP3−CD127+ T cells (p < 0.01) (Figure 2B left panel). Also, IFN-γ positivity in CD4+FoxP3+CD127− T cells was 2.2-fold higher when compared to CD4+FoxP3−CD127+ T cells (p < 0.01) (Figure 2B mid panel). Interestingly, higher (8.2 fold) frequencies of CD4+FoxP3+CD127− T cells that were both HIV-1 p24+ and IFN-γ+ as compared to CD4+FoxP3− CD127+ T cells (p < 0.05) were found (Figure 2B right panel).
Thus, CD127− Foxp3+ CD4 T cells that express HIV-1 p24+ are enriched in PFMC from HIV/TB co-infected patient. Further, almost 25% of HIV-1 p24+ CD127− Foxp3+ CD4 T cells are IFN-γ reactive.
Activation status of CD127− Foxp3+ CD4 T cells in PFMC from HIV/TB patients
Productive HIV-1 infection of CD4 T-cells is dependent on their status of cellular activation (29). At sites of HIV/TB co-infection HLA-DR+ CD4 T cells are expanded and HIV-1 originating from HLA-DR mononuclear cells in pleural fluid is higher than that in the blood (30). Here, we assessed the percentage of CD4+Foxp3+ CD127− T cells and CD4+Foxp3−CD127+ T cells in PFMC that were HLA-DR positive or negative. HLA-DR positivity among CD4+Foxp3+ CD127− T cells was 3 fold higher as compared to CD4+Foxp3−CD127+ T cells (p<0.001) (Figure 3).
Fig 3. Frequencies of HLADR expression by CD4+Foxp3+ CD127− versus CD4+Foxp3−CD127+ T cells.
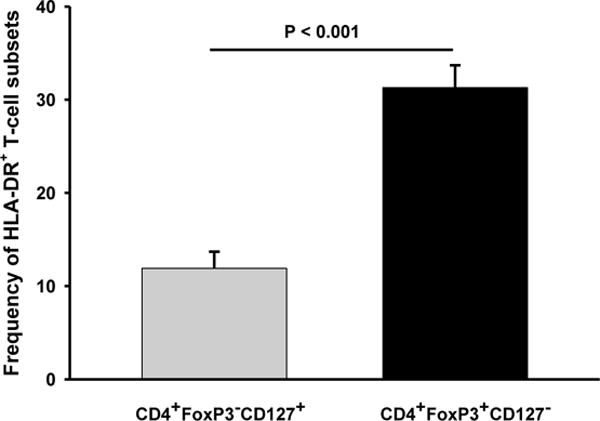
PFMC from HIV/TB co-infected patients were assessed for CD4, Foxp3+, CD127 and HLA-DR reactivity (n=16). Frequency of HLADR positive CD4+CD127−Foxp3+ T cells was significantly higher than CD4+ CD127 Foxp3− T cells (p<0.001).
These data identify Foxp3+ CD127− CD4 T cells in PFMC from HIV/TB co-infected patients with high levels of immune activation.
Expression of HIV co-receptors by CD4+Foxp3+PFMC
HIV-1 infection is dependent on expression of HIV-1 co-receptors (CCR5 and CXCR4) on CD4+ T-cells. In prior observations of PFMC from HIV/TB co-infected subjects we had found that T-cells characterized by high CD25 expression, had significantly higher expression of CCR5 as compared to CD4 T-cells that were low or negative in CD25 expression (unpublished data). Here we examined both CCR5 and CXCR4 expression by CD4+Foxp3+ PFMC as compared to CD4+Foxp3− PFMC (n=5). Fig 4A shows these results: expression of both CCR5 and CXCR4 was higher on CD4+Foxp3+ as compared to CD4+Foxp3− T-cells (P < 0.01, for either comparisons).
Figure 4. Expression of CCR5 and CXCR4 by Foxp3+ and Foxp3− CD4 T cells in PFMC and HIV-1 entry in PFMC.
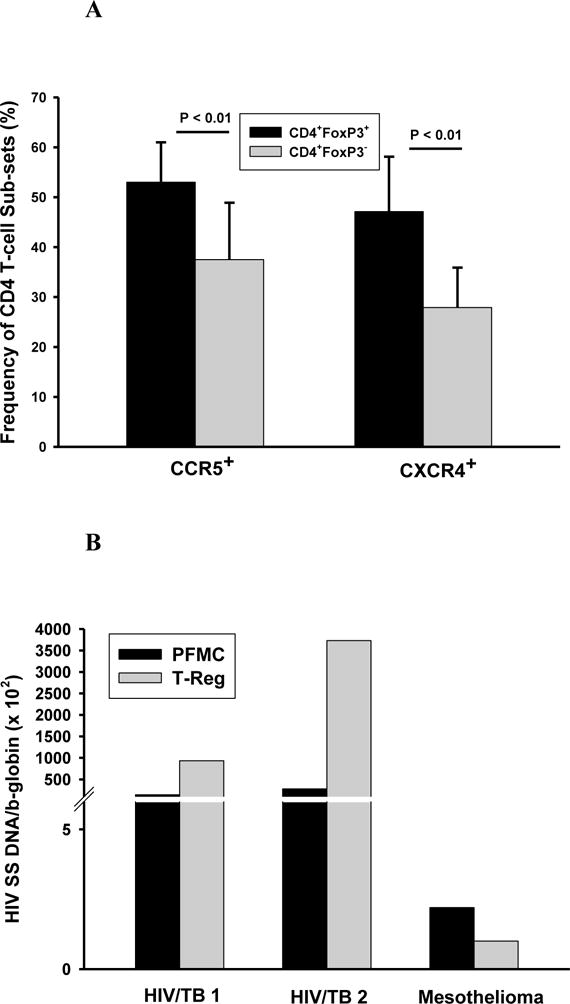
Foxp3+ and Foxp3− CD4 T cells in PFMC from HIV/TB co-infected patients were assessed for expression of HIV-1 co-receptors CCR5 and CXCR4 and HIV-1 entry. A. Expression of CCR5 and CXCR4 were increased in CD4+Foxp3+ as compared to CD4+Foxp3− T cells (p < 0.01 for both, n=5). B. HIV-1 entry was assessed by PCR for HIV-1 SS DNA following immune-magnetic separation of PFMC to obtain CD4+Foxp3+ CD127− T cells and compared to that in whole PFMC. Results are shown for 2 HIV/TB co-infected patients and one HIV-1 infected subject with pleural mesothelioma.
Next, we assessed successful HIV-1 entry in PFMC from HIV/TB co-infected patients (n=2) and one HIV-infected subject who proved later to have pleural mesothelioma. CD4+ CD25+ CD127− were purified from PFMC by immune-magnetic separation (Methods). Cellular DNA was extracted from each population and assessed for HIV-1 strong stop (SS) DNA, which represents all HIV-1 that has initiated reverse transcription post HIV-1 cell entry. HIV-1 SS DNA was enriched in the isolated CD4+ CD25+ CD127− T cells as compared to whole PFMC in the HIV/TB co-infected subjects, but not in the subject with pleural mesothelioma (Fig 4B).
Therefore, higher HIV-1 co-receptor expression and predisposition to successful HIV-1 infection are features of CD25+ CD127− CD4 T cells that are 90% Foxp3+ in PFMC from HIV/TB co-infected subjects.
Survival of HIV infected Foxp3+ T cells in PFMC
First, we assessed the apoptotic profile of CD4 T cells in PFMC from HIV/TB co-infected subjects. For these experiments TUNEL positivity of PFMC identified as CD4+CD25hi+ was compared to CD4+CD25lo+/− T-cells. Frequencies of TUNEL positive CD4+CD25lo+/− T-cells exceeded those of CD4+CD25hi+ T cells by 3.7 fold (P < 0.001) (Figure 5A). As noted above (Fig 1), 85% of CD4+CD25hi+ PFMC were Foxp3 positive.
Figure 5. Resistance to apoptosis and differential expression of Bcl2 in PFMC subpopulations in HIV/TB co-infected patients.
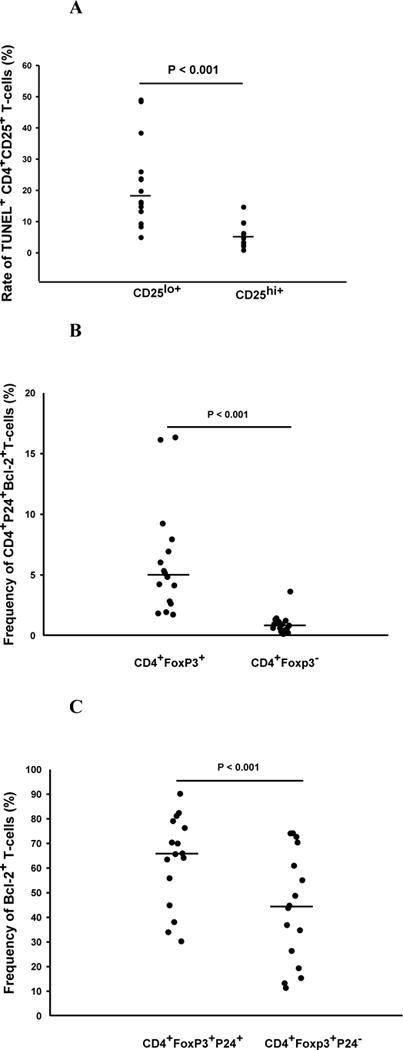
In A, apoptosis of CD4+CD25hi+T cells and CD4+CD25lo/− T cells were assessed by TUNEL methodology (n=10). In B and C PFMC from HIV/TB patients were assessed for co-expression of intra-cellular Foxp3, HIV-1 p24 and Bcl-2 (n=16). A. Frequencies of TUNEL positive CD4+CD25lo/− T cells exceeded those of CD4+CD25hi+ T cells by 3.7 fold (P < 0.001). B. Frequencies of HIV-1 p24+Foxp3+ CD4 T cells co-expressing Bcl-2 exceeded HIV-1 p24+Foxp3− T cells by more than 5 fold (p < 0.001). C. Among Foxp3 positive CD4 T cells, intracellular expression of Bcl-2 was enriched among HIV-1 p24+ as compared to HIV-1 p24− CD4 T-cell subsets (p<0.001) (n=16).
Next, the intracellular co-expression of the anti-apoptotic molecule, Bcl-2, and HIV-1 p24 in Foxp3+ and Foxp3− CD4T cells in PFMC were assessed. CD4+Foxp3+ T cells were 5 fold higher in co-expression of Bcl-2 and HIV-1 p24 as compared to CD4+Foxp3− T cells (P < 0.001) (Fig 5B). A correlation of Bcl-2 expression and Foxp3+ HIV-1 p24+ dual reactivity was found (R=0.551, p=0.02). Interestingly among CD4+Foxp3+ T cells, Bcl-2 reactivity was significantly higher among HIV-1 p24+ as compared to HIV-1 p24− cells (p<0.001) (Fig 5C).
Therefore, in PFMC from HIV/TB co-infected subjects HIV-1 p24 positive CD4+Foxp3+ T cells have a survival advantage over both HIV-1 p24+ Foxp3− CD4 T cells, and HIV-1 p24 negative CD4+Foxp3+ T cells.
Discussion
Current literature indicates that Foxp3+CD4 T cells are predisposed to HIV infection (5, 20), and therefore may contribute to HIV-1 disease progression through viral production. This is in addition to the role of CD4+Foxp3+T cells in suppression of antigen-specific HIV-1 T-cell responses (31). Here we found significantly higher numbers of CD4+Foxp3+ in PFMC as compared to autologous PBMC from HIV/TB co-infected patients with pleural disease. Higher HIV-1 p24 expression was found in Foxp3+ CD127− as compared to Foxp3− CD127+ CD4 T cells in PFMC. CD4+Foxp3+ T cells in PFMC were also characterized by both significantly higher cellular activation (HLA-DR reactivity) and expression of HIV-1 co-receptors (CCR5 and CXCR4) as compared to Foxp3− CD4 T cells, underscoring their susceptibility to productive HIV-1 infection. Interestingly, HIV-1 p24+ Foxp3+ CD4 T cells were highly Bcl-2 reactive as compared to both HIV-1 p24+ Foxp3− CD4 T cells and Foxp3+ CD4 T cells without HIV-1 expression. A significantly greater fraction of CD4+Foxp3+ CD127− T cells were both HIV-1 p24+ and IFN-γ+, when compared to Foxp3− CD4 T cells. Cumulatively, these data indicate CD4+Foxp3+ as a source of HIV-1 among CD4 T cell population at pleural sites of HIV/TB co-infection, with survival advantage over other CD4 T cells.
During in vitro infection of human T-cells by HIV-1 (6, 20), high viral replication is supported by Foxp3 expressing CD4 T cells. Here we identify Foxp3+ CD4 T cells as the subset with significantly higher expression of CCR5 and CXCR4 as compared to Foxp3− CD4 T cells in PFMC. Consistent with increased HIV-1 co-receptor expression, we found higher levels of HIV-1 SS DNA in immune-magnetically isolated CD4+CD25+CD127 −T cells as compared to un-separated PFMC. HIV-1 SS DNA was notably higher in CD4+CD25+CD127 −PFMC T cells from the two HIV-1 infected patients who were MTB culture positive as compared to the subject who proved to have pleural mesothelioma and not TB. All together, these data indicate that Foxp3+ CD4 T cells at sites of pleural HIV/TB co-infection are predisposed to successful and productive HIV-1 infection. Strong correlation of Foxp3 mRNA in PFMC with viral load in the pleural fluid of HIV/TB patients corroborates this contention. Thus, HIV-1 p24+ Foxp3+ CD4 T cells at sites of HIV/TB may be a significant source of HIV-1. However, only a modest and insignificant correlation of Foxp3 mRNA expression in PFMC with pleural fluid viral load was found in our own previous study (14). Whereas, the number of patients in the current study were higher (16 vs 10) than previously (14), differential cell handling may have contributed to this discrepancy also.
Here, HIV-1 p24+ Foxp3+ CD4 T cells in PFMC were higher in expression of the anti-apoptotic molecule, Bcl-2 as compared to HIV-1 p24+ Foxp3− CD4 T cells. Strong Th1 signaling provided at sites of HIV/TB co-infection (14, 19), in the presence of continuous TGF-β signaling necessary in maintaining expression of Foxp3 (32), likely underlies the expanded Bcl-2+ Foxp3+ CD4 T cells in PFMC. However, others have shown that among Foxp3 targeted genes, expression of Bcl-2 is actually down-regulated (33), possibly based on interaction of the transcriptional factor Eos with Foxp3 gene (34). While it is possible that at sites of HIV/TB co-infection, the interaction of Foxp3 and Eos is altered, this needs to be investigated. The higher Bcl-2 expression of HIV-1 p24+ Foxp3+ T-cells as compared to HIV-1 p24− Foxp3+ T-cells, and the correlation of Bcl-2 positivity with Foxp3+p24+ reactivity of PFMC T cells, implies a survival advantage of HIV-1 infected Foxp3+ CD4 T cells.
The stability of Foxp3 expression in natural T-reg has been ascribed to the highly de-methylated conserved non-coding DNA sequence 2 (CNS2) in the Foxp3 promoter, which is not present in induced T-reg. However, TGF-β has been shown to decrease methylation of CNS2 thus stabilizing Foxp3 expression in iT-reg (12, 13). It is possible that at sites of HIV/TB co-infection, high levels of bioactive TGF-β induce and maintain Fox p3 expression. Further, recent studies have shown that bystander inflammation, conditions naïve T cells to Foxp3 expression and reduced effector cell differentiation (35). The correlation of Foxp3 mRNA expression in PFMC from HIV/TB co-infected patients with pleural fluid levels of IL-6 and IL-8 and to a lesser extent TGF-β, but not at all with levels of IFN-γ (14), support this latter scenario. Overall, these data implicate induction of Foxp3 mRNA expression by inflammatory cytokines and maintenance of its expression by TGF-β at sites of HIV/TB co-infection.
Recently, it has been shown that CD4+Foxp3+ T-cells can produce IFN-γ when activated under a Th1 cytokine polarizing environment (15, 16). In fact, it has been suggested that IFN-γ production identifies pathogen epitope-specific CD4+Foxp3+ T-cells during viral infections (36). Here, over 25% of CD4+PFMC T cells that were Foxp3+CD127− T-cells were also IFN-γ+. Similar to the model of Graft-versus-Host Disease (37) IFN-γ expression by Foxp3+ CD4 T cells may be important to their immune-regulatory function. However, IFN-γ positivity may actually identify Foxp3+ CD4 T cells that are predisposed to loss of Foxp3 expression and conversion to a Th1 T cell phenotype (38, 39). Also, at sites of inflammation, certain Foxp3+ CD4 T cells may undergo rapid reprogramming into Th1 like T-cells, without loss of expression of Foxp3 (40). The true fate of IFN-γ producing Foxp3+ CD4 T cells at sites of dual HIV/TB co-infection remains to be explored.
In summary, high frequencies of HIV-1 infected Foxp3+ CD4 T cells that display features of survival at pleural sites of HIV/TB co-infection were found. Whether HIV-1 24 expressing Foxp3+ CD4 T cells convert to CD4 T cells with ‘silent’ HIV-1 infection upon resolution of TB needs to be further studied.
Acknowledgments
This study was supported by NIH grants HL 51636 and AI-1080313, and the Tuberculosis Research Unit grant (AI-70022).
Footnotes
None of the authors have a commercial or other association that might pose a conflict of interest.
References
- 1.Toossi Z, Johnson JL, Kanost RA, Wu M, Luzze H, Peters P, et al. Increased Replication of HIV-1 at Sites of Mycobacterium tuberculosis Infection: Potential Mechanisms of Viral Activation. J Acquir Immune Defic Syndr. 2001 Sep 1;28(1):1–8. doi: 10.1097/00042560-200109010-00001. [DOI] [PubMed] [Google Scholar]
- 2.Guyot-Revol V, Innes JA, Hackforth S, Hinks T, Lalvani A. Regulatory T Cells are Expanded in Blood and Disease Sites in Tuberculosis Patients. Am J Respir Crit Care Med. 2005 Dec 9; doi: 10.1164/rccm.200508-1294OC. [DOI] [PubMed] [Google Scholar]
- 3.Scott-Browne JP, Shafiani S, Tucker-Heard G, Ishida-Tsubota K, Fontenot JD, Rudensky AY, et al. Expansion and function of Foxp3-expressing T regulatory cells during tuberculosis. J Exp Med. 2007 Sep 3;204(9):2159–69. doi: 10.1084/jem.20062105. Epub 2007/08/22. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma PK, Saha PK, Singh A, Sharma SK, Ghosh B, Mitra DK. FoxP3+ regulatory T cells suppress effector T-cell function at pathologic site in miliary tuberculosis. Am J Respir Crit Care Med. 2009 Jun 1;179(11):1061–70. doi: 10.1164/rccm.200804-529OC. Epub 2009/02/28. eng. [DOI] [PubMed] [Google Scholar]
- 5.Moreno-Fernandez ME, Zapata W, Blackard JT, Franchini G, Chougnet CA. Human regulatory T cells are targets for human immunodeficiency Virus (HIV) infection, and their susceptibility differs depending on the HIV type 1 strain. J Virol. 2009 Dec;83(24):12925–33. doi: 10.1128/JVI.01352-09. Epub 2009/10/16. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oswald-Richter K, Grill SM, Shariat N, Leelawong M, Sundrud MS, Haas DW, et al. HIV infection of naturally occurring and genetically reprogrammed human regulatory T-cells. PLoS Biol. 2004 Jul;2(7):E198. doi: 10.1371/journal.pbio.0020198. Epub 2004/07/15. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity. 2009 May;30(5):626–35. doi: 10.1016/j.immuni.2009.05.002. Epub 2009/05/26. eng. [DOI] [PubMed] [Google Scholar]
- 8.Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25- T cells through Foxp3 induction and down-regulation of Smad7. J Immunol. 2004 May 1;172(9):5149–53. doi: 10.4049/jimmunol.172.9.5149. Epub 2004/04/22. eng. [DOI] [PubMed] [Google Scholar]
- 9.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003 Dec 15;198(12):1875–86. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Isomura I, Palmer S, Grumont RJ, Bunting K, Hoyne G, Wilkinson N, et al. c-Rel is required for the development of thymic Foxp3+ CD4 regulatory T cells. J Exp Med. 2009 Dec 21;206(13):3001–14. doi: 10.1084/jem.20091411. Epub 2009/12/10. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corbett EL. HIV and tuberculosis: surveillance revisited. Int J Tuberc Lung Dis. 2003 Aug;7(8):709. Epub 2003/08/19. eng. [PubMed] [Google Scholar]
- 12.Floess S, Freyer J, Siewert C, Baron U, Olek S, Polansky J, et al. Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol. 2007 Feb;5(2):e38. doi: 10.1371/journal.pbio.0050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lal G, Zhang N, van der Touw W, Ding Y, Ju W, Bottinger EP, et al. Epigenetic regulation of Foxp3 expression in regulatory T cells by DNA methylation. J Immunol. 2009 Jan 1;182(1):259–73. doi: 10.4049/jimmunol.182.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toossi Z, Hirsch CS, Wu M, Mayanja-Kizza H, Baseke J, Thiel B. Distinct cytokine and regulatory T cell profile at pleural sites of dual HIV/tuberculosis infection compared to that in the systemic circulation. Clin Exp Immunol. 2011 Mar;163(3):333–8. doi: 10.1111/j.1365-2249.2010.04269.x. Epub 2011/02/10. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dominguez-Villar M, Baecher-Allan CM, Hafler DA. Identification of T helper type 1-like, Foxp3+ regulatory T cells in human autoimmune disease. Nat Med. 2011 Jun;17(6):673–5. doi: 10.1038/nm.2389. Epub 2011/05/05. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei B, Baker S, Wieckiewicz J, Wood KJ. IFN-gamma triggered STAT1-PKB/AKT signalling pathway influences the function of alloantigen reactive regulatory T cells. Am J Transplant. 2010 Jan;10(1):69–80. doi: 10.1111/j.1600-6143.2009.02858.x. Epub 2009/11/06. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fragale A, Gabriele L, Stellacci E, Borghi P, Perrotti E, Ilari R, et al. IFN regulatory factor-1 negatively regulates CD4+ CD25+ regulatory T cell differentiation by repressing Foxp3 expression. J Immunol. 2008 Aug 1;181(3):1673–82. doi: 10.4049/jimmunol.181.3.1673. [DOI] [PubMed] [Google Scholar]
- 18.Zhou X, Jeker LT, Fife BT, Zhu S, Anderson MS, McManus MT, et al. Selective miRNA disruption in T reg cells leads to uncontrolled autoimmunity. J Exp Med. 2008 Sep 1;205(9):1983–91. doi: 10.1084/jem.20080707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barnes PF, Fong SJ, Brennan PJ, Twomey PE, Mazumder A, Modlin RL. Local production of tumor necrosis factor and IFN-gamma in tuberculous pleuritis. J Immunol. 1990;145(1):149–54. [PubMed] [Google Scholar]
- 20.Antons AK, Wang R, Oswald-Richter K, Tseng M, Arendt CW, Kalams SA, et al. Naive precursors of human regulatory T cells require FoxP3 for suppression and are susceptible to HIV infection. J Immunol. 2008 Jan 15;180(2):764–73. doi: 10.4049/jimmunol.180.2.764. Epub 2008/01/08. eng. [DOI] [PubMed] [Google Scholar]
- 21.Oswald-Richter K, Grill SM, Leelawong M, Unutmaz D. HIV infection of primary human T cells is determined by tunable thresholds of T cell activation. Eur J Immunol. 2004 Jun;34(6):1705–14. doi: 10.1002/eji.200424892. Epub 2004/05/27. eng. [DOI] [PubMed] [Google Scholar]
- 22.Holmes D, Knudsen G, Mackey-Cushman S, Su L. FoxP3 enhances HIV-1 gene expression by modulating NFkappaB occupancy at the long terminal repeat in human T cells. J Biol Chem. 2007 Jun 1;282(22):15973–80. doi: 10.1074/jbc.M702051200. Epub 2007/04/10. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grant C, Oh U, Fugo K, Takenouchi N, Griffith C, Yao K, et al. Foxp3 represses retroviral transcription by targeting both NF-kappaB and CREB pathways. PLoS Pathog. 2006 Apr;2(4):e33. doi: 10.1371/journal.ppat.0020033. Epub 2006/05/03. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Selliah N, Zhang M, White S, Zoltick P, Sawaya BE, Finkel TH, et al. FOXP3 inhibits HIV-1 infection of CD4 T-cells via inhibition of LTR transcriptional activity. Virology. 2008 Nov 25;381(2):161–7. doi: 10.1016/j.virol.2008.08.033. Epub 2008/10/03. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirsch CS, Toossi Z, Johnson JL, Luzze H, Ntambi L, Peters P, et al. Augmentation of Apoptosis and Interferon-gamma Production at Sites of Active Mycobacterium tuberculosis Infection in Human Tuberculosis. J Infect Dis. 2001;183(5):779–88. doi: 10.1086/318817. [DOI] [PubMed] [Google Scholar]
- 26.Toossi Z, Mayanja-Kizza H, Baseke J, Peters P, Wu M, Abraha A, et al. Inhibition of human immunodeficiency virus-1 (HIV-1) by beta-chemokine analogues in mononuclear cells from HIV-1-infected patients with active tuberculosis. Clin Exp Immunol. 2005 Nov;142(2):327–32. doi: 10.1111/j.1365-2249.2005.02913.x. Epub 2005/10/20. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fazekas de St Groth B, Zhu E, Asad S, Lee L. Flow cytometric detection of human regulatory T cells. Methods Mol Biol. 2011;707:263–79. doi: 10.1007/978-1-61737-979-6_17. Epub 2011/02/03. eng. [DOI] [PubMed] [Google Scholar]
- 28.Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006 Jul 10;203(7):1693–700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Z, Schuler T, Zupancic M, Wietgrefe S, Staskus KA, Reimann KA, et al. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science. 1999 Nov 12;286(5443):1353–7. doi: 10.1126/science.286.5443.1353. [DOI] [PubMed] [Google Scholar]
- 30.Lawn SD, Pisell TL, Hirsch CS, Wu M, Butera ST, Toossi Z. Anatomically compartmentalized human immunodeficiency virus replication in HLA-DR+ cells and CD14+ macrophages at the site of pleural tuberculosis coinfection. J Infect Dis. 2001 Nov 1;184(9):1127–33. doi: 10.1086/323649. [DOI] [PubMed] [Google Scholar]
- 31.Kinter AL, Hennessey M, Bell A, Kern S, Lin Y, Daucher M, et al. CD25(+)CD4(+) regulatory T cells from the peripheral blood of asymptomatic HIV-infected individuals regulate CD4(+) and CD8(+) HIV-specific T cell immune responses in vitro and are associated with favorable clinical markers of disease status. J Exp Med. 2004 Aug 2;200(3):331–43. doi: 10.1084/jem.20032069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li C, Ebert PJ, Li QJ. T cell receptor (TCR) and transforming growth factor beta (TGF-beta) signaling converge on DNA (cytosine-5)-methyltransferase to control forkhead box protein 3 (foxp3) locus methylation and inducible regulatory T cell differentiation. J Biol Chem. 2013 Jun 28;288(26):19127–39. doi: 10.1074/jbc.M113.453357. Epub 2013/05/21. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng Y, Josefowicz SZ, Kas A, Chu TT, Gavin MA, Rudensky AY. Genome-wide analysis of Foxp3 target genes in developing and mature regulatory T cells. Nature. 2007 Feb 22;445(7130):936–40. doi: 10.1038/nature05563. Epub 2007/01/24. eng. [DOI] [PubMed] [Google Scholar]
- 34.Pan F, Yu H, Dang EV, Barbi J, Pan X, Grosso JF, et al. Eos mediates Foxp3-dependent gene silencing in CD4+ regulatory T cells. Science. 2009 Aug 28;325(5944):1142–6. doi: 10.1126/science.1176077. Epub 2009/08/22. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thompson LJ, Lai JF, Valladao AC, Thelen TD, Urry ZL, Ziegler SF. Conditioning of naive CD4 T cells for enhanced peripheral Foxp3 induction by nonspecific bystander inflammation. Nat Immunol. 2016 Jan 11; doi: 10.1038/ni.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao J, Fett C, Trandem K, Fleming E, Perlman S. IFN-gamma- and IL-10-expressing virus epitope-specific Foxp3(+) T reg cells in the central nervous system during encephalomyelitis. J Exp Med. 2011 Aug 1;208(8):1571–7. doi: 10.1084/jem.20110236. Epub 2011/07/13. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koenecke C, Lee CW, Thamm K, Fohse L, Schafferus M, Mittrucker HW, et al. IFN-gamma production by allogeneic Foxp3+ regulatory T cells is essential for preventing experimental graft-versus-host disease. J Immunol. 2012 Sep 15;189(6):2890–6. doi: 10.4049/jimmunol.1200413. Epub 2012/08/08. eng. [DOI] [PubMed] [Google Scholar]
- 38.Chen Z, Barbi J, Bu S, Yang HY, Li Z, Gao Y, et al. The ubiquitin ligase Stub1 negatively modulates regulatory T cell suppressive activity by promoting degradation of the transcription factor Foxp3. Immunity. 2013 Aug 22;39(2):272–85. doi: 10.1016/j.immuni.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martinez-Llordella M, Ashby M, et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009 Sep;10(9):1000–7. doi: 10.1038/ni.1774. Epub 2009/07/28. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharma MD, Huang L, Choi JH, Lee EJ, Wilson JM, Lemos H, et al. An inherently bifunctional subset of Foxp3+ T helper cells is controlled by the transcription factor eos. Immunity. 2013 May 23;38(5):998–1012. doi: 10.1016/j.immuni.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]


