Abstract
Alcohol and nicotine are frequently co-used and co-abused, and use of both drugs alone can affect hepatic drug metabolism. We investigated the influences of chronic nicotine treatment and voluntary ethanol intake on the induction of rat hepatic cytochrome P450 (CYP) enzymes that metabolize ethanol and nicotine. Rats were trained to voluntarily drink ethanol (6% v/v, 1 h) with nicotine pretreatment for 10 days. Another group of rats were treated with the same nicotine doses alone. Hepatic CYP2E1, CYP2B1/2 and CYP2D1 proteins were assessed by immunoblotting. Nicotine pretreatment (0.4, 0.8 and 1.2 mg/kg) increased voluntary ethanol intake on day 10 by 1.8, 2.0, and 1.4 fold respectively compared to saline pretreatment (P<0.01–0.3). CYP2E1 was increased 1.7, 1.8, and 1.4 fold by the three doses of nicotine alone (P<0.02–0.21); CYP2E1 levels were increased by voluntary ethanol intake alone and a further 2.4, 2.2, and 1.8 fold by 0.4, 0.8, and 1.2 mg/kg nicotine respectively versus saline pretreatment (P<0.002–0.06). CYP2B1/2 proteins were not induced by nicotine alone, but were increased by 2.2–2.5 fold by ethanol drinking (P<0.05). CYP2E1 (r=0.67, P<0.001) and CYP2B1/2 levels (r=0.49, P=0.007) correlated with alcohol consumption on day 10. There was no change in CYP2D1. Chronic nicotine increased voluntary ethanol intake thereby enhancing CYP2E1 and CYP2B1/2 levels. Thus CYPs are regulated not only directly by nicotine and ethanol, but also indirectly via an increase in the ethanol consumption in the presence of nicotine pretreatment. Together this may contribute to the co-abuse of these drugs and alter the metabolism of clinical drugs and endogenous substrates.
Keywords: Ethanol self-administration, Cytochrome P450 2B1/2, Cytochrome P450 2E1, Nicotine, Alcohol
1. Introduction
Alcohol and nicotine are frequently co-used and co-abused by humans. Among alcoholics, approximately 90% smoke cigarettes and 70% are heavy smokers, compared to 30% of non-alcoholics (Batel et al., 1995); the occurrence of alcoholism is estimated to be 10 times higher among smokers compared to non-smokers (DiFranza and Guerrera, 1990). Nicotine treatment in animal models can also increase ethanol self-administration (Clark et al., 2001). The use of alcohol and nicotine alone is known to affect hepatic drug metabolism through the induction of a variety of cytochrome P450 (CYP) enzymes including CYP2E1 and CYP2B. The effects, however, of the co-administration of the two drugs on hepatic levels of these enzymes remains unknown, and needs to be assessed in order to understand the effects of the frequent co-use of alcohol and nicotine on drug metabolism. Previous studies have shown that co-administration of ethanol with other drugs can potentiate the induction of CYP2E1, as well as its toxicity (Hoet et al., 2002; Kessova et al., 2001).
CYP2E1 metabolizes numerous chemicals including cytotoxins, procarcinogens, and clinical drugs (Lieber, 1997). CYP2E1’s high NADPH oxidase activity results in increased free oxygen radicals production (Zhukov and Ingelman-Sundberg, 1999) causing lipid peroxidation and cell damage. CYP2E1 metabolizes approximately 20% of ethanol at low blood concentrations which increases to 60% at high concentrations (Lieber, 1994; Matsumoto et al., 1996). Hepatic CYP2E1 levels can be induced by ethanol administration in rats and chronic nicotine treatment also induces hepatic CYP2E1 protein and activity (Howard et al., 2001). The induction of hepatic CYP2E1, and the resultant increases in the rate of ethanol metabolic inactivation, could aid in the higher alcohol consumption in human smokers and in nicotine-treated animals (Schoedel and Tyndale, 2003).
CYP2B1/2 can metabolize numerous compounds including clinical drugs, pesticides, steroids, and drugs of abuse (Wang and Tompkins, 2008). In rats CYP2B1/2 are the primary nicotine-metabolizing enzymes (Nakayama et al., 1993). The constitutive expression of CYP2B1/2 is low and variable in rats. Chronic ethanol induces rat liver CYP2B1/2 levels, via a transcriptional mechanism, resulting in increased in vitro metabolism of nicotine in rat liver microsomes (Adir et al., 1980; Schoedel et al., 2001). Chronic nicotine treatment does not alter rat or monkey hepatic CYP2B levels (Lee et al., 2006; Miksys et al., 2000a) nor are the levels higher in human smokers (Hesse et al., 2004).
We investigated the influence of nicotine pretreatment on the voluntary intake of ethanol, and the combined effects of these drugs on the levels of CYP2E1 and CYP2B in rat liver, with CYP2D used as a control. We hypothesized that hepatic CYP2E1 levels will be induced by both nicotine and ethanol alone, and co-administration will have a greater effect than the two drugs alone; CYP2B1/2 will be induced by ethanol but not directly by nicotine, and CYP2D1 levels will remain unaffected. The effects of ethanol and nicotine co-administration on hepatic drug-metabolizing enzymes have not been previously reported, and this study will add to our knowledge of the effects of these commonly co-abused drugs on drug-metabolizing enzymes.
2. Methods
2.1. Animals
All experimental procedures were conducted in accordance with the Canadian and NIH guidelines for the care and use of laboratory animals and approved by the Animal Care Committee of the University of Toronto or the University of Pennsylvania. Alternatives to live animal models (e.g. cell lines) were considered, but deemed unsuitable for this study. Adult male Wistar rats were maintained on a 12 h artificial light/dark cycle (lights on at 6:00 AM) throughout the study period. Adult male rats were chosen to avoid any effects of estrous cycle as well as differences in growth-hormone secretion on hepatic CYP expression (Shapiro et al., 1995). For the ethanol self-administration portion of the study the rats used were between 325 and 350 g at the beginning of the study. For the rats that received nicotine alone, the animals were matched by age and weight with the rats from the self-administration study at the end of the study duration (about 190 days old, 450 to 500 g).
2.2. Drug treatment
Rats received two consecutive training periods before the evaluation of ethanol self-administration was begun. Initially rats had access to ethanol, but not water, 24 h/day in ascending concentrations from 2% to 4% to 6% v/v until stable drinking was established at 6% (30–40 days from start of training) as previously performed (Stromberg et al., 1998). 24 h after the end of continuous ethanol training, the rats were shifted to a 1-hour limited access paradigm; access to water was not restricted. Rats were given access to both ethanol and water bottles for 1 h, and these bottles were randomly rotated to avoid the development of side preference. This was continued until ethanol consumption during the limited access period was stabilized (less than 20% change in consumption across five consecutive days). To determine the effects of chronic nicotine on ethanol self-administration, rats were matched for baseline ethanol consumption and were assigned randomly to one of 4 groups (n=5–6 per group), so that the average baseline alcohol consumption in each experimental group was approximately equivalent. Baseline drinking was derived from the average of 5 days of ethanol self-administration prior to randomization. Rats were injected subcutaneously with nicotine bitartrate (0.0, 0.4, 0.8 and 1.2 mg base/kg, in sterile saline, pH 7.4) once per day for 10 days, 30 min before 1 h access to ethanol (6% v/v); this treatment paradigm has been previously used to assess the effects of drugs on ethanol self-administration (Stromberg, 2004). Rats will self-administer between 0.15 and 1.5 mg/kg of nicotine within self-administration sessions lasting 1–2 h (Matta et al., 2007), while human smokers, which are slower nicotine metabolizers than rats, consume approximately 0.5 mg/kg/day (Benowitz and Jacob, 1984). Together with plasma concentration data (Micu et al., 2003), this suggests that the doses used in this study are similar to the nicotine acquired from smoking approximately 10 cigarettes (Le Houezec et al., 1993). The rats voluntarily consumed between 0.4 and 1.5 g/kg of ethanol per day resulting in estimated peak ethanol levels of around 50–120 mg/dl (Buczek et al., 1997), which would be similar to plasma levels achieved in humans after 1–5 standard drinks (social drinking) (Wilkinson et al., 1977). Immediately following the last limited access ethanol intake session on the 10th day, the rats were sacrificed (1.5 h after the nicotine injections) and the livers removed, frozen immediately in liquid nitrogen, and stored at −80 °C. The total training and experimental period was around 80 days, and the rats were about 200 days old at sacrifice. There were no significant differences in body weight among the 4 treatment groups.
To evaluate the effects of nicotine treatment alone on hepatic CYP2E1 and CYP2B levels, another set of rats (n=6 per group) were injected subcutaneously daily for 10 days with nicotine (0.0, 0.4, 0.8 and 1.2 mg base/kg, in sterile saline, pH 7.4) and sacrificed at 1.5 h after the last injection. The livers were removed immediately and stored at −80 °C. In our previous studies, subcutaneous nicotine injections of 1.0 mg base/kg for 7 days lead to significant increases in hepatic CYP2E1 protein levels (Howard et al., 2001; Micu et al., 2003) while having no effect on CYP2B levels (Miksys et al., 2000a); 18 days of nicotine treatment did not further increase hepatic CYP2E1 over levels seen after 7 days treatment (Micu et al., 2003).
2.3. Microsomal membrane preparation and protein assay
Liver microsomal membranes were prepared as described previously (Miksys et al., 2000b). Briefly, portions of livers were homogenized manually in 100 mM Tris, pH 7.4, with 0.1 mM EDTA, 0.32 M sucrose, 0.1 mM dithiothreitol on ice. Homogenates were centrifuged at 9000 g for 20 min, and the supernatant was centrifuged at 110,000 g yielding a microsomal pellet. The pellets were aliquoted in storage solution (100 mM Tris (pH 7.4), 0.1 mM EDTA, 0.1 mM dithiothreitol, 1.15% w/v KCl and 20% v/v glycerol) and stored at −80 °C. The protein concentration was assayed with the Bradford technique using a Bio-Rad Protein Assay kit (Bio-Rad Laboratories, Mississauga, Canada).
2.4. Immunoblotting
Liver microsomal membranes were serially diluted to construct standard curves and establish the linear detection range for the immunoblotting assays. To determine cross-reactivity of the primary antibodies, cDNA-expressed rat CYP1A1, CYP1A2, CYP2A1, CYP2A2, CYP2B1, CYP2E1, CYP2C11, CYP2D1 and CYP3A2 (BD Biosciences, Mississauga, Canada) were used as negative or positive controls. In subsequent experiments 1.25, 5 or 0.5 μg of microsomal proteins were loaded for detection of CYP2E1, or CYP2B1/2 or CYP2D1 respectively. The proteins were separated by SDS-polyacrylamide gel electrophoresis (4% stacking and 8% separating gels), and then transferred overnight onto nitrocellulose membranes. To detect hepatic CYP2E1, the membranes were immunoblotted with polyclonal goat anti-rat CYP2E1 antibody (BD Biosciences), diluted 1:3000, for 1.5 h. Blots were then incubated with peroxidase-conjugated rabbit anti-sheep antibody (1:7000, Millipore, Billerica, MA), which also acts as an anti-goat antibody, for 1 h. The membranes were blocked with 1% skim milk in 50 mM Tris-buffered saline containing 0.1% w/v BSA, 0.1% v/v Triton X-100. For the detection of hepatic CYP2B1/2, the membranes were incubated with polyclonal rabbit anti-rat CYP2B1/2 antibody (1:750, Millipore) overnight at 4 °C. The membranes were then incubated with peroxidase-conjugated sheep anti-rabbit antibody (1:3000, Millipore) for 1 h. The membranes were blocked with 4% skim milk in 50 mM Tris-buffered saline containing 0.1% w/v BSA, 0.1% v/v Triton X-100. For the detection of CYP2D1, the membranes were blocked with 1% skim milk in 50 mM Tris-buffered saline containing 0.1% w/v BSA and 0.1% v/v Triton X-100 and were probed using polyclonal rabbit antihuman CYP2D antibody (A gift from A. Cribb and Merck & Co., Whitehouse Station, NJ) diluted 1:5000 for 1 h. Blots were then incubated with peroxidase-conjugated sheep anti-rabbit antibody (1:3000, Millipore). The equivalence of sample loading was confirmed by Coomassie blue staining. CYP proteins were visualized using chemiluminescence followed by exposure to autoradiography film. Immunoblots were analyzed using MCID Elite software (InterFocus Imaging Ltd., Linton, UK), and the relative density of each band was expressed as arbitrary density unit after subtracting background.
2.5. Statistical analyses
Ethanol intake data were expressed as g/kg/h and results were expressed as group mean±standard deviations. A repeated measures ANOVA was used to assess the effects of nicotine treatment and duration of treatment on voluntary ethanol drinking. One-way ANOVA followed by a post-hoc test (least significant difference, LSD) was used to test the differences in ethanol self-administration on individual treatment days among the different groups. Paired Student’s t-tests were used to test the differences in each group between ethanol intake on any treatment day and respective baseline consumption. The differences in the relative increases in ethanol drinking over baseline consumption on day 10 between rats pretreated with nicotine or saline were tested by a one-way ANOVA followed by a post-hoc test (LSD). For immunoblotting data, the average values were obtained from at least 4–6 separate assessments. One-way ANOVAs followed by post-hoc testing (LSD) were used to test the differences in CYP levels from rats treated with different doses of nicotine, or from rats pretreated with different doses of nicotine and then allowed to self-administer ethanol. The differences in CYP levels between rats in different groups (e.g. saline alone versus saline pretreatment and voluntary ethanol intake) were evaluated by unpaired Student’s t-tests. Pearson correlation coefficients were derived between the levels of ethanol consumed on day 10 and the levels of CYP proteins.
3. Results
3.1. The changes in voluntary ethanol intake after chronic nicotine treatment
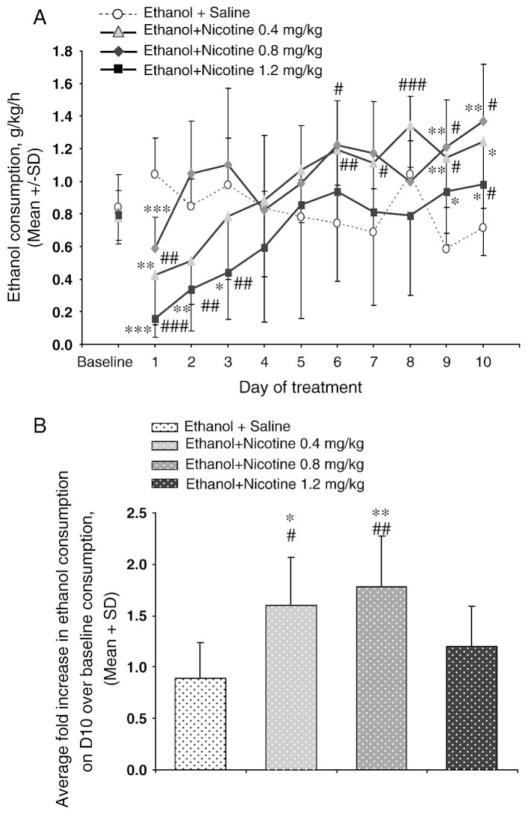
There was no significant difference in baseline ethanol drinking among the groups (Fig. 1A). Repeated measures ANOVA revealed a significant treatment×day interaction (F (30,190)=4.2, P<0.001), and a significant effect of day (F (10, 190)=10.6, P<0.001) with no significant effect of nicotine treatment (F (3, 19)=1.9, P=0.156). This indicates that the impact of nicotine pretreatment on ethanol intake was dependent on the treatment duration, consistent with the biphasic effect observed (Fig. 1A). On the first day, nicotine pretreatment (0.4, 0.8 or 1.2 mg/kg) significantly suppressed ethanol intake (P<0.05 versus their own baseline or versus saline pretreatment). Ethanol intake returned to baseline consumption in rats pretreated with 0.4 or 0.8 mg/kg of nicotine by days 2–4. By day 10 voluntary ethanol intake was increased by all doses of nicotine pretreatment over baseline consumption (Fig. 1B). The increases were 1.8 (P<0.05), 2.0 (P<0.01), and 1.4 fold (P=0.3) greater in the 0.4, 0.8, and 1.2 mg/kg nicotine-treated animals respectively, compared to the saline pretreated animals. The effect of the highest dose appeared more modest, consistent with the ethanol drinking returning to baseline by day 5 rather than earlier as seen with the lower doses (Fig. 1A). In rats with saline pretreatment, no significant change in ethanol intake was observed between the baseline and the post-treatment sessions on any treatment day as tested by paired Student’s t-test (Fig. 1A).
Fig. 1.
Nicotine pretreatment increases ethanol self-administration. A) Ethanol self-administration was initially suppressed by nicotine pretreatments, increasing with duration. Consumption values at baseline represent the average alcohol consumption over 5 days prior to testing period with saline or nicotine pretreatment. B) The average fold increase in ethanol self-administration, over their own baseline consumption, by day 10 was marked in rats pretreated with 0.4 and 0.8 mg/kg of nicotine and modest for rats pretreated with 1.2 mg/kg of nicotine. n=5–6 per group, ###P<0.001, ##P<0.01, #P<0.05 versus each groups respective baseline consumption, ***P<0.001, **P<0.01, *P<0.05 versus the saline pretreatment group.
3.2. The induction of CYP2E1 following voluntary ethanol intake and nicotine treatments
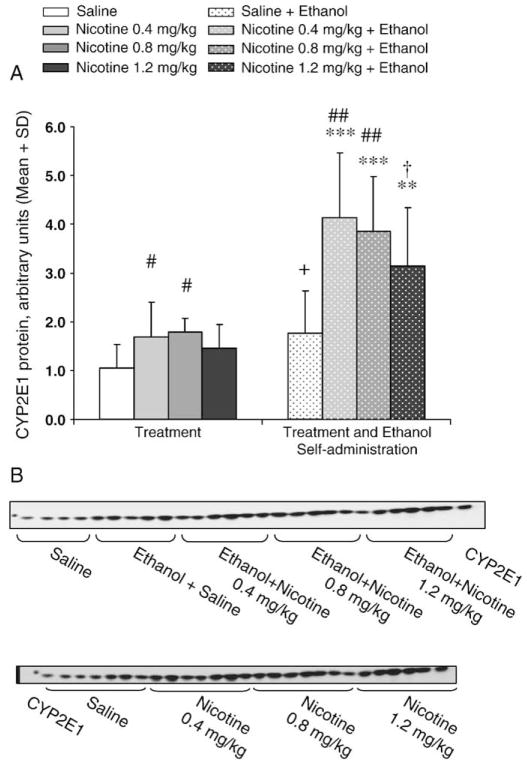
A quantitative immunoblotting assay was developed, and detection of CYP2E1 in serially diluted liver microsomal protein was shown to be linear (Fig. 2A). No cross-reactivity was observed with other cDNA-expressed rat CYPs, indicating the specificity of the polyclonal goat anti-rat CYP2E1 antibody under the conditions used here (Fig. 2B). The selectivity and linearity of the assays for CYP2E1, CYP2B1/2, and CYP2D1 were established to ensure that the manufacturer-stated specificity and selectivity of the antibody still applied to rat proteins under our assay conditions and to ensure that the assays provided linear detection for quantification. Rat liver CYP2E1 isoform co-migrated with cDNA-expressed rat CYP2E1 (Fig. 3B). To ensure that the increases seen in hepatic CYP2E1 levels were being measured accurately and were not being affected by the linear detection limits of the assay, samples were diluted to saline levels and reassayed. Any samples remaining elevated above saline levels indicated an initial underestimation of CYP2E1 and the data were adjusted accordingly. This adjustment would ensure that all samples assayed were within the linear response range of the assay. CYP2E1 levels were 1.7 (P=0.05, LSD), 1.8 (P=0.02, LSD), and 1.4 fold (P=0.21, LSD) higher in the rats treated with 0.4, 0.8, and 1.2 mg/kg nicotine respectively compared to the saline alone. CYP2E1 levels trended towards being higher in the rats that drank ethanol with saline pretreatment compared to saline treatment alone (1.8 fold, P=0.06) (Fig. 3A and Table 1). Among the rats allowed to voluntarily intake ethanol, CYP2E1 levels were increased by 2.4 fold (P=0.003, LSD) by 0.4 mg/kg nicotine pretreatment, 2.2 fold by 0.8 mg/kg nicotine pretreatment (P=0.007, LSD), and by 1.8 fold by 1.2 mg/kg nicotine pretreatment (P=0.06, LSD) compared to saline pretreatment. When the fold induction was compared to those with nicotine treatment alone, CYP2E1 levels in rats that had access to ethanol with 0.4, 0.8, and 1.2 mg/kg nicotine pretreatments were 2.5 (P=0.001), 2.2 (P=0.0007), and 2.2 fold (P=0.005) higher than the levels seen in rats treated with the corresponding doses of nicotine alone, respectively.
Fig. 2.
Quantification of rat liver CYP2E1 protein by immunoblotting. A) A dilution curve of microsomal proteins from a saline-treated animal indicates linear detection of CYP2E1 protein from 0.75 to 3.5 μg of protein loaded; the insert shows a representative immunoblot. B) The CYP2E1 antibody did not cross-react with cDNA-expressed rat CYP1A1, CYP1A2, CYP2A1, CYP2A2, CYP2B1, CYP2C11, CYP2D1 and CYP3A2, but detected a 5-fold lower amount of cDNA-expressed CYP2E1.
Fig. 3.
Hepatic CYP2E1 was induced by nicotine alone and by ethanol self-administration which was further enhanced by nicotine pretreatment. A) CYP2E1 was significantly induced by nicotine treatment versus respective saline treatments in rats treated with nicotine alone (0.4 and 0.8 mg/kg) and in rats pretreated with nicotine (0.4, 0.8, and 1.2 mg/kg) and allowed to self-administer (SA) ethanol. Ethanol self-administration following saline pretreatment also increased CYP2E1 relative to saline treatment alone. n=5–6 per group, ##P<0.01, #P<0.05, †P<0.06 versus saline within group using LSD, ***P<0.001, **P<0.01, +P<0.06 versus respective saline or nicotine treatment alone between groups by unpaired Student’s t-test. B) Representative immunoblots of CYP2E1 proteins from rats exposed to saline, nicotine and ethanol self-administration with or without nicotine pretreatment.
Table 1.
Average Relative Optical Density (O.D.) values for the expression and induction of hepatic CYP2E1, CYP2B1/2, and CYP2D1 in rats treated with nicotine and allowed to voluntarily drink alcohol.
| Treatment | CYP2E1
|
CYP2B1/2
|
CYP2D1
|
|||
|---|---|---|---|---|---|---|
| Adjusted
|
Adjusted
|
Adjusted
|
||||
| O.D. | S.D. | O.D. | S.D. | O.D. | S.D. | |
| Saline | 1.00 | 0.48 | 1.00 | 0.86 | 1.00 | 0.24 |
| Nicotine 0.4 mg/kg | 1.67 | 0.72 | 0.86 | 0.56 | 1.05 | 0.26 |
| Nicotine 0.8 mg/kg | 1.77 | 0.29 | 0.92 | 0.92 | 0.83 | 0.15 |
| Nicotine 1.2 mg/kg | 1.44 | 0.50 | 0.93 | 0.35 | 0.95 | 0.22 |
| Saline+ethanol | 1.75 | 0.87 | 2.23 | 1.27 | 1.07 | 0.40 |
| Nicotine 0.4 mg/kg+ ethanol | 4.13 | 1.31 | 2.80 | 2.20 | 1.19 | 0.26 |
| Nicotine 0.8 mg/kg+ ethanol | 3.83 | 1.12 | 2.76 | 1.29 | 0.98 | 0.29 |
| Nicotine 1.2 mg/kg+ ethanol | 3.12 | 1.20 | 2.40 | 1.18 | 0.89 | 0.29 |
The optical density values represented in this table were adjusted so that the values for the saline-treated animals would be 1.00 for each isoform, and all other treatments are represented as a fold induction with their corresponding standard deviation (S.D.).
3.3. The regulation of CYP2B1/2 following voluntary ethanol intake and nicotine treatments
An immunoblotting assay was developed to measure hepatic CYP2B1/2 proteins; detection of CYP2B1/2 was linear in serially diluted liver microsomal membranes (Fig. 4A and Table 1). The specificity of polyclonal rabbit anti-rat CYP2B1/2 antibody under the conditions used in this study was confirmed; no cross-reactivity was observed with other cDNA-expressed rat CYPs (Fig. 4B) and rat liver CYP2B co-migrated with cDNA-expressed rat CYP2B1 (Fig. 5B). Compared with saline treatment alone, the levels of CYP2B1/2 were significantly increased by 2.2 fold (P=0.05) in rats exposed to ethanol with saline pretreatment; the levels of CYP2B1/2 in the rats that drank ethanol with nicotine pretreatment did not differ significantly from those seen in the rats exposed to ethanol with saline pretreatment (Fig. 5A). Nicotine treatment alone (0.4, 0.8 and 1.2 mg/kg) did not alter hepatic CYP2B1/2 protein levels (Fig. 5A) as seen previously (Miksys et al., 2000a).
Fig. 4.
Quantification of rat liver CYP2B1/2 protein by immunoblotting. A) A dilution curve of membrane proteins from a saline-treated animal shows linear CYP2B1/2 protein detection from 5 to 25 μg of protein; the insert shows a representative immunoblot. B) The CYP2B1/2 antibody did not cross-react with cDNA-expressed rat CYP1A1, CYP1A2, CYP2A1, CYP2A2, CYP2C11, CYP2D1, CYP2E1 and CYP3A2, but detected a 5-fold lower amount of cDNA-expressed CYP2B1.
Fig. 5.
Hepatic CYP2B1/2 was induced by ethanol self-administration. A) Ethanol self-administration following saline pretreatment increased CYP2B1/2 relative to saline treatment alone. No induction of CYP2B1/2 proteins was seen by nicotine treatment alone, and nicotine pretreatment followed by ethanol self-administration did not enhance CYP2B1/2 levels versus respective saline or nicotine treatments alone (**P<0.01, *P<0.05 between group by unpaired Student’s t-test). B) Representative immunoblots of CYP2B1/2 proteins from rats exposed to saline, nicotine and ethanol self-administration with or without nicotine pretreatment. Coomassie blue staining is included to illustrate equal loading of protein underscoring the large inter-animal variation in hepatic CYP2B1/2 protein levels.
3.4. The regulation of CYP2D1 following voluntary ethanol intake and nicotine treatments
As a control for potential other effects of ethanol or nicotine (e.g. toxic, proliferative, degradative) on CYP expression in hepatic tissues we also assessed CYP2D1 as hepatic CYP2D6 is unaltered in smokers and alcohol drinkers and is generally not thought to be an inducible enzyme (Edwards et al., 2003; Mann et al., 2008; Rae et al., 2001; Yue et al., 2008). Linearity and specificity were determined in the same manner as CYP2E1 and CYP2B1/2 (Fig. 6A). No differences in the levels of CYP2D1 were seen between any of the treatment or ethanol drinking groups (Fig. 6B and Table 1).
Fig. 6.
CYP2D1 was unaffected by exposure to nicotine and/or ethanol. A) A dilution curve of membrane proteins from a saline-treated animal shows linear CYP2D1 protein detection from 0.4 to 1.2 μg of protein; the insert shows a representative immunoblot. The CYP2D1 antibody did not cross-react with cDNA-expressed rat CYP1A1, CYP1A2, CYP2A1, CYP2A2, CYP2B1, CYP2C11, CYP2E1 and CYP3A2, but detected a 16-fold lower amount of cDNA-expressed CYP2D1. B) Neither nicotine alone, nor ethanol self-administration following saline or nicotine pretreatment increased hepatic CYP2D1. n=5–6 per group; inset illustrates a representative immunoblot of CYP2D1 proteins from rats exposed to saline, nicotine and ethanol self-administration with or without nicotine pretreatment.
3.5. Individual levels of ethanol consumption were positively correlated with hepatic levels of both CYP2E1 and CYP2B1/2 protein, but not with CYP2D1
Among the total group of rats allowed to consume ethanol there was a significant positive correlation (r=0.67, P<0.001) between CYP2E1 protein levels and ethanol intake on the last day (Fig. 7A). While no group differences in CYP2B1/2 levels were seen among the rats allowed to drink ethanol (Fig. 5A), there was a significant positive correlation between CYP2B1/2 protein levels and ethanol self-administration on the last day (r=0.49, P=0.007, Fig. 7B) suggesting a relationship between the degree of ethanol exposure and induction of CYP2B1/2. No correlation was seen between hepatic CYP2D1 levels and ethanol consumption (Fig. 7C).
Fig. 7.
Individual levels of ethanol consumption were positively correlated with hepatic levels of both A) CYP2E1 and B) CYP2B1/2 proteins, but not with C) CYP2D1. Among the 4 groups of animals which self-administered ethanol, there was a significant and positive correlation between the amount of ethanol consumed on day 10 and the levels of hepatic CYP2E1 and CYP2B1/2.
4. Discussion
Alcohol and nicotine are commonly co-abused (Batel et al., 1995), and the individual use of both these drugs alters hepatic levels of cytochrome P450 enzymes (Schoedel et al., 2001); (Micu et al., 2003; Schoedel and Tyndale, 2003). Increases in hepatic CYP2E1 and CYP2B enzymes can alter the metabolism of clinical drugs and toxins and may play a role in the increase in ethanol consumption seen in the presence of chronic nicotine treatment.
Consistent with earlier studies the voluntary intake of ethanol was increased by nicotine pretreatment. We also observed a highly significant correlation between CYP2E1 levels and ethanol consumption on day 10 (immediately prior to sacrifice). The greater increase in CYP2E1 levels seen with nicotine versus saline pretreatment prior to ethanol drinking relative to nicotine treatment versus saline treatment alone suggests either 1) a synergistic effect of the two drugs on hepatic CYP2E1 levels or 2) that nicotine pretreatment increases CYP2E1 both directly and by increasing ethanol consumption which in turn further increases the induction of CYP2E1. While the highest nicotine dose (1.2 mg/kg) alone slightly, although not significantly, increased CYP2E1, it had a greater effect on enhancing CYP2E1 when in combination with ethanol self-administration. Even though this highest nicotine dose had a very modest effect on the intake of ethanol, the increase in CYP2E1 levels was greater than the sum of the effects bythe2 drugs alone, supporting the first contention that there is a synergistic effect of the two drugs on CYP2E1 induction.
The regulation of CYP2E1 is considered to involve multiple mechanisms including transcriptional activation (Ueno and Gonzalez, 1990), stabilization of mRNA (Song et al., 1987), posttranslational modifications (Song et al., 1989), and substrate stabilization of the protein against degradation (Eliasson et al., 1988). The induction of hepatic CYP2E1 proteins by ethanol can occur at transcriptional and/or post-transcriptional levels; ligand-mediated protein stabilization is thought to be the primary mechanism at relatively low ethanol doses (Eliasson et al., 1988). The induction of both hepatic and cerebral CYP2E1 by chronic nicotine treatment is post-transcriptional which may be due to the protein stabilization or translational activation (Howard et al., 2001; Joshi and Tyndale, 2006). Thus, ethanol and chronic nicotine can induce hepatic CYP2E1 via post-transcriptional methods, although while ethanol may stabilize the CYP2E1 protein (Roberts et al., 1995), nicotine does not (Micu et al., 2003).
CYP2E1 generates a high level of reactive oxygen species, without needing a ligand, which can damage cells via the initiation of lipid peroxidation and DNA strand breaks (Caro and Cederbaum, 2004) as well as contributing to the toxicity and carcinogenicity of industrial and environmental chemicals (Gonzalez, 2005). CYP2E1 can also activate tobacco-specific nitrosamines and its induction by nicotine may contribute to the development of tobacco-related cancers (Lieber, 1997; Woodcroft and Novak, 1998). Nicotine-treated rats had a significant increase in reactive oxygen species generation and lipid peroxidation was observed in the pancreatic tissues from these animals (Wetscher et al., 1995; Woodcroft and Novak, 1997), with nicotine-induced CYP2E1 possibly playing a role in this increase. Additionally CYP2E1 has been implicated in the pathogenesis of alcoholic liver disease (ALD), through the production of hydroxyethyl radicals, reactive oxygen species in heavy drinkers (Lieber, 1999). CYP2E1 can also play a role in the development of non-alcoholic steatohepatitis through an oxidative stress mechanism (Leclercq et al., 2000). Thus, the addition of cigarette smoking to the consumption of alcohol may elevate hepatic CYP2E1 and increase the risk for CYP2E1-mediated toxicity beyond the use of either drug alone. The induction of hepatic CYP2E1 by nicotine not only increases ethanol consumption and ethanol-mediated liver damage, but can also increase the risk for a number of non-ethanol associated liver diseases. Elevated CYP2E1 levels could also alter the metabolism of clinically relevant substrates of CYP2E1 such as acetaminophen (Patten et al., 1993), chlorzoxazone (Peter et al., 1990), isoniazid (Ryan et al., 1986), tamoxifen (Styles et al., 1994), and halothane (Spracklin et al., 1997). The altered metabolism of these drugs could alter the therapeutic efficacy for some drugs and even cause toxicity in the case of drugs with a narrow therapeutic index.
The greater CYP2E1 induction seen after chronic nicotine pretreatment and ethanol drinking could in turn contribute to greater clearance of ethanol, and result in increased ethanol consumption. The impact of pretreatment with nicotine on ethanol self-administration was biphasic with an initial suppression followed by an increase in consumption with time. This reduction in ethanol intake during acute nicotine treatment, or in the early days of treatment as we have observed, may be due to initial actions of nicotine as a depressant (Clarke and Kumar, 1983; Stolerman, 1990; Stolerman et al., 1995). These effects were also seen in this study where after initial exposure to nicotine, the rats had modestly difficult breathing as well as reduced locomotion. The rats, however, become tolerant to these effects after 2–4 nicotine injections. Metabolic cross-tolerance through CYP2E1 induction by chronic nicotine treatment might be playing a greater role in the enhancement of ethanol self-administration by chronic nicotine, since chronic (7 days), and not acute, treatment with nicotine was shown to induce hepatic CYP2E1 (Micu et al., 2003), and 6–7 days of nicotine treatment were required for the 0.4 and 0.8 mg/kg nicotine pretreated animals to drink more ethanol than they did at baseline. In addition to metabolic cross-tolerance a variety of factors including genetic factors may contribute to the dependence on both nicotine and ethanol (Bierut et al., 2004; Goldman et al., 2005; Swan et al., 1997; True et al., 1999; Tyndale, 2003). Additionally, other physiological mechanisms could include the ability of nicotine and ethanol to induce dopamine release in the mesolimbic dopamine system, thereby potentiating each other’s rewarding effects (Corrigall et al., 1994; Gonzales et al., 2004), as well as a possible role of activation of nicotinic acetylcholine receptors (Blomqvist et al., 1996; Le et al., 2000). Our study demonstrates that the higher levels of hepatic CYP2E1 after chronic nicotine pretreatment and ethanol self-administration were accompanied by an increase in ethanol consumption and it remains to be tested whether metabolic cross-tolerance directly contributes to this.
CYP2B1/2 was not induced by nicotine treatment (Fig. 5) as previously observed (Miksys et al., 2000a) but was increased by ethanol consumption. As nicotine pretreatment had some effects on ethanol self-administration we examined the relationship between the levels of ethanol consumption in all animals and the levels of CYP2B1/2. While no effect of nicotine treatment alone was seen on CYP2B1/2 levels, an indirect impact of nicotine on CYP2B1/2 exclusively via altering ethanol self-administration is supported by a strong correlation seen between CYP2B1/2 levels and the ethanol consumption on day 10 (r=0.49 P=0.007). Therefore, it seems likely that the influence of chronic nicotine on ethanol-induced CYP2B1/2 proteins was indirect via a regulation of ethanol self-administration. The induction of hepatic CYP2B by xenobiotics is generally via transcriptional activation through orphan nuclear receptors such as the constitutive androstane receptor, pregnane X receptor and glucocorticoid receptors (Stoltz et al., 1998; Wang and Negishi, 2003). The induction of hepatic CYP2B1/2 by ethanol can involve both protein and mRNA-mediated regulation (Kocarek et al., 1990; Schoedel and Tyndale, 2003). While chronic nicotine has no effect on hepatic CYP2B1/2 levels, it can induce brain CYP2B protein which is associated with elevated mRNA levels (Miksys et al., 2000a). Thus, the regulation of CYP2B by ethanol and nicotine are tissue-specific and may use different mechanisms. Exposure to ethanol alone, and in combination with nicotine, could increase the hepatic metabolism of CYP2B1/2 substrates thereby affecting their efficacy and/or toxicity. This may be of clinical importance for a number of drugs metabolized by CYP2B such as bupropion (Hesse et al., 2000), efavirenz (Ward et al., 2003), and cyclophosphamide (Chang et al., 1993), where alterations in plasma levels can result in altered therapeutic outcomes and toxicities (Hesse et al., 2004; Rotger et al., 2005).
As a negative control, hepatic CYP2D1 was also measured, and no effect of either ethanol or nicotine treatment was seen as expected (Fig. 6). We also found that neither ethanol alone, nor ethanol with nicotine pretreatments (all doses) affected the expression of hepatic CYP2A (data not shown).
In summary, while chronic nicotine alone can induce some CYP enzymes, it can also increase CYPs indirectly by increasing voluntary ethanol intake in a duration and dose-dependent manner. The induction of hepatic levels of drug-metabolizing enzymes may increase CYP2E1-and CYP2B1/2-mediated metabolism, thereby altering drug talking behaviors, the therapeutic efficacy of clinical drugs, and potentially increasing the risk of toxicity by xenobiotics.
Acknowledgments
We are grateful to Dr. Mike Stromberg for his assistance and expertise with the behavioral component of this study, as well as Kevin Sorokin for his technical assistance. This work was supported by the CIHR grant MT14173. We are grateful for additional support from the Centre for Addiction and Mental Health and a Canada Research Chair to RFT.
Footnotes
Conflict of interest
Dr. Rachel F. Tyndale is a shareholder in Nicogen Research Inc, a company focused on the development of novel smoking cessation treatment approaches. None of the data contained in this manuscript alters or improves any commercial aspect of Nicogen and no Nicogen funds were used in this work. The manuscript was also not reviewed by anyone associated with Nicogen before submission or revision.
References
- Adir J, Wildfeuer W, Miller RP. Effect of ethanol pretreatment on the pharmacokinetics of nicotine in rats. J Pharmacol Exp Ther. 1980;212:274–279. [PubMed] [Google Scholar]
- Batel P, Pessione F, Maitre C, Rueff B. Relationship between alcohol and tobacco dependencies among alcoholics who smoke. Addiction. 1995;90:977–980. doi: 10.1046/j.1360-0443.1995.90797711.x. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Jacob P., III Daily intake of nicotine during cigarette smoking. Clin Pharmacol Ther. 1984;35:499–504. doi: 10.1038/clpt.1984.67. [DOI] [PubMed] [Google Scholar]
- Bierut LJ, Rice JP, Goate A, Hinrichs AL, Saccone NL, Foroud T, Edenberg HJ, Cloninger CR, Begleiter H, Conneally PM, Crowe RR, Hesselbrock V, Li TK, Nurnberger JI, Jr, Porjesz B, Schuckit MA, Reich T. A genomic scan for habitual smoking in families of alcoholics: common and specific genetic factors in substance dependence. Am J Med Genet, A. 2004;124:19–27. doi: 10.1002/ajmg.a.20329. [DOI] [PubMed] [Google Scholar]
- Blomqvist O, Ericson M, Johnson DH, Engel JA, Soderpalm B. Voluntary ethanol intake in the rat: effects of nicotinic acetylcholine receptor blockade or subchronic nicotine treatment. Eur J Pharmacol. 1996;314:257–267. doi: 10.1016/s0014-2999(96)00583-3. [DOI] [PubMed] [Google Scholar]
- Buczek Y, Tomkins DM, Le AD, Sellers EM. Opposite effects of Ro 15-4513 on acquisition and maintenance of ethanol drinking behavior in male Wistar rats. Alcohol Clin Exp Res. 1997;21:1667–1675. [PubMed] [Google Scholar]
- Caro AA, Cederbaum AI. Oxidative stress, toxicology, and pharmacology of CYP2E1. Annu Rev Pharmacol Toxicol. 2004;44:27–42. doi: 10.1146/annurev.pharmtox.44.101802.121704. [DOI] [PubMed] [Google Scholar]
- Chang TK, Weber GF, Crespi CL, Waxman DJ. Differential activation of cyclophosphamide and ifosphamide by cytochromes P-450 2B and 3A in human liver microsomes. Cancer Res. 1993;53:5629–5637. [PubMed] [Google Scholar]
- Clark A, Lindgren S, Brooks SP, Watson WP, Little HJ. Chronic infusion of nicotine can increase operant self-administration of alcohol. Neuropharmacology. 2001;41:108–117. doi: 10.1016/s0028-3908(01)00037-5. [DOI] [PubMed] [Google Scholar]
- Clarke PB, Kumar R. The effects of nicotine on locomotor activity in non-tolerant and tolerant rats. Br J Pharmacol. 1983;78:329–337. doi: 10.1111/j.1476-5381.1983.tb09398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Adamson KL. Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res. 1994;653:278–284. doi: 10.1016/0006-8993(94)90401-4. [DOI] [PubMed] [Google Scholar]
- DiFranza JR, Guerrera MP. Alcoholism and smoking. J Stud Alcohol. 1990;51:130–135. doi: 10.15288/jsa.1990.51.130. [DOI] [PubMed] [Google Scholar]
- Edwards RJ, Price RJ, Watts PS, Renwick AB, Tredger JM, Boobis AR, Lake BG. Induction of cytochrome P450 enzymes in cultured precision-cut human liver slices. Drug Metab Dispos. 2003;31:282–288. doi: 10.1124/dmd.31.3.282. [DOI] [PubMed] [Google Scholar]
- Eliasson E, Johansson I, Ingelman-Sundberg M. Ligand-dependent maintenance of ethanol-inducible cytochrome P-450 in primary rat hepatocyte cell cultures. Biochem Biophys Res Commun. 1988;150:436–443. doi: 10.1016/0006-291x(88)90539-6. [DOI] [PubMed] [Google Scholar]
- Goldman D, Oroszi G, Ducci F. The genetics of addictions: uncovering the genes. Nat Rev, Genet. 2005;6:521–532. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- Gonzalez FJ. Role of cytochromes P450 in chemical toxicity and oxidative stress: studies with CYP2E1. Mutat Res. 2005;569:101–110. doi: 10.1016/j.mrfmmm.2004.04.021. [DOI] [PubMed] [Google Scholar]
- Gonzales RA, Job MO, Doyon WM. The role of mesolimbic dopamine in the development and maintenance of ethanol reinforcement. Pharmacol Ther. 2004;103:121–146. doi: 10.1016/j.pharmthera.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Hesse LM, Venkatakrishnan K, Court MH, von Moltke LL, Duan SX, Shader RI, Greenblatt DJ. CYP2B6 mediates the in vitro hydroxylation of bupropion: potential drug interactions with other antidepressants. Drug Metab Dispos. 2000;28:1176–1183. [PubMed] [Google Scholar]
- Hesse LM, He P, Krishnaswamy S, Hao Q, Hogan K, von Moltke LL, Greenblatt DJ, Court MH. Pharmacogenetic determinants of interindividual variability in bupropion hydroxylation by cytochrome P450 2B6 in human liver microsomes. Pharmacogenetics. 2004;14:225–238. doi: 10.1097/00008571-200404000-00002. [DOI] [PubMed] [Google Scholar]
- Hoet P, Buchet JP, Sempoux C, Haufroid V, Rahier J, Lison D. Potentiation of 2,2-dichloro-1,1,1-trifluoroethane (HCFC-123)-induced liver toxicity by ethanol in guinea-pigs. Arch Toxicol. 2002;76:707–714. doi: 10.1007/s00204-002-0389-8. [DOI] [PubMed] [Google Scholar]
- Howard LA, Micu AL, Sellers EM, Tyndale RF. Low doses of nicotine and ethanol induce CYP2E1 and chlorzoxazone metabolism in rat liver. J Pharmacol Exp Ther. 2001;299:542–550. [PubMed] [Google Scholar]
- Joshi M, Tyndale RF. Induction and recovery time course of rat brain CYP2E1 after nicotine treatment. Drug Metab Dispos. 2006;34:647–652. doi: 10.1124/dmd.105.008029. [DOI] [PubMed] [Google Scholar]
- Kessova IG, Leo MA, Lieber CS. Effect of beta-carotene on hepatic cytochrome P-450 in ethanol-fed rats. Alcohol Clin Exp Res. 2001;25:1368–1372. [PubMed] [Google Scholar]
- Kocarek TA, Schuetz EG, Guzelian PS. Differentiated induction of cytochrome P450b/e and P450p mRNAs by dose of phenobarbital in primary cultures of adult rat hepatocytes. Mol Pharmacol. 1990;38:440–444. [PubMed] [Google Scholar]
- Le AD, Corrigall WA, Harding JW, Juzytsch W, Li TK. Involvement of nicotinic receptors in alcohol self-administration. Alcohol Clin Exp Res. 2000;24:155–163. doi: 10.1111/j.1530-0277.2000.tb04585.x. [DOI] [PubMed] [Google Scholar]
- Le Houezec J, Jacob P, 3rd, Benowitz NL. A clinical pharmacological study of subcutaneous nicotine. Eur J Clin Pharmacol. 1993;44:225–230. doi: 10.1007/BF00271362. [DOI] [PubMed] [Google Scholar]
- Leclercq IA, Farrell GC, Field J, Bell DR, Gonzalez FJ, Robertson GR. CYP2E1 and CYP4A as microsomal catalysts of lipid peroxides in murine nonalcoholic steatohepatitis. J Clin Invest. 2000;105:1067–1075. doi: 10.1172/JCI8814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AM, Miksys S, Palmour R, Tyndale RF. CYP2B6 is expressed in African Green monkey brain and is induced by chronic nicotine treatment. Neuropharmacology. 2006;50:441–450. doi: 10.1016/j.neuropharm.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Lieber CS. Mechanisms of ethanol–drug–nutrition interactions. J Toxicol, Clin Toxicol. 1994;32:631–681. doi: 10.3109/15563659409017974. [DOI] [PubMed] [Google Scholar]
- Lieber CS. Cytochrome P-4502E1: its physiological and pathological role. Physiol Rev. 1997;77:517–544. doi: 10.1152/physrev.1997.77.2.517. [DOI] [PubMed] [Google Scholar]
- Lieber CS. Microsomal ethanol-oxidizing system (MEOS): the first 30 years (1968–1998)—a review. Alcohol Clin Exp Res. 1999;23:991–1007. [PubMed] [Google Scholar]
- Mann A, Miksys S, Lee A, Mash DC, Tyndale RF. Induction of the drug metabolizing enzyme CYP2D in monkey brain by chronic nicotine treatment. Neuropharmacology. 2008;55:1147–1155. doi: 10.1016/j.neuropharm.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H, Matsubayashi K, Fukui Y. Evidence that cytochrome P-4502E1 contributes to ethanol elimination at low doses: effects of diallyl sulfide and 4-methyl pyrazole on ethanol elimination in the perfused rat liver. Alcohol Clin Exp Res. 1996;20:12A–16A. doi: 10.1111/j.1530-0277.1996.tb01719.x. [DOI] [PubMed] [Google Scholar]
- Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Damaj MI, Donny EC, Gardiner PS, Grady SR, Heberlein U, Leonard SS, Levin ED, Lukas RJ, Markou A, Marks MJ, McCallum SE, Parameswaran N, Perkins KA, Picciotto MR, Quik M, Rose JE, Rothenfluh A, Schafer WR, Stolerman IP, Tyndale RF, Wehner JM, Zirger JM. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology (Berl) 2007;190:269–319. doi: 10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- Micu AL, Miksys S, Sellers EM, Koop DR, Tyndale RF. Rat hepatic CYP2E1 is induced by very low nicotine doses: an investigation of induction, time course, dose response, and mechanism. J Pharmacol Exp Ther. 2003;306:941–947. doi: 10.1124/jpet.103.052183. [DOI] [PubMed] [Google Scholar]
- Miksys S, Hoffmann E, Tyndale RF. Regional and cellular induction of nicotine-metabolizing CYP2B1 in rat brain by chronic nicotine treatment. Biochem Pharmacol. 2000a;59:1501–1511. doi: 10.1016/s0006-2952(00)00281-1. [DOI] [PubMed] [Google Scholar]
- Miksys S, Rao Y, Sellers EM, Kwan M, Mendis D, Tyndale RF. Regional and cellular distribution of CYP2D subfamily members in rat brain. Xenobiotica. 2000b;30:547–564. doi: 10.1080/004982500406390. [DOI] [PubMed] [Google Scholar]
- Nakayama H, Okuda H, Nakashima T, Imaoka S, Funae Y. Nicotine metabolism by rat hepatic cytochrome P450s. Biochem Pharmacol. 1993;45:2554–2556. doi: 10.1016/0006-2952(93)90238-r. [DOI] [PubMed] [Google Scholar]
- Patten CJ, Thomas PE, Guy RL, Lee M, Gonzalez FJ, Guengerich FP, Yang CS. Cytochrome P450 enzymes involved in acetaminophen activation by rat and human liver microsomes and their kinetics. Chem Res Toxicol. 1993;6:511–518. doi: 10.1021/tx00034a019. [DOI] [PubMed] [Google Scholar]
- Peter R, Bocker R, Beaune PH, Iwasaki M, Guengerich FP, Yang CS. Hydroxylation of chlorzoxazone as a specific probe for human liver cytochrome P-450IIE1. Chem Res Toxicol. 1990;3:566–573. doi: 10.1021/tx00018a012. [DOI] [PubMed] [Google Scholar]
- Rae JM, Johnson MD, Lippman ME, Flockhart DA. Rifampin is a selective, pleiotropic inducer of drug metabolism genes in human hepatocytes: studies with cDNA and oligonucleotide expression arrays. J Pharmacol Exp Ther. 2001;299:849–857. [PubMed] [Google Scholar]
- Roberts BJ, Song BJ, Soh Y, Park SS, Shoaf SE. Ethanol induces CYP2E1 by protein stabilization. Role of ubiquitin conjugation in the rapid degradation of CYP2E1. J Biol Chem. 1995;270:29632–29635. doi: 10.1074/jbc.270.50.29632. [DOI] [PubMed] [Google Scholar]
- Rotger M, Colombo S, Furrer H, Bleiber G, Buclin T, Lee BL, Keiser O, Biollaz J, Decosterd L, Telenti A. Influence of CYP2B6 polymorphism on plasma and intracellular concentrations and toxicity of efavirenz and nevirapine in HIV-infected patients. Pharmacogenet Genomics. 2005;15:1–5. doi: 10.1097/01213011-200501000-00001. [DOI] [PubMed] [Google Scholar]
- Ryan DE, Koop DR, Thomas PE, Coon MJ, Levin W. Evidence that isoniazid and ethanol induce the same microsomal cytochrome P-450 in rat liver, an isozyme homologous to rabbit liver cytochrome P-450 isozyme 3a. Arch Biochem Biophys. 1986;246:633–644. doi: 10.1016/0003-9861(86)90319-x. [DOI] [PubMed] [Google Scholar]
- Schoedel KA, Tyndale RF. Induction of nicotine-metabolizing CYP2B1 by ethanol and ethanol-metabolizing CYP2E1 by nicotine: summary and implications. Biochim Biophys Acta. 2003;1619:283–290. doi: 10.1016/s0304-4165(02)00487-7. [DOI] [PubMed] [Google Scholar]
- Schoedel KA, Sellers EM, Tyndale RF. Induction of CYP2B1/2 and nicotine metabolism by ethanol in rat liver but not rat brain. Biochem Pharmacol. 2001;62:1025–1036. doi: 10.1016/s0006-2952(01)00744-4. [DOI] [PubMed] [Google Scholar]
- Shapiro BH, Agrawal AK, Pampori NA. Gender differences in drug metabolism regulated by growth hormone. Int J Biochem Cell Biol. 1995;27:9–20. doi: 10.1016/1357-2725(94)00056-5. [DOI] [PubMed] [Google Scholar]
- Song BJ, Matsunaga T, Hardwick JP, Park SS, Veech RL, Yang CS, Gelboin HV, Gonzalez FJ. Stabilization of cytochrome P450j messenger ribonucleic acid in the diabetic rat. Mol Endocrinol. 1987;1:542–547. doi: 10.1210/mend-1-8-542. [DOI] [PubMed] [Google Scholar]
- Song BJ, Veech RL, Park SS, Gelboin HV, Gonzalez FJ. Induction of rat hepatic N-nitrosodimethylamine demethylase by acetone is due to protein stabilization. J Biol Chem. 1989;264:3568–3572. [PubMed] [Google Scholar]
- Spracklin DK, Hankins DC, Fisher JM, Thummel KE, Kharasch ED. Cytochrome P450 2E1 is the principal catalyst of human oxidative halothane metabolism in vitro. J Pharmacol Exp Ther. 1997;281:400–411. [PubMed] [Google Scholar]
- Stolerman IP. Behavioural pharmacology of nicotine: implications for multiple brain nicotinic receptors. Ciba Found Symp. 1990;152:3–16. doi: 10.1002/9780470513965.ch2. discussion 16–22. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Garcha HS, Mirza NR. Dissociations between the locomotor stimulant and depressant effects of nicotinic agonists in rats. Psychopharmacology (Berl) 1995;117:430–437. doi: 10.1007/BF02246215. [DOI] [PubMed] [Google Scholar]
- Stoltz C, Vachon MH, Trottier E, Dubois S, Paquet Y, Anderson A. The CYP2B2 phenobarbital response unit contains an accessory factor element and a putative glucocorticoid response element essential for conferring maximal phenobarbital responsiveness. J Biol Chem. 1998;273:8528–8536. doi: 10.1074/jbc.273.14.8528. [DOI] [PubMed] [Google Scholar]
- Stromberg MF. The effect of baclofen alone and in combination with naltrexone on ethanol consumption in the rat. Pharmacol Biochem Behav. 2004;78:743–750. doi: 10.1016/j.pbb.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Stromberg MF, Casale M, Volpicelli L, Volpicelli JR, O’Brien CP. A comparison of the effects of the opioid antagonists naltrexone, naltrindole, and beta-funaltrexamine on ethanol consumption in the rat. Alcohol. 1998;15:281–289. doi: 10.1016/s0741-8329(97)00131-6. [DOI] [PubMed] [Google Scholar]
- Styles JA, Davies A, Lim CK, De Matteis F, Stanley LA, White IN, Yuan ZX, Smith LL. Genotoxicity of tamoxifen, tamoxifen epoxide and toremifene in human lymphoblastoid cells containing human cytochrome P450s. Carcinogenesis. 1994;15:5–9. doi: 10.1093/carcin/15.1.5. [DOI] [PubMed] [Google Scholar]
- Swan GE, Carmelli D, Cardon LR. Heavy consumption of cigarettes, alcohol and coffee in male twins. J Stud Alcohol. 1997;58:182–190. doi: 10.15288/jsa.1997.58.182. [DOI] [PubMed] [Google Scholar]
- True WR, Xian H, Scherrer JF, Madden PA, Bucholz KK, Heath AC, Eisen SA, Lyons MJ, Goldberg J, Tsuang M. Common genetic vulnerability for nicotine and alcohol dependence in men. Arch Gen Psychiatry. 1999;56:655–661. doi: 10.1001/archpsyc.56.7.655. [DOI] [PubMed] [Google Scholar]
- Tyndale RF. Genetics of alcohol and tobacco use in humans. Ann Med. 2003;35:94–121. doi: 10.1080/07853890310010014. [DOI] [PubMed] [Google Scholar]
- Ueno T, Gonzalez FJ. Transcriptional control of the rat hepatic CYP2E1 gene. Mol Cell Biol. 1990;10:4495–4505. doi: 10.1128/mcb.10.9.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Negishi M. Transcriptional regulation of cytochrome p450 2B genes by nuclear receptors. Curr Drug Metab. 2003;4:515–525. doi: 10.2174/1389200033489262. [DOI] [PubMed] [Google Scholar]
- Wang H, Tompkins LM. CYP2B6: new insights into a historically overlooked cytochrome P450 isozyme. Curr Drug Metab. 2008;9:598–610. doi: 10.2174/138920008785821710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward BA, Gorski JC, Jones DR, Hall SD, Flockhart DA, Desta Z. The cytochrome P450 2B6 (CYP2B6) is the main catalyst of efavirenz primary and secondary metabolism: implication for HIV/AIDS therapy and utility of efavirenz as a substrate marker of CYP2B6 catalytic activity. J Pharmacol Exp Ther. 2003;306:287–300. doi: 10.1124/jpet.103.049601. [DOI] [PubMed] [Google Scholar]
- Wetscher GJ, Bagchi M, Bagchi D, Perdikis G, Hinder PR, Glaser K, Hinder RA. Free radical production in nicotine treated pancreatic tissue. Free Radic Biol Med. 1995;18:877–882. doi: 10.1016/0891-5849(94)00221-5. [DOI] [PubMed] [Google Scholar]
- Wilkinson PK, Sedman AJ, Sakmar E, Kay DR, Wagner JG. Pharmacokinetics of ethanol after oral administration in the fasting state. J Pharmacokinet Biopharm. 1977;5:207–224. doi: 10.1007/BF01065396. [DOI] [PubMed] [Google Scholar]
- Woodcroft KJ, Novak RF. Insulin effects on CYP2E1, 2B, 3A, and 4A expression in primary cultured rat hepatocytes. Chem Biol Interact. 1997;107:75–91. doi: 10.1016/s0009-2797(97)00075-6. [DOI] [PubMed] [Google Scholar]
- Woodcroft KJ, Novak RF. Xenobiotic-enhanced expression of cytochromes P450 2E1 and 2B in primary cultured rat hepatocytes. Drug Metab Dispos. 1998;26:372–378. [PubMed] [Google Scholar]
- Yue J, Miksys S, Hoffmann E, Tyndale RF. Chronic nicotine treatment induces rat CYP2D in the brain but not in the liver: an investigation of induction and time course. J Psychiatry Neurosci. 2008;33:54–63. [PMC free article] [PubMed] [Google Scholar]
- Zhukov A, Ingelman-Sundberg M. Relationship between cytochrome P450 catalytic cycling and stability: fast degradation of ethanol-inducible cytochrome P450 2E1 (CYP2E1) in hepatoma cells is abolished by inactivation of its electron donor NADPH-cytochrome P450 reductase. Biochem J. 1999;340:453–458. [PMC free article] [PubMed] [Google Scholar]