Abstract
Salivary glands are useful gene transfer target sites for the production of therapeutic proteins, and can secrete proteins into both saliva and the bloodstream. The mechanisms involved in this differential protein sorting are not well understood, although it is believed, at least in part, to be based on the amino acid sequence of the encoded protein. We hypothesized that a transgenic protein, human erythropoietin (hEpo), normally sorted from murine salivary glands into the bloodstream, could be redirected into saliva by fusing it with human growth hormone (hGH). After transfection, the hEpo–hGH fusion protein was expressed and glycosylated in both HEK 293 and A5 cells. When packaged in an adenovirus serotype 5 vector and delivered to murine submandibular cells in vivo via retroductal cannulation, the hEpo–hGH fusion protein was also expressed, albeit at ~26% of the levels of hEpo expression. Importantly, in multiple experiments with different cohorts of mice, the hEpo–hGH fusion protein was sorted more frequently into saliva, versus the bloodstream, than was the hEpo protein (p < 0.001). These studies show it is possible to redirect the secretion of a transgenic constitutive pathway protein from salivary gland cells after gene transfer in vivo, a finding that may facilitate developing novel treatments for certain upper gastrointestinal tract disorders.
INTRODUCTION
Salivary glands are considered an attractive target for gene transfer when using genes as drugs (Baum et al., 2004). This is because they are easily accessible and not critical-for-life organs that can produce and secrete large amounts of proteins locally into the oral cavity and gastrointestinal tract or into the bloodstream systemically (Kagami et al., 1996; O’Connell et al., 1996; He et al., 1998; Baum et al., 1999). Theoretically, the secretion of therapeutic transgenic proteins from salivary glands can be useful for many clinical conditions. For example, to manage emergent antibiotic-resistant oral and oropharyngeal bacterial infections (Bryskier, 2002; Huang et al., 2002; Martin et al., 2002), transfer of genes encoding antimicrobial peptides capable of being continuously secreted into the saliva may prove beneficial. Further, because there are no suitable treatments for iatrogenic oral ulcerations resulting from cancer radiation and chemotherapy, the transfer of genes encoding various cytokines or growth factors to salivary glands, and their subsequent secretion into saliva, may be therapeutically useful (Sonis et al., 2000; Spielberger et al., 2004). Conversely, secretion of transgenic proteins from salivary glands into the bloodstream may be valuable for managing several systemic single-protein deficiency disorders, such as erythropoietin-responsive anemia and growth hormone deficiency (Baum et al., 2004; Voutetakis et al., 2005).
In order for salivary gland gene transfer to be clinically useful in this manner, it is important to understand, and potentially modify, the intracellular sorting pathways used by encoded transgenic proteins produced after salivary gland gene transfer. Several different protein secretion pathways have been identified in salivary gland cells, including both major and minor regulated pathways, apical and basolateral constitutive pathways, and a constitutive-like pathway (Castle and Castle, 1998; Gorr et al., 2005). Ideally, control over protein entry into any of these pathways could provide a means for selectively directing transgene products to a desired therapeutic site, that is, either into the bloodstream or into the upper gastrointestinal tract via saliva, as appropriate. This issue appears to be a significant practical concern (Baum et al., 1999; Hoque et al., 2001). For example, human growth hormone (hGH), a protein normally secreted via the regulated pathway into the bloodstream from the pituitary gland, is secreted almost entirely into the saliva, apparently by the major regulated pathway, from rodent and miniature pig salivary glands, while it is secreted minimally into the bloodstream, the site where it would be therapeutically useful (Baum et al., 1999; Hoque et al., 2001; Yan et al., 2007). Conversely, a protein such as interleukin-11, which may be beneficial for treating iatrogenic mucositis as described above, is not normally secreted via the regulated pathway and thus is unlikely to be secreted at high levels into saliva, where it would be needed therapeutically to function.
To date, the sorting mechanisms employed for secretory proteins within polarized epithelial cells are not well understood and are clearly complex (Arvan and Castle, 1998). In a general sense, secretory protein sorting in endocrine and exocrine cells previously was shown to occur either constitutively or via the regulated secretory pathway (Gumbiner and Kelly, 1982; Kelly, 1985). It was postulated that proteins destined for regulated pathway secretion in polarized cells require a sorting signal directing them to or retaining them within secretory granules where they await an external secretory signal for their release. In contrast, proteins secreted by the constitutive pathway are considered to do so continuously, essentially at the same rate as their translation, and without regard to changes in extracellular signals (Dannies, 1999; Loh et al., 2002; Tekirian, 2002). This pathway is generally considered a default secretory route. Available evidence can support both the “sorting-for-entry” and “sorting-by-retention” hypotheses for sorting of secretory proteins (Griffiths and Simons, 1986; Arvan and Castle, 1992, 1998; Arvan, 2004; Gorr et al., 2005). While in some neuroendocrine cells targeting of prohormones to the regulated pathway appears likely to involve specific sorting motifs, interactions of proteins with the immature granule membrane and aggregation/condensation of proteins (Lou et al., 2007), at present no universal sorting signals for secretory proteins have been identified in any cell type. In addition, most studies of secretory protein sorting have been performed in transformed cell lines in vitro, with few studies performed in vivo or with acutely prepared primary cells (Alexander et al., 1989; Trahair et al., 1989; Arvan, 2004; Arvan and Halban, 2004). It is clear that specific proteins can be sorted differently in various cell types, and transformed cell lines may exhibit dramatically different secretory protein-sorting behaviors than primary cells or the native cells in situ (Arvan, 2004; Arvan and Halban, 2004).
Given the complexity of secretory pathways in polarized epithelial cells, and the lack of a common sorting mechanism for secretory proteins, the prospect of redirecting secretory proteins to alternative sorting pathways in salivary glands is not trivial. Nonetheless, in principle, we have shown the partial resorting of a C terminus-mutated hGH from the regulated (exocrine) to the constitutive (endocrine) pathway in rat submandibular glands in vivo after adenoviral vector-mediated gene transfer (Wang et al., 2005). Also, concomitant administration of the U.S. Food and Drug Administration (FDA)-approved drug hydroxychloroquine to rats administered an adenoviral vector encoding wild-type hGH increased dramatically the secretion of transgenic hGH into the bloodstream (Hoque et al., 2001). Comparable studies, however, have not been performed with secretory proteins normally secreted primarily into the bloodstream, that is, presumably via the constitutive pathway. Interestingly, past studies in the AtT20 mouse pituitary cell line demonstrated that fusion of the C terminus of hGH to the C terminus of a viral glycoprotein substantially redirected the glycoprotein from the constitutive into the regulated pathway (Moore and Kelly, 1986).
The purpose of the present report was to investigate the possibility of redirecting a model transgenic constitutive pathway protein (human erythropoietin; hEpo) from its principal pathway of secretion from salivary glands, that is, into the bloodstream, into saliva instead (Voutetakis et al., 2004; Zufferey and Aebischer, 2004). We hypothesized that fusion of hGH at the C terminus of hEpo would redirect the chimeric protein more frequently into saliva instead of into the bloodstream. We constructed a cDNA encoding the hEpo–hGH fusion protein and initially tested its production in vitro. We then packaged the chimeric cDNA into an adenovirus serotype 5 vector (AdCMVhEpo-hGH) and delivered it to murine submandibular cells to study fusion protein sorting in vivo.
MATERIALS AND METHODS
Plasmids
The coding sequences of hEpo and hGH (hEpo upstream of hGH) were fused by polymerase chain reaction (PCR), using splicing by overlap extension (Advantage HF PCR kit; Clontech, Palo Alto, CA) (Vallejo et al., 1995). Oligonucleotides spanning the desired fusion site and encoding EcoRI and BamHI restriction sites were designed (hEpo: forward, 5′-CGGAATTCCGATGGGGGTGCACGAATG-3′; reverse, 5′-GGGAGCCTGCAGCCATTCTGTCCCCTGTCCTGCA-3′; hGH: forward, 5′-ATGGCTGCAGGCTCCC-3′; reverse, 5′-CGCGGATCCGCGCTAGAAGCCACAGCTGCC-3′) and used to produce two initial PCR products. These products were hybridized with the flanking primers (forward, 5′-CGGAATTCCGATGGGGGTGCACGAATG-3′; reverse, 5′-CGCGGATCCGCGCTAGAAGCCACAGCTGCC-3′) to produce the full-length chimeric cDNA of hEpo–hGH. The PCR product was directionally cloned into the expression vector pAC-CMV-pLpA and the resultant plasmid, pAC-CMV-hEpo-hGH, was sequenced and confirmed to contain the correct coding sequences.
Recombinant adenoviral vector
First-generation, E1−, recombinant adenoviral (serotype 5) vectors encoding either hEpo (AdCMVhEpo) or hEpo–hGH (AdCMVhEpo-hGH) were generated, as previously reported (He et al., 1998), by cotransfection of the shuttle plasmid pAC-CMV-pLpA with the corresponding transgenes, together with the adenoviral plasmid pJM17 into 293 human embryonic kidney (HEK) cells. Construction of the vector AdCMVhGH was previously reported (Wang et al., 2005). In addition to the hEpo, hGH, or fusion cDNA, the vectors contained the simian virus 40 (SV40) polyadenylation signal, and were driven by the human cytomegalovirus (CMV) promoter/enhancer. The vectors were amplified in HEK 293 cells and purified by two rounds of CsCl gradient centrifugation as described (Delporte et al., 1996). Purified vectors were dialyzed for 4 hr at 4°C against 4 liters of dialysis buffer containing 10% glycerol, 0.1 M Tris (pH 7.4), and 5 mM MgCl2 and stored in aliquots at −80°C for later use. Vectors were titered by quantitative PCR (ABI PRISM 7700; Applied Biosystems, Foster City, CA) with primers from the adenoviral E2 region: E2q1 (5′-GCAGAACCACCAGCACAGTGT-3′) and E2q2 (5′-TCCACGCATTTCCTTCTAAGCTA-3′).
Cell culture, plasmid transfection, and Western blot analysis
Correct expression of the hEpo–hGH fusion protein initially was evaluated in HEK 293 and A5 cells. 293 cells were grown in IMEM (improved minimal essential medium, Eagle’s) supplemented with 10% bovine serum, penicillin G (100 U/ml), and streptomycin (100 µg/ml) (all from Invitrogen Biosource, Camarillo, CA) at 37°C in a humidified, 5% CO2 atmosphere incubator. A5 cells were grown similarly, but using McCoy’s 5A medium (Invitrogen, Carlsbad, CA). pAC-CMV-hEpohGH, pAC-CMV-hEpo, or pAC-CMV-hGH was transfected into HEK 293 cells in 6-well plates with Lipofectamine 2000 (Invitrogen). Forty-eight hours after transfection the medium was collected and centrifuged at 3000 rpm for 3 min, and the resulting supernatants were used for hEpo (Stemcell Technologies, Vancouver, BC, Canada) and hGH (Anogen, Mississauga, ON, Canada) enzyme-linked immunosorbent assays (ELISAs). The cells were rinsed with phosphate-buffered saline (PBS) and harvested with M-PER extraction reagent (Pierce Biotechnology, Rockford, IL). After centrifugation at 1000 × g to remove debris, the supernatants were used for Western blot analyses after being treated or not with N-glycosidase F (New England BioLabs, Ipswich, MA) to determine whether the expressed constructs were glycosylated. For Western blot analyses proteins were separated with 12% precast polyacrylamide gels (Bio-Rad, Hercules, CA) and transferred to nitrocellulose membranes (GE Healthcare Life Sciences, Buckinghamshire, UK). The membranes were then blocked with 5% dry milk (Bio-Rad) in Tris-buffered saline with 0.1% Tween 20 (TBST) for 1 hr, followed by incubation for 1.5 hr at room temperature with either anti-hEpo rabbit polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA) or anti-hGH rabbit polyclonal antibody (Genetex, San Antonio, TX), diluted 1:200 in the same blocking solution. After several washes in TBST, the membranes were incubated with a 1:5000 dilution of horseradish peroxidase (HRP)-conjugated secondary antibodies (GE Healthcare) for 1 hr at room temperature. The signal was detected by enhanced chemiluminescence, by incubating the blot with excess detection system (GE Healthcare) for 3 min, followed by exposure to X-ray film and development.
Vector delivery to murine salivary glands
Animal experiments were approved by the Animal Care and Use Committee of the National Institute of Dental and Craniofacial Research and by the Biosafety Committee of the National Institutes of Health. Forty-six male BALB/c mice from 4 different cohorts were used in the study. For animals administered AdCMVhEpo-hGH, the cohorts contained 5, 8, 10, and 9 mice, respectively. For animals administered AdCMVhEpo, the cohorts contained 3, 3, 4, and 4 mice, respectively. All cohorts yielded similar results. Animals were anesthetized with a mixed solution of ketamine (60 mg/ml; Phoenix Scientific, St. Joseph, MO) and xylazine (8 mg/ml; Phoenix Scientific) given intramuscularly (1 µl/g body weight) followed by cannulation of submandibular gland ducts with modified polyethylene tubing (Intramedic PE-10; BD Diagnostic Systems, Sparks, MD). Atropine (intramuscular, 0.5 mg/kg body weight; Sigma-Aldrich, St. Louis, MO) was administered to decrease salivary flow. Ten minutes later, 1010 viral particles/gland of either AdCMVhEpo or AdCMVhEpo-hGH was administered by retrograde ductal delivery into submandibular glands. Note that for initial dosing experiments other mice, that is, not included in the 46 presented herein, were administered either vector at 109 viral particles/gland. Vectors were delivered in a 50-µl volume. Forty-eight hours later, mice were anesthetized and administered a subcutaneous injection of pilocarpine (0.5 mg/ml, 1 µl/g body weight; Sigma-Aldrich). Whole saliva and blood were collected and after separation of serum were stored at −80°C until assayed for hEpo, using the above-mentioned hEpo ELISA. Aqueous extracts of submandibular glands were prepared by mechanical homogenization in the presence of PBS and a protease inhibitor cocktail (complete; Roche, Indianapolis, IN). Samples were then centrifuged for 10 min at 10,000 × g (4°C) and the supernatant was collected and assayed for immunoreactive hEpo by ELISA, and by Western blot analysis. Protein was determined by Bradford assay.
Calculations and statistical analysis
The levels of immunoreactive hEpo in saliva and serum are generally shown as ratios of their concentrations present in each fluid. Data from in vivo experiments were analyzed with SigmaStat software (version 2.03; Systat Software, San Jose, CA), using the Mann–Whitney test, and are presented unless otherwise stated as means ± SEM. Differences with p < 0.05 were considered to be statistically significant.
RESULTS
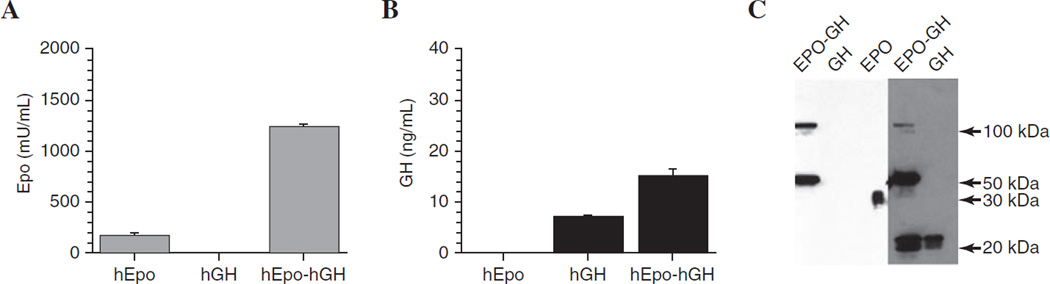
Fusion of the hEpo and hGH cDNAs was performed by PCR, using splicing by overlap extension. The hEpo and hGH cDNAs are 578 and 666 bp, respectively. Thus, the size of the final chimeric cDNA product was ~1.2 kb. After directional cloning into pAC-CMV-pLpA and sequence confirmation, the expression vector initially was tested in HEK 293 cells. Similar results also were obtained in experiments with A5 submandibular epithelial cells (data not shown). Forty-eight hours after transfection, media and whole cell lysates were collected. Media were analyzed for immunoreactive products with hEpo and hGH ELISAs and lysates were reacted with anti-hEpo and hGH antibodies in Western blots. The results are presented in Fig. 1. Medium from cells transfected with pAC-CMV-hEpo reacted positively with antibodies for hEpo but not with hGH antibodies in ELISAs. Conversely, medium from pAC-CMV-hGH-transfected cells reacted only in the hGH ELISAs. However, medium from pAC-CMV-hEpo-hGH-transfected cells reacted positively with antibodies in both the hEpo and hGH ELISAs (Fig. 1).
FIG. 1.
Immunoreactive hEpo and hGH detected in media and lysates of transfected HEK 293 cells. Forty-eight hours after transfection either with pAC-CMV-hGH, pAC-CMV-hEpo, or pAC-CMV-hEpo-hGH, media were collected and subjected to (A) hEpo ELISA or (B) hGH ELISA. Media from hEpo- and hGH-transfected cells were immunoreactive only in their respective ELISA. Media from hEpo–hGH-transfected cells reacted positively with both hEpo and hGH ELISAs. Data are presented as means ± SEM (n = 3). Western blots (C) from lysates of cells transfected with plasmids encoding hEpo–hGH reacted with both anti-hEpo (left) and anti-hGH (right) antibodies. hGH and hEpo have apparent molecular masses of ~22 and ~34 kDa, respectively. The predicted size of the chimeric hEpo–hGH fusion protein is ~56 kDa. The arrows to the right indicate the molecular masses of simultaneously electrophoresed protein standards. See Materials and Methods for additional details.
Proteins from the cell lysates were separated on polyacryl-amide gels and transferred to nitrocellulose membranes for immunoblotting. The apparent masses of hEpo and hGH are ~34 and ~22 kDa, respectively and the expected size of the fusion protein is thus ~56 kDa. Lysates from cells transfected with pAC-CMV-hEpo-hGH contained a protein with a size of ~56 kDa, which reacted with both anti-hEpo and anti-hGH antibodies (Fig. 1C). Detection of the ~56-kDa fusion protein in cell lysates with both antibodies indicated that hEpo–hGH likely was expressed correctly. In addition, two immunoreactive bands (~22 and ~110 kDa) were also detected with the anti-hGH antibody. The 110-kDa band likely represents the dimeric form of the fusion protein, as it is immunoreactive with both the hEpo and hGH antisera. The ~22-kDa product is about the size of hGH and conceivably could have been generated through proteolytic processing of the fusion protein in these cells. To determine whether the hEpo–hGH fusion protein was glycosylated, protein samples from cell lysates were treated or not with N-glycosidase F to remove N-linked oligosaccharides. As shown in Fig. 2, N-glycosidase F-treated samples migrated at a lower molecular mass than untreated samples, indicating that the native hEpo, the chimeric hEpo–hGH, and its apparent dimer form are all glycosylated. Similar results were obtained with A5 cells (data not shown).
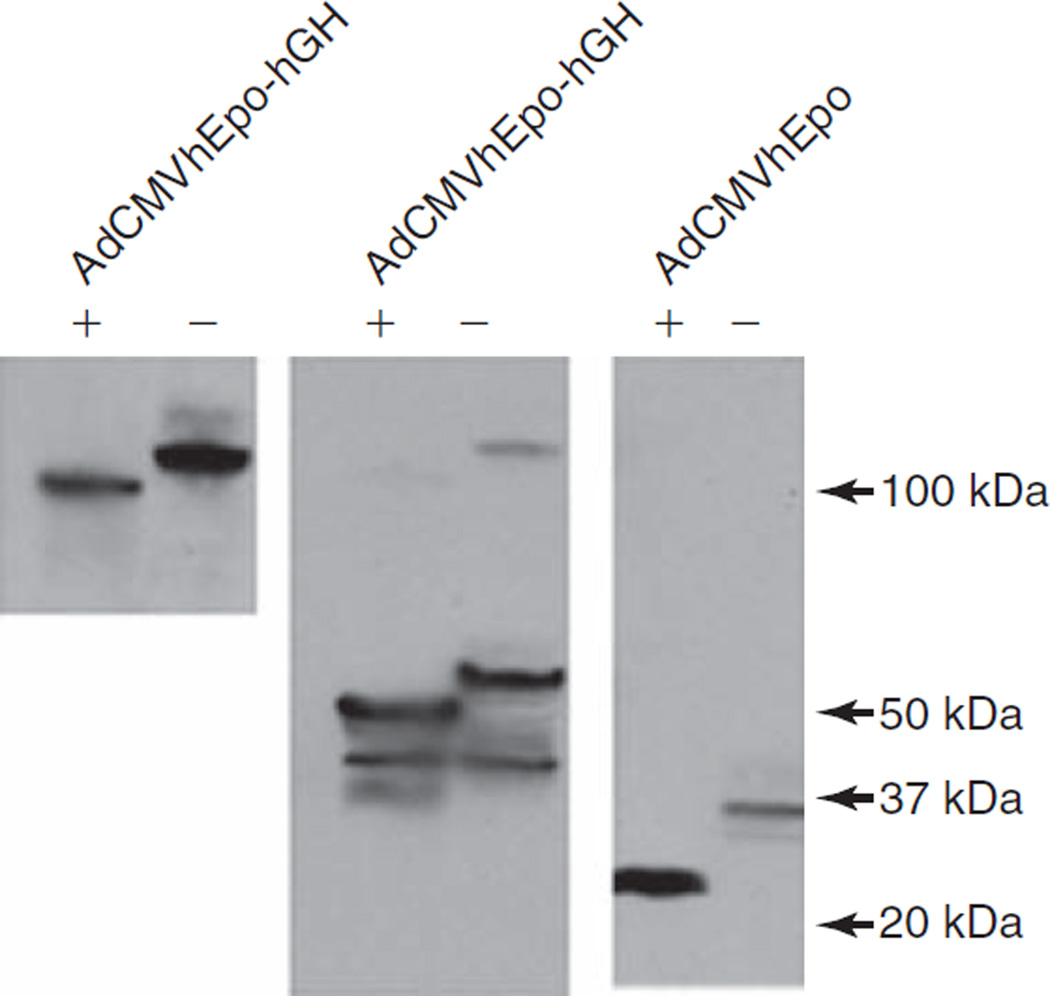
FIG. 2.
Western blot analyses of the glycosylation state of hEpo and hEpo–hGH protein. Forty-eight hours after transduction with AdCMVhEpo or AdCMVhEpo-hGH lysates were collected from transduced HEK 293 cells and either treated with N-glycosidase F (+) or left untreated (−). Proteins were then separated by gel electrophoresis, blotted, and reacted with anti-hEpo antibody. On the basis of the shift in electrophoretic mobility after incubation with N-glycosidase F, hEpo, the fusion protein hEpo–hGH, and the likely dimer of hEpo–hGH all were present in their glycosylated form with apparent molecular masses of ~34, ~56, and ~110 kDa, respectively. See Materials and Methods for additional details.
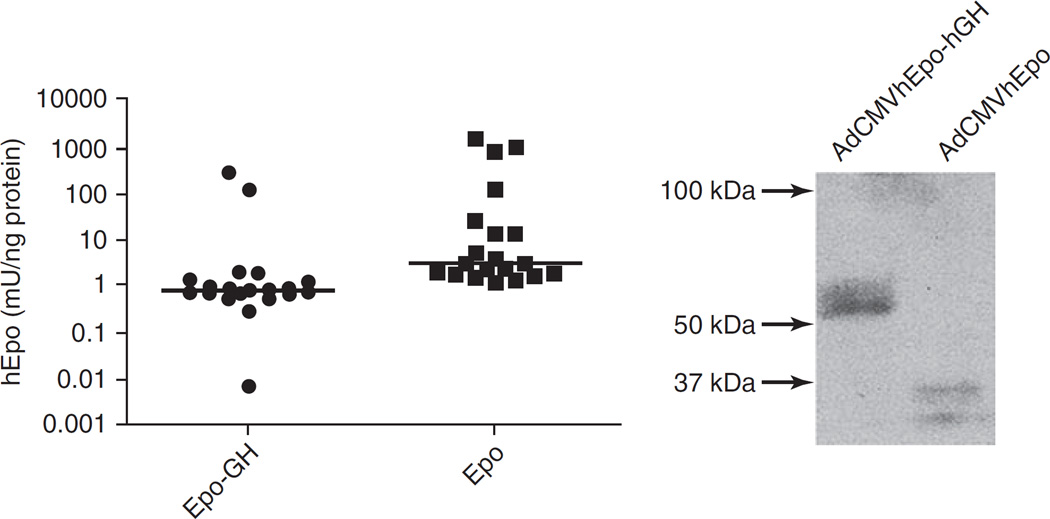
Next, we initiated in vivo studies to examine sorting of the chimeric protein in transduced salivary glands. Initially, we delivered, at 109 particles/gland, either AdCMVhEpo-hGH or AdCMVhEpo (as a negative control vector) to 20 animals (10 in each group) via retroductal cannulation of Wharton’s duct. Forty-eight hours after vector administration serum and saliva were collected, and assayed for the level of immunoreactive hEpo. Serum and saliva from AdCMVhEpo-transduced animals (n = 10) contained 79.1 ± 30.1 and 0.7 ± 0.6 mU/ml, respectively, of immunoreactive hEpo. Animals transduced with AdCMVhEpo-hGH (n = 10) had low levels of immunoreactive hEpo, 4.4 ± 4.4 and 0.1 ± 0.1 mU/ml in serum and saliva, respectively. Thus, we decided that this initial dose was too low for evaluating the sorting of the hEpo–hGH fusion protein and we increased the dose of administered vector to 1010 particles/gland for all other experiments reported here. As noted above, four separate cohorts of mice were tested. Mice were administered the 1010 viral particles/gland dose of either AdCMVhEpo-hGH or AdCMVhEpo to the submandibular glands. Forty-eight hours later serum and saliva were collected, aqueous extracts of salivary glands were prepared, and all were assayed for the levels of immunoreactive hEpo. In extracts of 20 glands from the 10 animals transduced with AdCMVhEpo the median level of immunoreactive hEpo was 2.99 mU/ng protein (range, 1.18–1292.4 mU/ng protein; Fig. 3, left), whereas for the 20 glands from the 10 animals transduced with AdCMVhEpo-hGH the median level was 0.79 mU/ng protein (range, 0.01–255.6 mU/ng protein). This difference was statistically significant (p < 0.001). In addition, proteins from the aqueous extracts were separated on polyacrylamide gels and transferred to nitrocellulose membranes for immunoblotting with anti-hEpo antibody. Extracts from glands transduced with AdCMVhEpo contained immunoreactive proteins of ~32–34 kDa. In contrast, extracts from glands transduced with AdCMVhEpo-hGH contained immunoreactive proteins of ~56–58 kDa (about the expected size of the fusion protein; Fig. 3, right). Unlike the immunoblots from lysates of cells transduced in vitro with AdCMVhEpo-hGH, extracts from glands transduced in vivo did not contain additional immunoreactive bands, that is, ~110 and 22 kDa (Fig. 3, right).
FIG. 3.
Immunoreactive hEpo in aqueous extracts of mouse submandibular glands. Left: Forty-eight hours after administration of AdCMVhEpo-GH or AdCMVhEpo at 1010 viral particles/gland, glands were harvested and frozen at −80°C. Thereafter, glands were homogenized and levels of immunoreactive hEpo were determined in gland extracts by ELISA. Glands transduced with AdCMVhEpo had a median protein level of 2.99 mU/ng protein (range, 1.18–1292.4 mU/ng protein), whereas glands transduced with AdCMVhEpo-hGH showed a median protein level of 0.79 mU/ng protein (range, 001–255.6 mU/ng protein). The horizontal bar represent the median value (n = 20, p < 0.001). Right: Extracts from glands were electrophoresed as described in Materials and Methods, blotted, and then reacted with anti-hEpo antibody. Mouse glands transduced with AdCMVhEpo-hGH or AdCMVhEpo expressed immunoreactive hEpo–hGH and hEpo protein bands with apparent molecular sizes of ~56–58 and ~32–34 kDa, respectively.
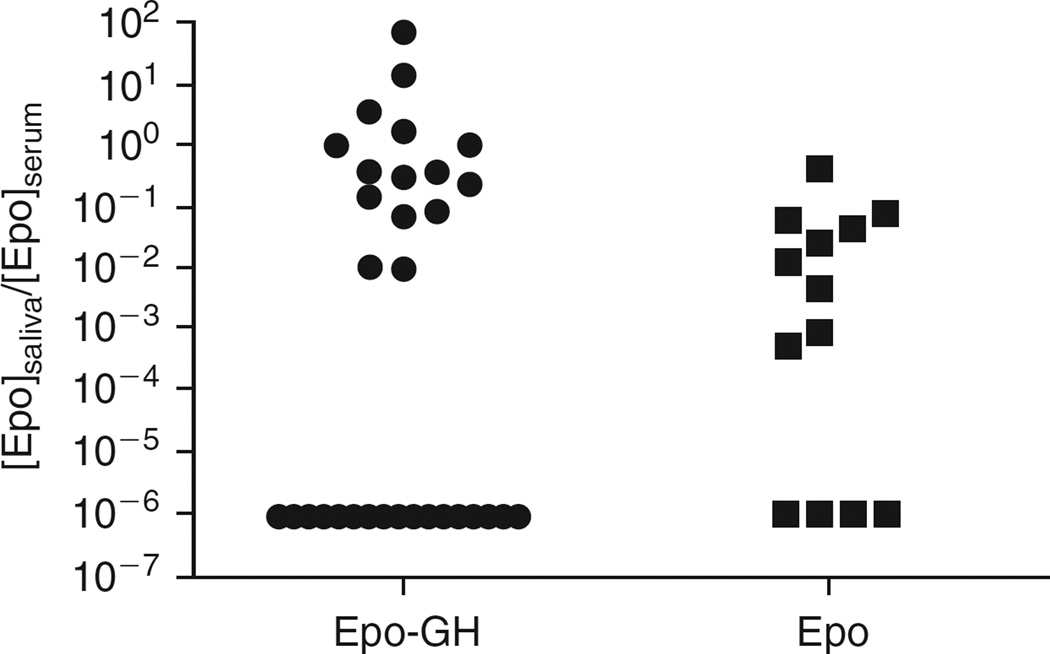
To evaluate the sorting pathways used by hEpo and the fusion protein, we next examined the distribution of these transgenic proteins by comparing ratios of immunoreactive hEpo in saliva and serum in both experimental groups. The total amounts of immunoreactive hEpo in serum (1 ml) and saliva (100 µl) were 1233 ± 735 and 6 ± 5.4 mU, respectively, for the hEpo group, and 39.2 ± 16 and 3.3 ± 1.85 mU, respectively, for the hEpo–hGH group. The range of saliva:serum immunoreactive hEpo ratios observed for most animals was considerable, ~3 orders of magnitude (Fig. 4). This variability likely was a result of both differences in cannulation efficacy in ~20-g mice, and true interanimal differences. Nonetheless, it is clear from the data that immunoreactive hEpo levels in mice transduced with AdCMVhEpo were more frequently higher in the bloodstream (saliva:serum ratios, <<0.1), whereas for mice transduced with AdCMVhEpo-hGH the immunoreactive hEpo was found more often in saliva (saliva:serum ratios more frequently >0.1), as generally hypothesized. This pattern was independent of stratification of individual animals on the basis of the levels of immunoreactive hEpo production, that is, whether they were “high or low expressers” (data not shown). Furthermore, when animals in both treatment groups were ranked according to their saliva:serum immunoreactive hEpo ratios, the values at the 50th, 75th, and 90th percentiles for the AdCMVhEpo group were 0.003, 0.041, and 0.072, respectively, whereas for the AdCMVhEpo-hGH group these values were 0.095, 0.355, and 3.14, respectively. Indeed, for five mice in the AdCMVhEpo-hGH experimental group the immunoreactive hEpo saliva:serum ratio was >>1. The differences in immunoreactive hEpo sorting between the two groups were significantly different (p < 0.001).
FIG. 4.
Ratios of immunoreactive hEpo products in saliva and serum from mice transduced with either AdCMVhEpo-hGH or AdCMVhEpo at 1010 particles/gland. Mice were administered either vector via the submandibular glands. After 2 days serum and saliva were collected and hEpo-immunoreactive products were measured as described in Materials and Methods. Animals transduced with AdCMVhEpo-hGH had significantly higher saliva:serum ratios of immunoreactive hEpo products compared with those transduced with AdCMVhEpo (p < 0.001), that is, relatively higher levels of immunoreactive hEpo were being secreted into saliva. For these analyses, the number of mice tested was n = 32 for hEpo–hGH and n = 14 for hEpo.
DISCUSSION
To use salivary glands effectively for both local (upper gastrointestinal tract) and systemic gene therapeutics applications, it is important to understand and eventually be able to influence the sorting pathways used by transgenic secretory proteins in salivary glands. Most proteins secreted from salivary cells follow an exocrine, regulated pathway into saliva; however, endocrine secretion into the bloodstream, most likely via a constitutive-type pathway, clearly occurs (Kagami et al., 1996; Isenman et al., 1999). The specific mechanisms that underlie the sorting of secretory proteins into this pathway are not, however, well understood (Castle and Castle, 1998; Gorr et al., 2005). Studies of trafficking in other cell types with some prohormones and proneuropeptides have provided evidence that sorting of these molecules into the regulated pathway involves a receptor-mediated mechanism (Cool et al., 1997; Loh et al., 2004). Thus, in endocrine cells, sorting motifs of pro-opiomelanocortin, brain-derived neurotrophic factor, and proinsulin have been shown to interact with membrane-bound carboxypeptidase E, which can act as a sorting or retention receptor to target these prohormones to the regulated pathway (Lou et al., 2005). However, no universal regulated pathway-sorting motifs or signals have been identified (Arvan and Castle, 1998; Arvan and Halban, 2004). Secretion via the constitutive pathway has been little studied (Arvan and Castle, 1998; Ponnambalam and Baldwin, 2003) and was long considered a default pathway for secretion. However, more recently in vitro studies have shown that secretion through this pathway is more complex (Huang et al., 2001; Feng and Arvan, 2003; Lara-Lemus et al., 2006).
It was the purpose of the present study to redirect a constitutive pathway protein (hEpo), which is readily secreted as a transgene product from mouse salivary glands into the bloodstream (Voutetakis et al., 2005), into an exocrine pathway, that is, into saliva. As noted earlier, such redirection of a constitutive pathway secretory protein may be beneficial for treating certain upper gastrointestinal tract disorders such as oropharyngeal mucositis and antibiotic-resistant infections. We constructed a chimeric protein fusing hEpo to hGH, and hypothesized that this hGH addition to the C terminus of hEpo would lead to an increase in immunoreactive hEpo secreted into saliva after delivery of the vector to mouse submandibular glands. Mice provide a particularly good model for testing our hypothesis, because previously we reported that transduction of mouse salivary glands with a vector encoding hEpo resulted in the overwhelmingly endocrine secretion of this transgene product (~10:1, serum vs. saliva) (Voutetakis et al., 2005).
In the present study, we demonstrate that significant redirection of hEpo can occur in mouse salivary glands, from the bloodstream into saliva, after fusing hGH to the C terminus of hEpo. Although it has previously been shown in vitro that fusion of the C terminus of hGH to a model constitutive pathway viral protein (a truncated vesicular stomatitis virus G sequence) can redirect the viral protein into the regulated pathway in the AtT20 model endocrine cell line (Moore and Kelly, 1986), this is the first demonstration of such a sorting manipulation in vivo. Our results clearly suggest that fusion of hGH to the C terminus of hEpo facilitates diversion of hEpo from an endocrine pathway (i.e., toward the bloodstream) into an exocrine pathway (i.e., into saliva). Mice administered the AdCMVhEpo-hGH vector had significantly higher saliva:serum ratios of immunoreactive hEpo compared with those of mice receiving AdCMVhEpo (Fig. 4). Importantly, the absolute amount of immunoreactive hEpo produced in glands from mice transduced with the AdCMVhEpo-hGH vector was about one-fourth that found in glands from mice transduced with the AdCMVhEpo vector (Fig. 3). This generally comparable expression level of immunoreactive hEpo with both vectors makes it highly unlikely that redirection of hEpo–hGH into saliva was simply the result of a “spillover” of excess fusion protein from the endocrine to an exocrine pathway. Indeed, whereas the levels of immunoreactive hEpo detected in saliva were similar in both groups, the levels found in serum of the hEpo group were 10-fold higher than those of the hEpo–hGH group. It is likely, but not proven from our present studies, that the exocrine pathway taken by the fusion protein was either the major or minor regulated secretory pathway found in salivary gland cells (Castle and Castle, 1998; Gorr et al., 2005). Interestingly, some animals from the AdCMVhEpo-hGH group showed high saliva:serum ratios, and there also was some overlap in these ratios between animals in both treatment groups. At present we do not understand the intragroup variation between individual animals. It is possible that differences in the expression of genes associated with secretory protein-sorting mechanisms could in part underlie this phenomenon; a hypothesis that is testable. At present, we are trying to define the minimal hGH sequence(s) that is sufficient to redirect hEpo from the bloodstream into saliva.
In conclusion, the present gene transfer studies in male mice show that in principle a normally constitutively secreted protein, hEpo, can be substantially directed from an endocrine into an exocrine secretory pathway of salivary gland cells. Understanding the precise signals and mechanisms responsible for such redirection in the sorting of secretory proteins should be beneficial for developing novel treatments for several upper gastrointestinal tract disorders.
Acknowledgments
This research was supported by the Intramural Research Programs of the National Institute of Dental and Craniofacial Research and the National Institute of Child Health and Human Development. The authors thank Dr. M. Nahid Parvin for generous help with the N-glycosidase F experiments.
Footnotes
AUTHOR DISCLOSURE STATEMENT
No competing financial interests exist.
REFERENCES
- Alexander JM, Hsu D, Penchuk L, Heinrich G. Cell-specific and developmental regulation of a nerve growth factor-human growth hormone fusion gene in transgenic mice. Neuron. 1989;3:133–139. doi: 10.1016/0896-6273(89)90122-0. [DOI] [PubMed] [Google Scholar]
- Arvan P. Secretory protein trafficking: Genetic and biochemical analysis. Cell Biochem. Biophys. 2004;40:169–178. doi: 10.1385/cbb:40:3:169. [DOI] [PubMed] [Google Scholar]
- Arvan P, Castle D. Protein sorting and secretion granule formation in regulated secretory cells. Trends Cell Biol. 1992;2:327–331. doi: 10.1016/0962-8924(92)90181-l. [DOI] [PubMed] [Google Scholar]
- Arvan P, Castle D. Sorting and storage during secretory granule biogenesis: Looking backward and looking forward. Biochem. J. 1998;332:593–610. doi: 10.1042/bj3320593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvan P, Halban PA. Sorting ourselves out: Seeking consensus on trafficking in the beta-cell. Traffic. 2004;5:53–61. doi: 10.1111/j.1600-0854.2004.00152.x. [DOI] [PubMed] [Google Scholar]
- Baum BJ, Berkman ME, Marmary Y, Goldsmith CM, Baccaglini L, Wang S, Wellner RB, Hoque AT, Atkinson JC, Yamagishi H, Kagami H, Parlow AF, Chao J. Polarized secretion of transgene products from salivary glands in vivo. Hum. Gene Ther. 1999;10:2789–2797. doi: 10.1089/10430349950016528. [DOI] [PubMed] [Google Scholar]
- Baum BJ, Voutetakis A, Wang J. Salivary glands: Novel target sites for gene therapeutics. Trends Mol. Med. 2004;10:585–590. doi: 10.1016/j.molmed.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Bryskier A. Viridans group streptococci: A reservoir of resistant bacteria in oral cavities. Clin. Microbiol. Infect. 2002;8:65–69. doi: 10.1046/j.1198-743x.2001.00398.x. [DOI] [PubMed] [Google Scholar]
- Castle D, Castle A. Intracellular transport and secretion of salivary proteins. Crit. Rev. Oral Biol. Med. 1998;9:4–22. doi: 10.1177/10454411980090010301. [DOI] [PubMed] [Google Scholar]
- Cool DR, Normant E, Shen F, Chen HC, Pannell L, Zhang Y, Loh YP. Carboxypeptidase E is a regulated secretory pathway sorting receptor: Genetic obliteration leads to endocrine disorders in Cpefat mice. Cell. 1997;88:73–83. doi: 10.1016/s0092-8674(00)81860-7. [DOI] [PubMed] [Google Scholar]
- Dannies PS. Protein hormone storage in secretory granules: Mechanisms for concentration and sorting. Endocr. Rev. 1999;20:3–21. doi: 10.1210/edrv.20.1.0354. [DOI] [PubMed] [Google Scholar]
- Delporte C, O’Connell BC, He X, Ambudkar IS, Agre P, Baum BJ. Adenovirus-mediated expression of aquaporin-5 in epithelial cells. J. Biol. Chem. 1996;271:22070–22075. doi: 10.1074/jbc.271.36.22070. [DOI] [PubMed] [Google Scholar]
- Feng L, Arvan P. The trafficking of α1-antitrypsin, a post-Golgi secretory pathway marker, in INS-1 pancreatic beta cells. J. Biol. Chem. 2003;278:31486–31494. doi: 10.1074/jbc.M305690200. [DOI] [PubMed] [Google Scholar]
- Gorr SU, Venkatesh SG, Darling DS. Parotid secretory granules: Crossroads of secretory pathways and protein storage. J. Dent. Res. 2005;84:500–509. doi: 10.1177/154405910508400604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G, Simons K. The trans Golgi network: Sorting at the exit site of the Golgi complex. Science. 1986;234:438–443. doi: 10.1126/science.2945253. [DOI] [PubMed] [Google Scholar]
- Gumbiner B, Kelly RB. Two distinct intracellular pathways transport secretory and membrane glycoproteins to the surface of pituitary tumor cells. Cell. 1982;28:51–59. doi: 10.1016/0092-8674(82)90374-9. [DOI] [PubMed] [Google Scholar]
- He X, Goldsmith CM, Marmary Y, Wellner RB, Parlow AF, Nieman LK, Baum BJ. Systemic action of human growth hormone following adenovirus-mediated gene transfer to rat submandibular glands. Gene Ther. 1998;5:537–541. doi: 10.1038/sj.gt.3300622. [DOI] [PubMed] [Google Scholar]
- Hoque AT, Baccaglini L, Baum BJ. Hydroxychloroquine enhances the endocrine secretion of adenovirus-directed growth hormone from rat submandibular glands in vivo. Hum. Gene Ther. 2001;12:1333–1341. doi: 10.1089/104303401750270986. [DOI] [PubMed] [Google Scholar]
- Huang AY, Castle AM, Hinton BT, Castle JD. Resting (basal) secretion of proteins is provided by the minor regulated and constitutive-like pathways and not granule exocytosis in parotid acinar cells. J. Biol. Chem. 2001;276:22296–22306. doi: 10.1074/jbc.M100211200. [DOI] [PubMed] [Google Scholar]
- Huang GTJ, Zhang H-B, Kim D, Liu L, Ganz T. A model for antimicrobial gene therapy: Demonstration of human beta-defensin 2 antimicrobial activities in vivo. Hum. Gene Ther. 2002;13:2017–2025. doi: 10.1089/10430340260395875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenman L, Liebow C, Rothman S. The endocrine secretion of mammalian digestive enzymes by exocrine glands. Am. J. Physiol. 1999;276:E223–E232. doi: 10.1152/ajpendo.1999.276.2.E223. [DOI] [PubMed] [Google Scholar]
- Kagami H, O’Connell BC, Baum BJ. Evidence for the systemic delivery of a transgene product from salivary glands. Hum. Gene Ther. 1996;7:2177–2184. doi: 10.1089/hum.1996.7.17-2177. [DOI] [PubMed] [Google Scholar]
- Kelly RB. Pathways of protein secretion in eukaryotes. Science. 1985;230:25–32. doi: 10.1126/science.2994224. [DOI] [PubMed] [Google Scholar]
- Lara-lemus R, Liu M, Turner MD, Scherer P, Stenbeck G, Iyengar P, Arvan P. Lumenal protein sorting to the constitutive secretory pathway of a regulated secretory cell. J. Cell Sci. 2006;119:1833–1842. doi: 10.1242/JCS.02905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh YP, Maldonado A, Zhang C, Tam WH, Cawley N. Mechanism of sorting proopiomelanocortin and proenkephalin to the regulated secretory pathway of neuroendocrine cells. Ann. N. Y. Acad. Sci. 2002;971:416–425. doi: 10.1111/j.1749-6632.2002.tb04504.x. [DOI] [PubMed] [Google Scholar]
- Loh YP, Kim T, Rodriguez YM, Cawley NX. Secretory granule biogenesis and neuropeptide sorting to the regulated secretory pathway in neuroendocrine cells. J. Mol. Neurosci. 2004;22:63–71. doi: 10.1385/JMN:22:1-2:63. [DOI] [PubMed] [Google Scholar]
- Lou H, Kim SK, Zaitsev E, Snell CR, Lu B, Loh YP. Sorting and activity-dependent secretion of BDNF require interaction of a specific motif with the sorting receptor carboxypeptidase E. Neuron. 2005;45:245–255. doi: 10.1016/j.neuron.2004.12.037. [DOI] [PubMed] [Google Scholar]
- Lou H, Smith AM, Coates LC, Cawley NX, Loh YP, Birch NP. The transmembrane domain of the pro-hormone convertase PC3: A key motif for targeting to the regulated secretory pathway. Mol. Cell. Endocrinol. 2007;267:17–25. doi: 10.1016/j.mce.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JM, Green M, Barbadora KA, Wald ER. Erythromycin-resistant group A streptococci in schoolchildren in Pittsburgh. N. Engl. J. Med. 2002;346:1200–1206. doi: 10.1056/NEJMoa013169. [DOI] [PubMed] [Google Scholar]
- Moore HH, Kelly RB. Re-routing of a secretory protein by fusion with human growth hormone sequences. Nature. 1986;321:443–446. doi: 10.1038/321443a0. [DOI] [PubMed] [Google Scholar]
- O’Connell BC, Xu T, Walsh TJ, Sein T, Mastrangeli A, Crystal RG, Oppenheim FG, Baum BJ. Transfer of a gene encoding the anticandidal protein histatin 3 to salivary glands. Hum. Gene Ther. 1996;7:2255–2261. doi: 10.1089/hum.1996.7.18-2255. [DOI] [PubMed] [Google Scholar]
- Ponnambalam S, Baldwin SA. Constitutive protein secretion from the trans-Golgi network to the plasma membrane. Mol. Membr. Biol. 2003;20:129–139. doi: 10.1080/0968768031000084172. [DOI] [PubMed] [Google Scholar]
- Sonis ST, Peterson RL, Edwards LJ, Lucey CA, Wang L, Mason L, Login G, Ymamkawa M, Moses G, Bouchard P, Hayes LL, Bedrosian C, Dorner AJ. Defining mechanisms of action of interleukin-11 on the progression of radiation-induced oral mucositis in hamsters. Oral Oncol. 2000;36:373–381. doi: 10.1016/s1368-8375(00)00012-9. [DOI] [PubMed] [Google Scholar]
- Spielberger R, Stiff P, Bensinger W, Gentile T, Weisdorf D, Kewalramani T, Shea T, Yanovich S, Hansen K, Noga S, Mccarty J, Lemaistre CF, Sung EC, Blazar BR, Elhardt D, Chen M-G, Emmanouilides C. Palifermin for oral mucositis after intensive therapy for hematologic cancers. N. Engl. J. Med. 2004;351:2590–2598. doi: 10.1056/NEJMoa040125. [DOI] [PubMed] [Google Scholar]
- Tekirian TL. The central role of the trans-Golgi network as a gateway of the early secretory pathway: Physiologic vs non-physiologic protein transit. Exp. Cell Res. 2002;281:9–18. doi: 10.1006/excr.2002.5656. [DOI] [PubMed] [Google Scholar]
- Trahair JF, Neutra MR, Gordon JI. Use of transgenic mice to study the routing of secretory proteins in intestinal epithelial cells: Analysis of human growth hormone compartmentalization as a function of cell type and differentiation. J. Cell Biol. 1989;109:3231–3242. doi: 10.1083/jcb.109.6.3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejo AN, Pogulis RJ, Pease LR. Mutagenesis and Synthesis of Novel Recombinant Genes Using PCR. Plainview, NY: Cold Spring Harbor Laboratory Press; 1995. [Google Scholar]
- Voutetakis A, Kok MR, Zheng C, Bossis I, Wang J, Cotrim AP, Marracino N, Goldsmith CM, Chiorini JA, Loh YP, Nieman LK, Baum BJ. Reengineered salivary glands are stable endogenous bioreactors for systemic gene therapeutics. Proc. Natl. Acad. Sci. U.SA. 2004;101:3053–3058. doi: 10.1073/pnas.0400136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voutetakis A, Bossis I, Kok MR, Zhang W, Wang J, Cotrim AP, Zheng C, Chiorini JA, Nieman LK, Baum BJ. Salivary glands as a potential gene transfer target for gene therapeutics of some monogenetic endocrine disorders. J. Endocrinol. 2005;185:363–372. doi: 10.1677/joe.1.06171. [DOI] [PubMed] [Google Scholar]
- Wang J, Cawley NX, Voutetakis A, Rodriguez YM, Goldsmith CM, Nieman LK, Hoque AT, Frank SJ, Snell CR, Loh YP, Baum BJ. Partial redirection of transgenic human growth hormone secretion from rat salivary glands. Hum. Gene Ther. 2005;16:571–583. doi: 10.1089/hum.2005.16.571. [DOI] [PubMed] [Google Scholar]
- Yan X, Voutetakis A, Zheng C, Hai B, Zhang C, Baum BJ, Wang S. Sorting of transgenic secretory proteins in miniature pig parotid glands following adenoviral-mediated gene transfer. J. Gene Med. 2007;9:779–787. doi: 10.1002/jgm.1081. [DOI] [PubMed] [Google Scholar]
- Zufferey R, Aebischer P. Salivary glands and gene therapy: The mouth waters. Gene Ther. 2004;11:1425–1426. doi: 10.1038/sj.gt.3302321. [DOI] [PubMed] [Google Scholar]