Abstract
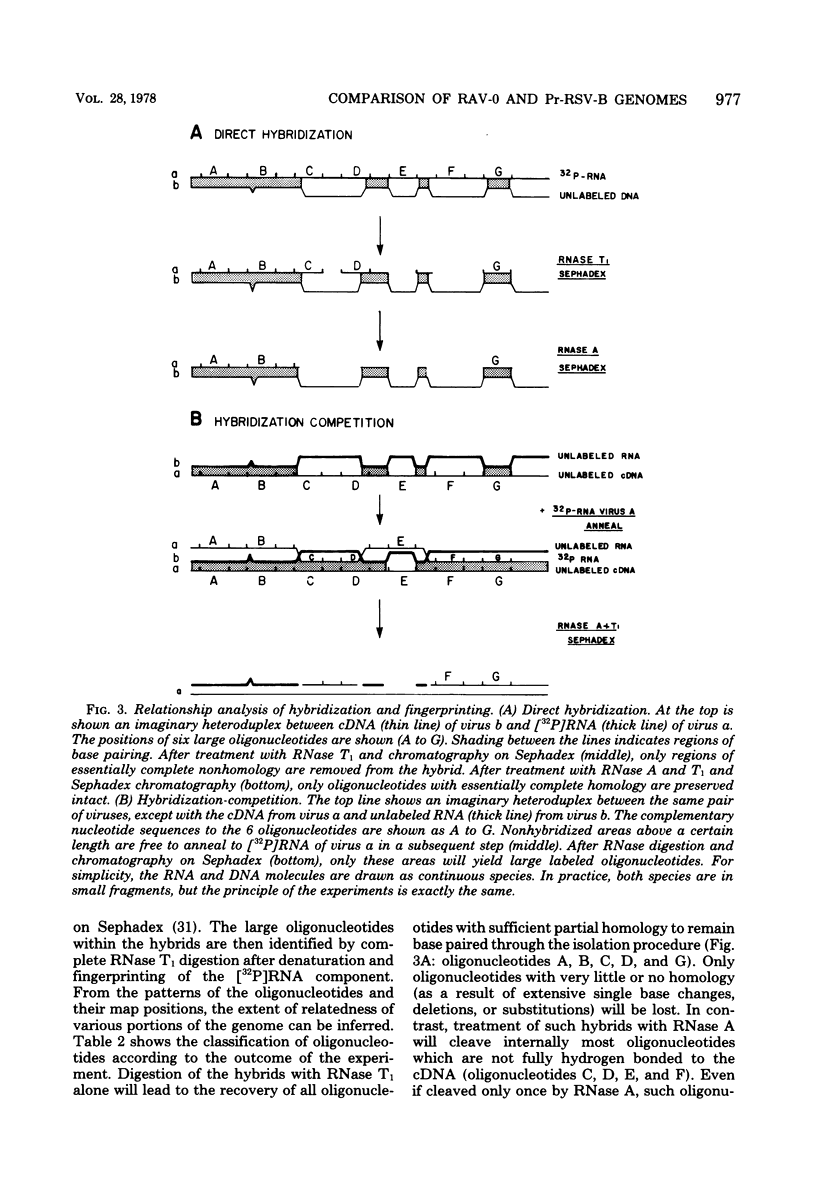
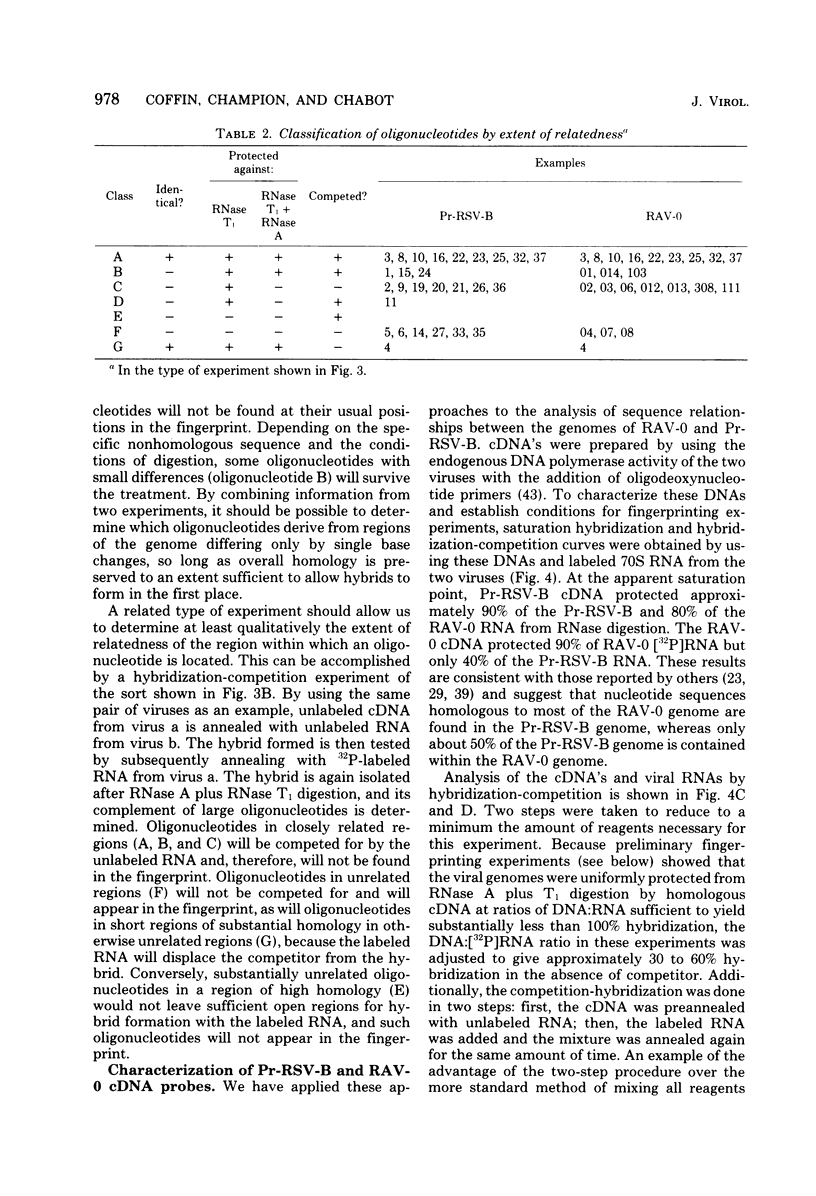
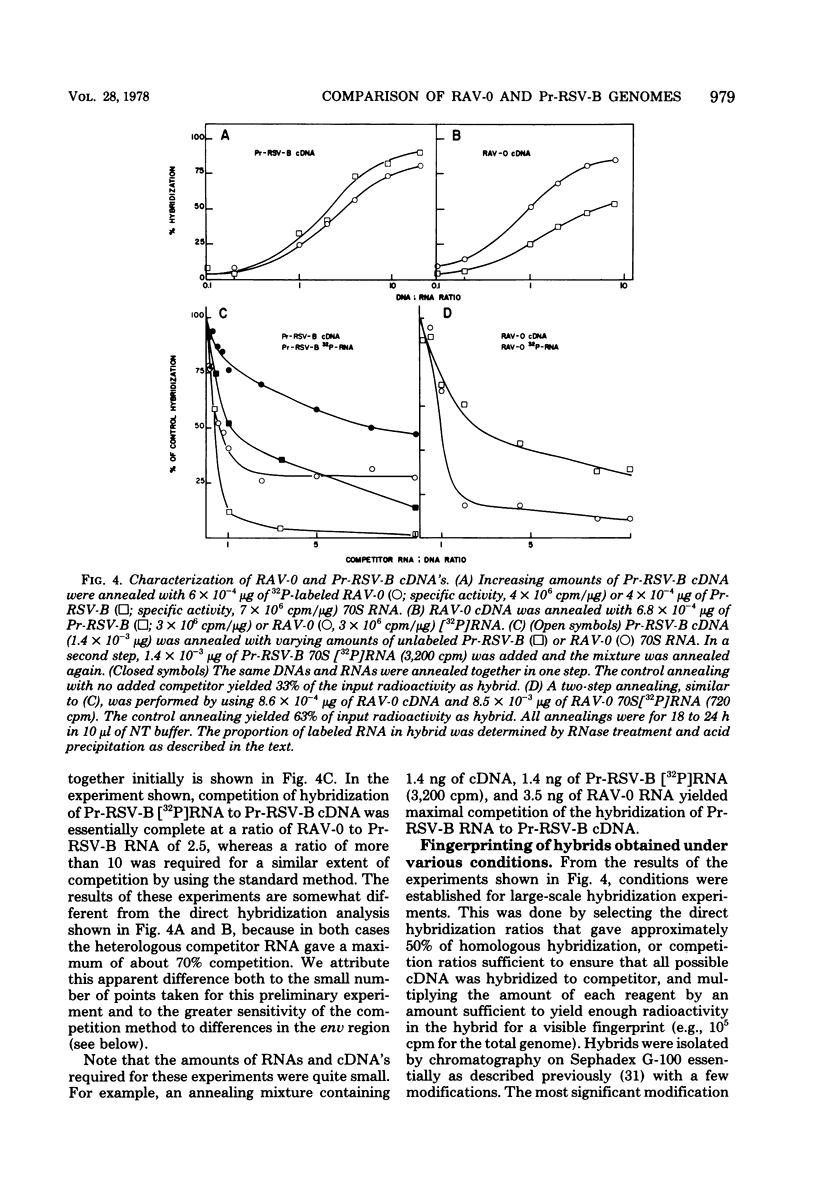
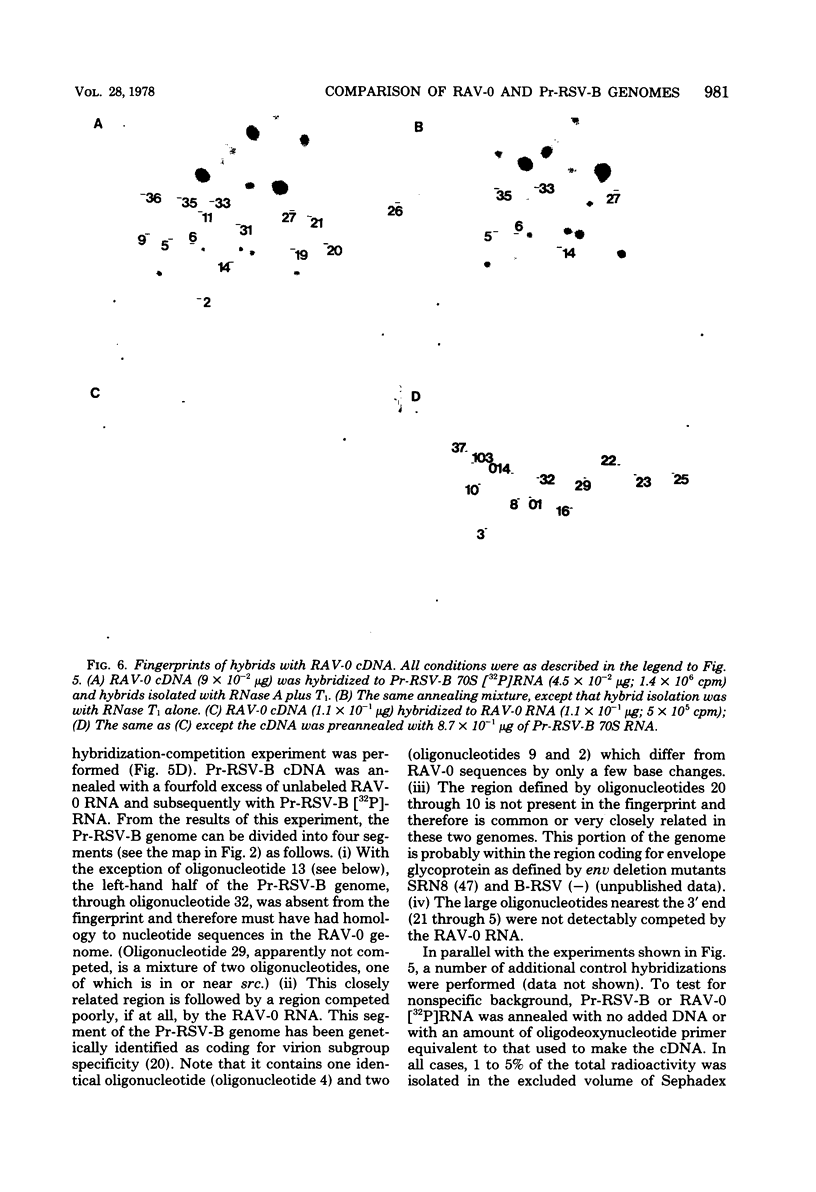
We have used mapping of large T1 oligonucleotides to examine the genome of Rous-associated virus-O (RAV-O), an endogenous virus of chickens, and to compare it with that of Prague strain Rous sarcoma virus, subgroup B, (Pr-RSV-B), an exogenous sarcoma virus. To extend the sensitivity of such comparisons, we have developed a system of nucleic acid hybridization and hybridization-competition combined with fingerprinting. This method allows us to estimate the relative degree of relatedness of various portions of the viral genomes. From the results of this study, we have concluded that the genomes of Pr-RSV-B and RAV-O are related in the following way. The 5'-terminal half of the genomes (corresponding to the gag and pol regions) is virtually identical, with only scattered single nucleotide differences. This region is followed by a region comprising 25 to 30% of the genome (the env region) which contains substantial nucleotide sequence differences, most or all of which are due to single base changes. The env-coding region can be further subdivided into three regions: a more variable region probably containing sequences coding for subgroup specificity, flanked by relatively common sequences on each side. To the 3' side of the env region, the RAV-O genome contains a very short sequence not found in Pr-RSV-B, whereas the Pr-RSV-B genome contains a much longer unrelated sequence. The central portion of this sequence comprises the src gene as defined by transformation-defective mutants. Particularly striking is the absence, in the RAV-O genome, of any nucleotide sequence related to the "c region" found very near the 3' end of all exogenous tumor viruses. Both the Pr-RSV-B and RAV-O genomes contain the identical terminally redundant sequence of 21 nucleotides near each end of the genome.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beemon K., Duesberg P., Vogt P. Evidence for crossing-over between avian tumor viruses based on analysis of viral RNAs. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4254–4258. doi: 10.1073/pnas.71.10.4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashion L. M., Joho R. H., Planitz M. A., Billeter M. A., Weissmann C. Initiation sites of Rous sarcoma virus RNA-directed DNA synthesis in vitro. Nature. 1976 Jul 15;262(5565):186–190. doi: 10.1038/262186a0. [DOI] [PubMed] [Google Scholar]
- Chen J. H., Hanafusa H. Detection of a protein of avian leukoviruses in uninfected chick cells by radioimmunoassay. J Virol. 1974 Feb;13(2):340–346. doi: 10.1128/jvi.13.2.340-346.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin J. M., Billeter M. A. A physical map of the Rous sarcoma virus genome. J Mol Biol. 1976 Jan 25;100(3):293–318. doi: 10.1016/s0022-2836(76)80065-4. [DOI] [PubMed] [Google Scholar]
- Coffin J. M. Genes responsible for transformation by avian RNA tumor viruses. Cancer Res. 1976 Nov;36(11 Pt 2):4282–4288. [PubMed] [Google Scholar]
- Coffin J. M., Hageman T. C., Maxam A. M., Haseltine W. A. Structure of the genome of Moloney murine leukemia virus: a terminally redundant sequence. Cell. 1978 Apr;13(4):761–773. doi: 10.1016/0092-8674(78)90226-x. [DOI] [PubMed] [Google Scholar]
- Coffin J. M., Haseltine W. A. Nucleotide sequence of Rous sarcoma virus RNA at the initiation site of DNA synthesis: The 102nd nucleotide is U. J Mol Biol. 1977 Dec 15;117(3):805–814. doi: 10.1016/0022-2836(77)90071-7. [DOI] [PubMed] [Google Scholar]
- Coffin J. M., Haseltine W. A. Terminal redundancy and the origin of replication of Rous sarcoma virus RNA. Proc Natl Acad Sci U S A. 1977 May;74(5):1908–1912. doi: 10.1073/pnas.74.5.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crittenden L. B., Smith E. J., Weiss R. A., Sarma P. S. Host gene control of endogenous avian leukosis virus production. Virology. 1974 Jan;57(1):128–138. doi: 10.1016/0042-6822(74)90114-7. [DOI] [PubMed] [Google Scholar]
- Galehouse D. M., Duesberg P. H. Glycoproteins of avian tumor virus recombinants: evidence for intragenic crossing-over. J Virol. 1978 Jan;25(1):86–96. doi: 10.1128/jvi.25.1.86-96.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern M. S., Bolognesi D. P., Friis R. R., Mason W. S. Expression of the Major Viral Glycoprotein of Avian Tumor Virus in Cells of chf(+) Chicken Embryos. J Virol. 1975 May;15(5):1131–1140. doi: 10.1128/jvi.15.5.1131-1140.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseltine W. A., Kleid D. G., Panet A., Rothenberg E., Baltimore D. Ordered transcription of RNA tumor virus genomes. J Mol Biol. 1976 Sep 5;106(1):109–131. doi: 10.1016/0022-2836(76)90303-x. [DOI] [PubMed] [Google Scholar]
- Haseltine W. A., Maxam A. M., Gilbert W. Rous sarcoma virus genome is terminally redundant: the 5' sequence. Proc Natl Acad Sci U S A. 1977 Mar;74(3):989–993. doi: 10.1073/pnas.74.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward W. S. Size and genetic content of viral RNAs in avian oncovirus-infected cells. J Virol. 1977 Oct;24(1):47–63. doi: 10.1128/jvi.24.1.47-63.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joho R. H., Billeter M. A., Weissmann C. Mapping of biological functions on RNA of avian tumor viruses: location of regions required for transformation and determination of host range. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4772–4776. doi: 10.1073/pnas.72.12.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junghans R. P., Hu S., Knight C. A., Davidson N. Heteroduplex analysis of avian RNA tumor viruses. Proc Natl Acad Sci U S A. 1977 Feb;74(2):477–481. doi: 10.1073/pnas.74.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C. Y., Temin H. M. Lack of sequence homology among RNAs of avian leukosis-sarcoma viruses, reticuloendotheliosis viruses, and chicken endogenous RNA-directed DNA polymerase activity. J Virol. 1973 Dec;12(6):1314–1324. doi: 10.1128/jvi.12.6.1314-1324.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linial M., Mason W. S. Characterization of two conditional early mutants of Rous sarcoma virus. Virology. 1973 May;53(1):258–273. doi: 10.1016/0042-6822(73)90484-4. [DOI] [PubMed] [Google Scholar]
- Linial M., Neiman P. E. Infection of chick cells by subgroup E viruses. Virology. 1976 Sep;73(2):508–520. doi: 10.1016/0042-6822(76)90412-8. [DOI] [PubMed] [Google Scholar]
- Mellon P., Duesberg P. H. Subgenomic, cellular Rous sarcoma virus RNAs contain oligonucleotides from the 3' half and the 5' terminus of virion RNA. Nature. 1977 Dec 15;270(5638):631–634. doi: 10.1038/270631a0. [DOI] [PubMed] [Google Scholar]
- Motta J. V., Crittenden L. B., Purchase H. G., Stone H. A., Witter R. L. Low oncogenic potential of avian endogenous RNA tumor virus infection or expression. J Natl Cancer Inst. 1975 Sep;55(3):685–689. doi: 10.1093/jnci/55.3.685. [DOI] [PubMed] [Google Scholar]
- Neiman P. E., Das S., Macdonnell D., McMillin-Helsel C. Organization of shared and unshared sequences in the genomes of chicken endogenous and sarcoma viruses. Cell. 1977 Jun;11(2):321–329. doi: 10.1016/0092-8674(77)90048-4. [DOI] [PubMed] [Google Scholar]
- Oppermann H., Bishop J. M., Varmus H. E., Levintow L. A joint produce of the genes gag and pol of avian sarcoma virus: a possible precursor of reverse transcriptase. Cell. 1977 Dec;12(4):993–1005. doi: 10.1016/0092-8674(77)90164-7. [DOI] [PubMed] [Google Scholar]
- Parsons J. T., Coffin J. M., Haroz R. K., Bromley P. A., Weissmann C. Quantitative determination and location of newly synthesized virus-specific ribonucleic acid in chicken cells infected with Rous sarcoma virus. J Virol. 1973 May;11(5):761–774. doi: 10.1128/jvi.11.5.761-774.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purchio A. F., Erikson E., Erikson R. L. Translation of 35S and of subgenomic regions of avian sarcoma virus RNA. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4661–4665. doi: 10.1073/pnas.74.10.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettenmier C. W., Hanafusa H. Structural protein markers in the avian oncoviruses. J Virol. 1977 Dec;24(3):850–864. doi: 10.1128/jvi.24.3.850-864.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson H. L. Intracellular restriction on the growth of induced subgroup E avian type C viruses in chicken cells. J Virol. 1976 Jun;18(3):856–866. doi: 10.1128/jvi.18.3.856-866.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson H. L., Swanson C. A., Hruska J. F., Crittenden L. B. Production of unique C-type viruses by chicken cells grown in bromodeoxyuridine. Virology. 1976 Jan;69(1):63–74. doi: 10.1016/0042-6822(76)90194-x. [DOI] [PubMed] [Google Scholar]
- Schwartz D. E., Zamecnik P. C., Weith H. L. Rous sarcoma virus genome is terminally redundant: the 3' sequence. Proc Natl Acad Sci U S A. 1977 Mar;74(3):994–998. doi: 10.1073/pnas.74.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh R., Linial M., Coffin J., Eisenman R. Recombinant avian oncoviruses. I. Alterations in the precursor to the internal structural proteins. Virology. 1978 Jun 15;87(2):326–338. doi: 10.1016/0042-6822(78)90138-1. [DOI] [PubMed] [Google Scholar]
- Shine J., Czernilofsky A. P., Friedrich R., Bishop J. M., Goodman H. M. Nucleotide sequence at the 5' terminus of the avian sarcoma virus genome. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1473–1477. doi: 10.1073/pnas.74.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoyab M., Baluda M. A. Ribonucleotide sequence homology among avian oncornaviruses. J Virol. 1975 Jan;17(1):106–113. doi: 10.1128/jvi.17.1.106-113.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehelin D., Varmus H. E., Bishop J. M., Vogt P. K. DNA related to the transforming gene(s) of avian sarcoma viruses is present in normal avian DNA. Nature. 1976 Mar 11;260(5547):170–173. doi: 10.1038/260170a0. [DOI] [PubMed] [Google Scholar]
- Tal J., Fujita D. J., Kawai S., Varmus H. E., Bishop J. M. Purification of DNA complementary to the env gene of avian sarcoma virus and analysis of relationships among the env genes of avian leukosis-sarcoma viruses. J Virol. 1977 Feb;21(2):497–505. doi: 10.1128/jvi.21.2.497-505.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. M., Illmensee R. Site on the RNA of an avian sarcoma virus at which primer is bound. J Virol. 1975 Sep;16(3):553–558. doi: 10.1128/jvi.16.3.553-558.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. M., Illmensee R., Summers J. Efficeint transcription of RNA into DNA by avian sarcoma virus polymerase. Biochim Biophys Acta. 1976 Sep 6;442(3):324–330. doi: 10.1016/0005-2787(76)90307-5. [DOI] [PubMed] [Google Scholar]
- Vogt P. K., Friis R. R. An avian leukosis virus related to RSV(O): properties and evidence for helper activity. Virology. 1971 Jan;43(1):223–234. doi: 10.1016/0042-6822(71)90240-6. [DOI] [PubMed] [Google Scholar]
- Wang L. H., Duesberg P. H., Kawai S., Hanafusa H. Location of envelope-specific and sarcoma-specific oligonucleotides on RNA of Schmidt-Ruppin Rous sarcoma virus. Proc Natl Acad Sci U S A. 1976 Feb;73(2):447–451. doi: 10.1073/pnas.73.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Duesberg P., Beemon K., Vogt P. K. Mapping RNase T1-resistant oligonucleotides of avian tumor virus RNAs: sarcoma-specific oligonucleotides are near the poly(A) end and oligonucleotides common to sarcoma and transformation-defective viruses are at the poly(A) end. J Virol. 1975 Oct;16(4):1051–1070. doi: 10.1128/jvi.16.4.1051-1070.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. A., Friis R. R., Katz E., Vogt P. K. Induction of avian tumor viruses in normal cells by physical and chemical carcinogens. Virology. 1971 Dec;46(3):920–938. doi: 10.1016/0042-6822(71)90091-2. [DOI] [PubMed] [Google Scholar]
- Weiss S. R., Varmus H. E., Bishop J. M. The size and genetic composition of virus-specific RNAs in the cytoplasm of cells producing avian sarcoma-leukosis viruses. Cell. 1977 Dec;12(4):983–992. doi: 10.1016/0092-8674(77)90163-5. [DOI] [PubMed] [Google Scholar]
- de Wachter R., Fiers W. Preparative two-dimensional polyacrylamide gel electrophoresis of 32 P-labeled RNA. Anal Biochem. 1972 Sep;49(1):184–197. doi: 10.1016/0003-2697(72)90257-6. [DOI] [PubMed] [Google Scholar]