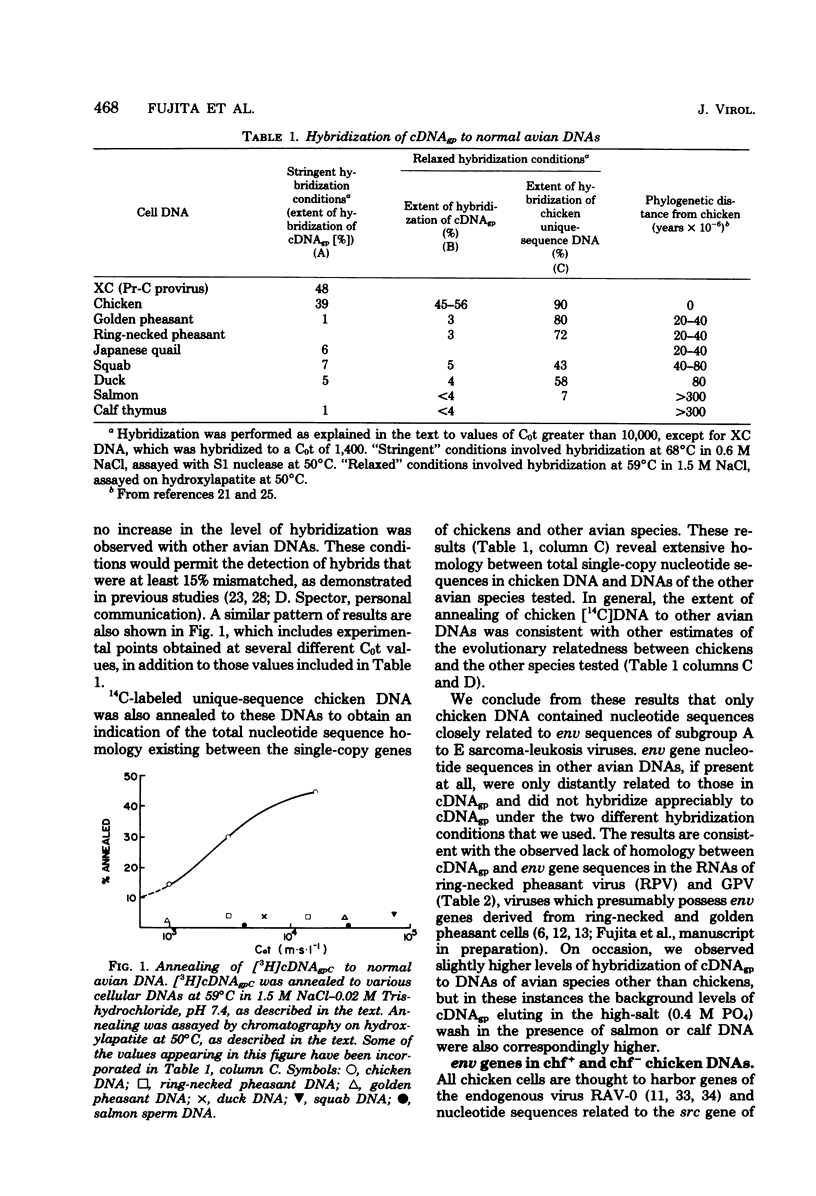
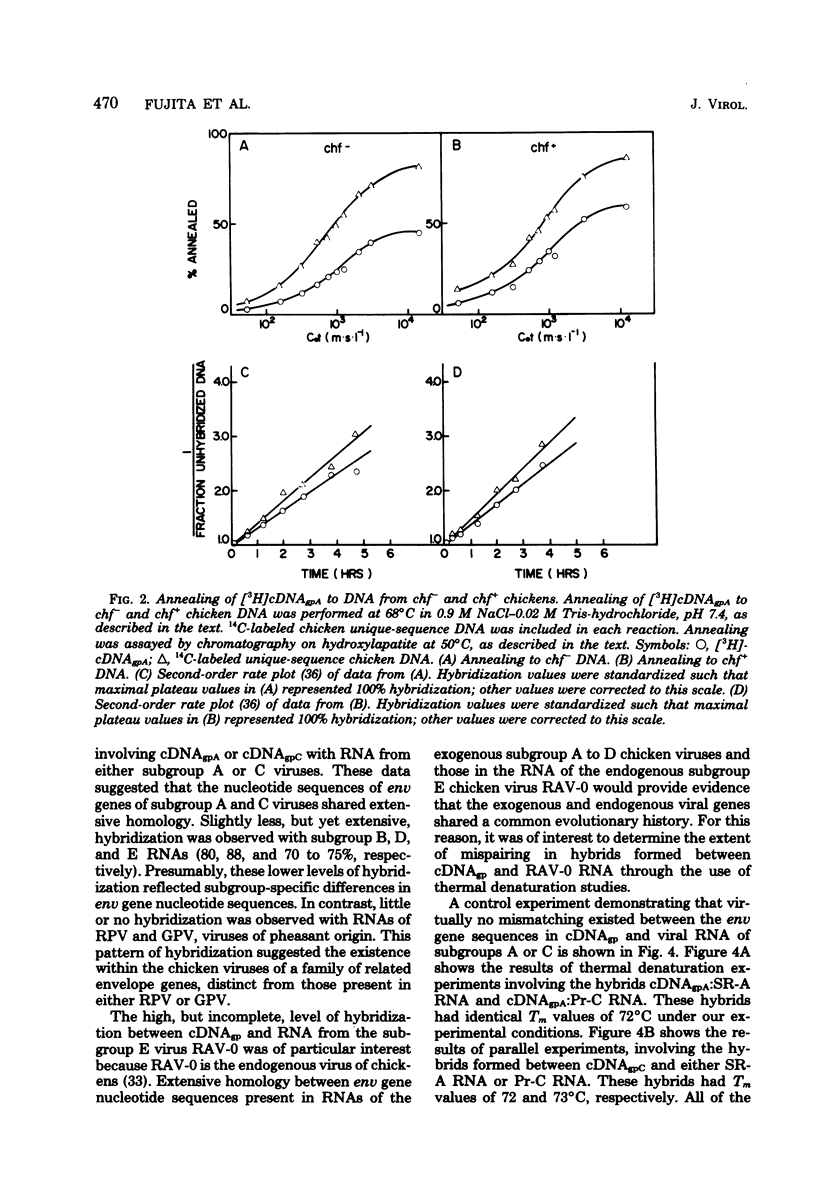
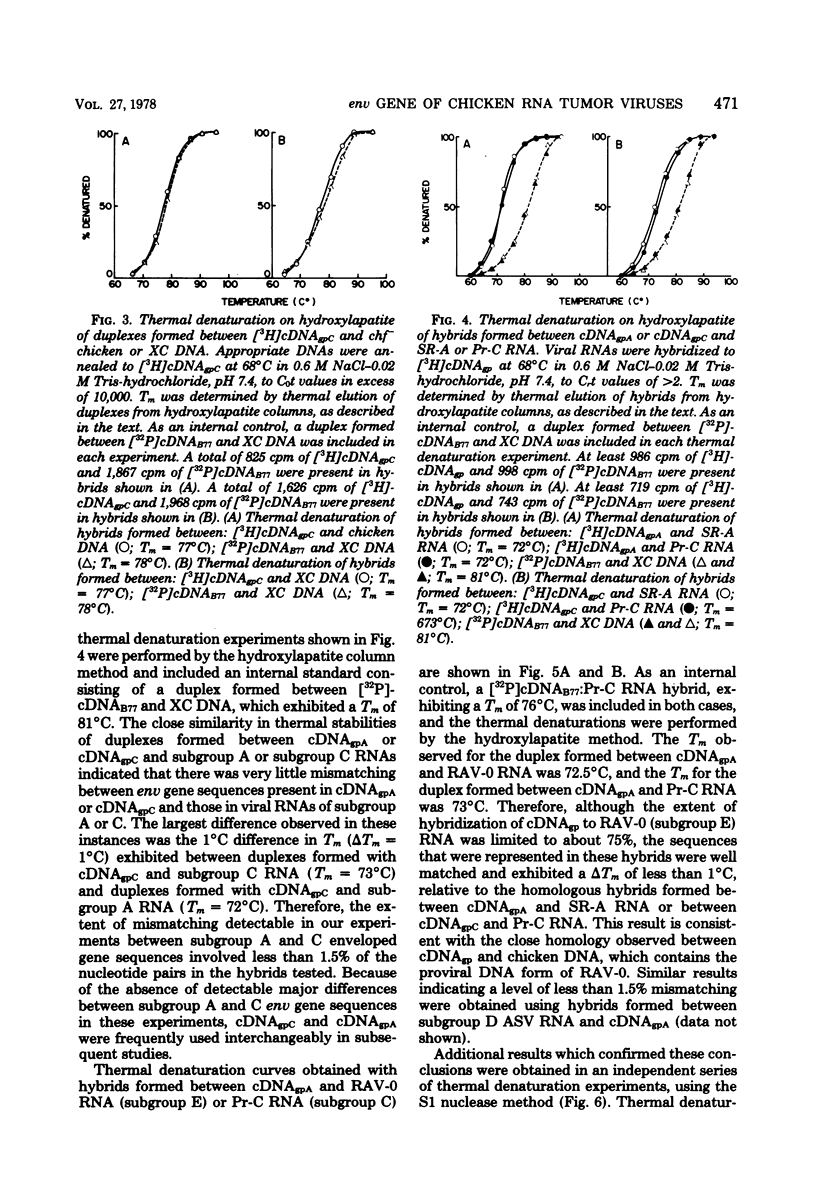
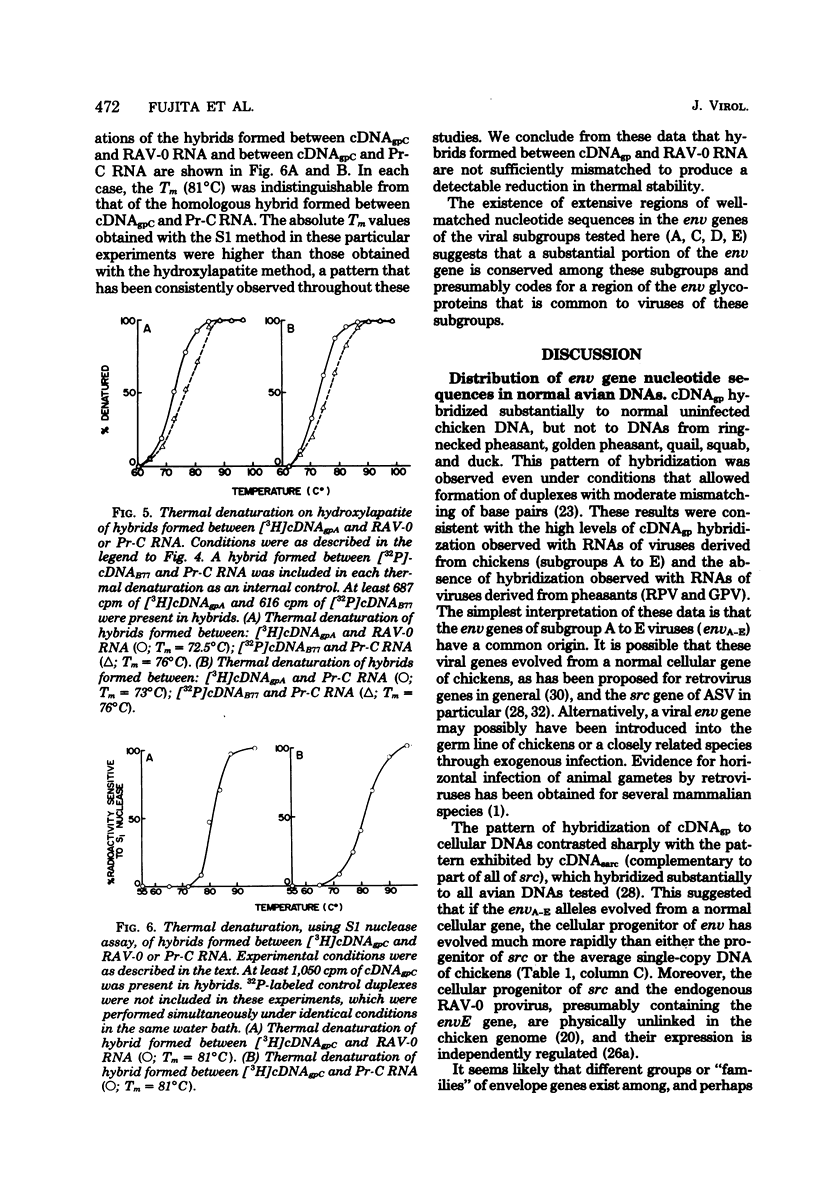
Abstract
The env gene of avian sarcoma-leukosis viruses codes for envelope glycoproteins that determine viral host range, antigenic specificity, and interference patterns. We used molecular hybridization to analyze the natural distribution and possible origins of the nucleotide sequences that encode env; our work exploited the availability of radioactive DNA (cDNAgp) complementary to most or all of env. env sequences were detectable in the DNAs of chickens which synthesized an env gene product (chick helper factor positive) encoded by an endogenous viral gene and also in the DNAs of chickens which synthesized little or no env gene product (chick helper factor negative). env sequences were not detectable in DNAs from Japanese quail, ring-necked pheasant, golden pheasant, duck, squab, salmon sperm, or calf thymus. The detection of sequences closely related to viral env only in chicken DNA contrasts sharply with the demonstration that the transforming gene (src) of avian sarcoma viruses has readily detectable homologues in the DNAs of all avian species tested [D. Stehelin, H. E. Varmus, J. M. Bishop, and P. K. Vogt, Nature (London) 260: 170-173, 1976] and in the DNAs of other vertebrates (D. Spector, personal communication). Thermal denaturation studies on duplexes formed between cDNAgp and chicken DNA and also between cDNAgp and RNAs of subgroup A to E viruses derived from chickens indicated that these duplexes were well matched. In contrast, cDNAgp did not form stable hybrids with RNAs of viruses which were isolated from ring-necked and golden pheasants. We conclude that substantial portions of nucleotide sequences within the env genes of viruses of subgroups A to E are closely related and that these genes probably have a common, perhaps cellular, evolutionary origin.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benveniste R. E., Todaro G. J. Evolution of C-type viral genes: inheritance of exogenously acquired viral genes. Nature. 1974 Dec 6;252(5483):456–459. doi: 10.1038/252456a0. [DOI] [PubMed] [Google Scholar]
- Bishop J. M., Levinson W. E., Sullivan D., Fanshier L., Quintrell N., Jackson J. The low molecular weight RNAs of Rous sarcoma virus. II. The 7 S RNA. Virology. 1970 Dec;42(4):927–937. doi: 10.1016/0042-6822(70)90341-7. [DOI] [PubMed] [Google Scholar]
- Chen Y. C., Vogt P. K. Endogenous leukosis viruses in the avian family Phasianidae. Virology. 1977 Feb;76(2):740–750. doi: 10.1016/0042-6822(77)90255-0. [DOI] [PubMed] [Google Scholar]
- Fanshier L., Garapin A. C., McDonnell J., Faras A., Levinson W., Bishop J. M. Deoxyribonucleic acid polymerase associated with avian tumor viruses: secondary structure of the deoxyribonucleic acid product. J Virol. 1971 Jan;7(1):77–86. doi: 10.1128/jvi.7.1.77-86.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich R., Kung H. J., Baker B., Varmus H. E., Goodman H. M., Bishop J. M. Characterization of DNA complementary to nucleotide sequences at the 5'-terminus of the avian sarcoma virus genome. Virology. 1977 Jun 1;79(1):198–215. doi: 10.1016/0042-6822(77)90345-2. [DOI] [PubMed] [Google Scholar]
- Galehouse D. M., Duesberg P. H. Glycoproteins of avian tumor virus recombinants: evidence for intragenic crossing-over. J Virol. 1978 Jan;25(1):86–96. doi: 10.1128/jvi.25.1.86-96.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garapin A. C., Varmus H. E., Faras A. J., Levinson W. E., Bishop J. M. RNA-directed DNA synthesis by virions of Rous sarcoma virus: further characterization of the templates and the extent of their transcription. Virology. 1973 Mar;52(1):264–274. doi: 10.1016/0042-6822(73)90414-5. [DOI] [PubMed] [Google Scholar]
- Guntaka R. V., Richards O. C., Shank P. R., Kung H. J., Davidson N. Covalently closed circular DNA of avian sarcoma virus: purification from nuclei of infected quail tumor cells and measurement by electron microscopy and gel electrophoresis. J Mol Biol. 1976 Sep 15;106(2):337–357. doi: 10.1016/0022-2836(76)90090-5. [DOI] [PubMed] [Google Scholar]
- Halpern M. S., Bolognesi D. P., Friis R. R., Mason W. S. Expression of the Major Viral Glycoprotein of Avian Tumor Virus in Cells of chf(+) Chicken Embryos. J Virol. 1975 May;15(5):1131–1140. doi: 10.1128/jvi.15.5.1131-1140.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa H., Miyamoto T., Hanafusa T. A cell-associated factor essential for formation of an infectious form of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1970 Jun;66(2):314–321. doi: 10.1073/pnas.66.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa T., Hanafusa H. Isolation of leukosis-type virus from pheasant embryo cells: possible presence of viral genes in cells. Virology. 1973 Jan;51(1):247–251. doi: 10.1016/0042-6822(73)90388-7. [DOI] [PubMed] [Google Scholar]
- Hanafusa T., Hanafusa H., Metroka C. E., Hayward W. S., Rettenmier C. W., Sawyer R. C., Dougherty R. M., Distefano H. S. Pheasant virus: new class of ribodeoxyvirus. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1333–1337. doi: 10.1073/pnas.73.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward W. S., Hanafusa H. Independent regulation of endogenous and exogenous avian RNA tumor virus genes. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2259–2263. doi: 10.1073/pnas.73.7.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaki R., Vogt P. K. Immunological relationships among envelope antigens of avian tumor viruses. Virology. 1966 Nov;30(3):375–387. doi: 10.1016/0042-6822(66)90116-4. [DOI] [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. Isolation of defective mutant of avian sarcoma virus. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3493–3497. doi: 10.1073/pnas.70.12.3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshet E., Temin H. M. Nucleotide sequences derived from pheasant DNA in the genome of recombinant avian leukosis viruses with subgroup F specificity. J Virol. 1977 Nov;24(2):505–513. doi: 10.1128/jvi.24.2.505-513.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong J. A., Garapin A. C., Jackson N., Fanshier L., Levinson W., Bishop J. M. Virus-specific ribonucleic acid in cells producing rous sarcoma virus: detection and characterization. J Virol. 1972 Jun;9(6):891–902. doi: 10.1128/jvi.9.6.891-902.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. A., Kazan P. M., Varmus H. E. The importance of DNA size for successful transfection of chicken embryo fibroblasts. Virology. 1974 Sep;61(1):297–302. doi: 10.1016/0042-6822(74)90265-7. [DOI] [PubMed] [Google Scholar]
- Padgett T. G., Stubbledield E., Varmus H. E. Chicken macrochromosomes contain an endogenous provirus and microchromosomes contain sequences related to the transforming gene of ASV. Cell. 1977 Apr;10(4):649–657. doi: 10.1016/0092-8674(77)90098-8. [DOI] [PubMed] [Google Scholar]
- Prager E. M., Brush A. H., Nolan R. A., Nakanishi M., Wilson A. C. Slow evolution of transferrin and albumin in birds according to micro-complement fixation analysis. J Mol Evol. 1974;3(4):243–262. doi: 10.1007/BF01796041. [DOI] [PubMed] [Google Scholar]
- Reitz M. S., Jr, Abrell J. W., Trainor C. D., Gallo R. C. Precipitation of nucleic acids with cetyltrimethylammonium bromide: a method for preparing viral and cellular DNA polymerase products for cesium sulfate density gradient analysis. Biochem Biophys Res Commun. 1972 Oct 6;49(1):30–38. doi: 10.1016/0006-291x(72)90005-8. [DOI] [PubMed] [Google Scholar]
- Rohrschneider L., Bauer H., Bolognesi D. P. Group-specific antigenic determinants of the large envelope glycoprotein of avian oncornaviruses. Virology. 1975 Sep;67(1):234–241. doi: 10.1016/0042-6822(75)90420-1. [DOI] [PubMed] [Google Scholar]
- Schäfer W., Fischinger P. J., Collins J. J., Bolognesi D. P. Role of carbohydrate in biological functions of Friend murine leukemia virus gp71. J Virol. 1977 Jan;21(1):35–40. doi: 10.1128/jvi.21.1.35-40.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector D. H., Smith K., Padgett T., McCombe P., Roulland-Dussoix D., Moscovici C., Varmus H. E., Bishop J. M. Uninfected avian cells contain RNA related to the transforming gene of avian sarcoma viruses. Cell. 1978 Feb;13(2):371–379. doi: 10.1016/0092-8674(78)90205-2. [DOI] [PubMed] [Google Scholar]
- Stehelin D., Guntaka R. V., Varmus H. E., Bishop J. M. Purification of DNA complementary to nucleotide sequences required for neoplastic transformation of fibroblasts by avian sarcoma viruses. J Mol Biol. 1976 Mar 5;101(3):349–365. doi: 10.1016/0022-2836(76)90152-2. [DOI] [PubMed] [Google Scholar]
- Stehelin D., Varmus H. E., Bishop J. M., Vogt P. K. DNA related to the transforming gene(s) of avian sarcoma viruses is present in normal avian DNA. Nature. 1976 Mar 11;260(5547):170–173. doi: 10.1038/260170a0. [DOI] [PubMed] [Google Scholar]
- Tal J., Fujita D. J., Kawai S., Varmus H. E., Bishop J. M. Purification of DNA complementary to the env gene of avian sarcoma virus and analysis of relationships among the env genes of avian leukosis-sarcoma viruses. J Virol. 1977 Feb;21(2):497–505. doi: 10.1128/jvi.21.2.497-505.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temin H. M. On the origin of RNA tumor viruses. Annu Rev Genet. 1974;8:155–177. doi: 10.1146/annurev.ge.08.120174.001103. [DOI] [PubMed] [Google Scholar]
- Ullman J. S., McCarthy B. J. The relationship between mismatched base pairs and the thermal stability of DNA duplexes. I. Effects of depurination and chain scission. Biochim Biophys Acta. 1973 Feb 4;294(1):405–415. doi: 10.1016/0005-2787(73)90095-6. [DOI] [PubMed] [Google Scholar]
- Vogt P. K., Friis R. R. An avian leukosis virus related to RSV(O): properties and evidence for helper activity. Virology. 1971 Jan;43(1):223–234. doi: 10.1016/0042-6822(71)90240-6. [DOI] [PubMed] [Google Scholar]
- Vogt P. K. The emerging genetics of RNA tumor viruses. J Natl Cancer Inst. 1972 Jan;48(1):3–9. [PubMed] [Google Scholar]
- Weiss R. A., Mason W. S., Vogt P. K. Genetic recombinants and heterozygotes derived from endogenous and exogenous avian RNA tumor viruses. Virology. 1973 Apr;52(2):535–552. doi: 10.1016/0042-6822(73)90349-8. [DOI] [PubMed] [Google Scholar]
- Wetmur J. G., Davidson N. Kinetics of renaturation of DNA. J Mol Biol. 1968 Feb 14;31(3):349–370. doi: 10.1016/0022-2836(68)90414-2. [DOI] [PubMed] [Google Scholar]



