Abstract
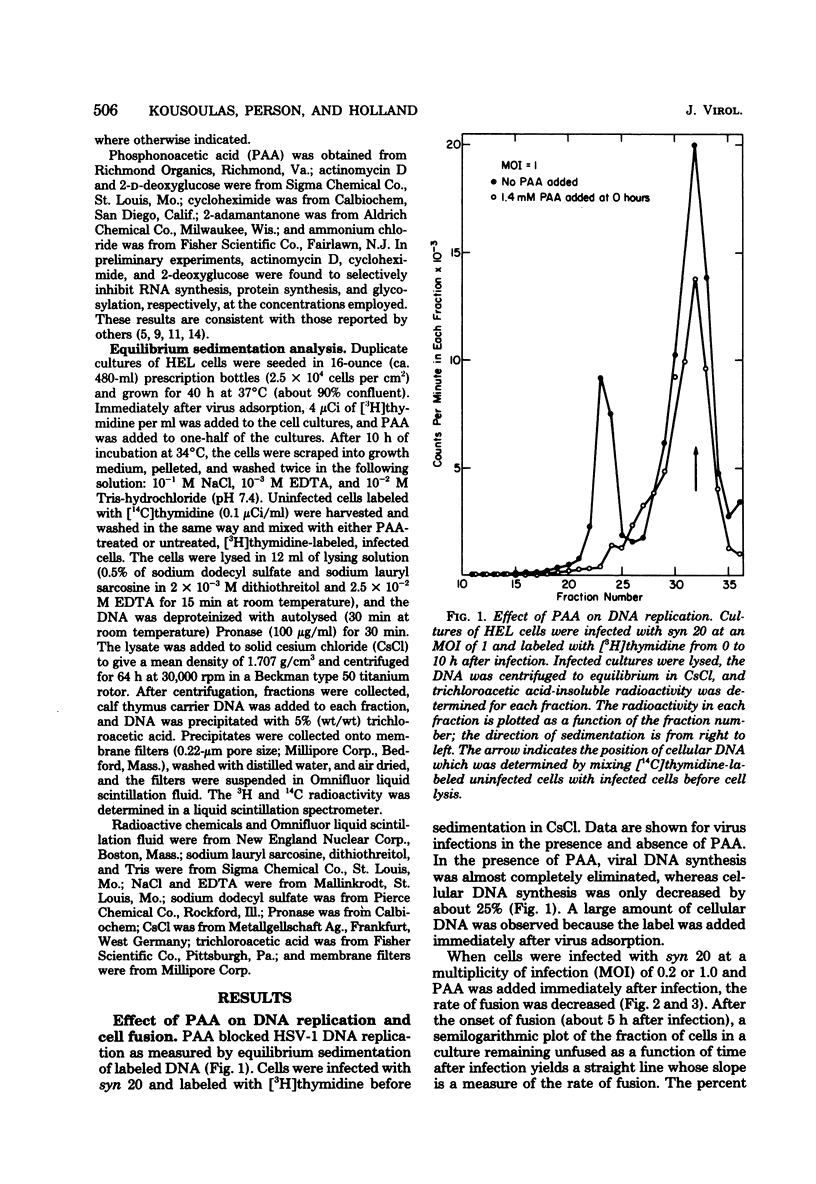
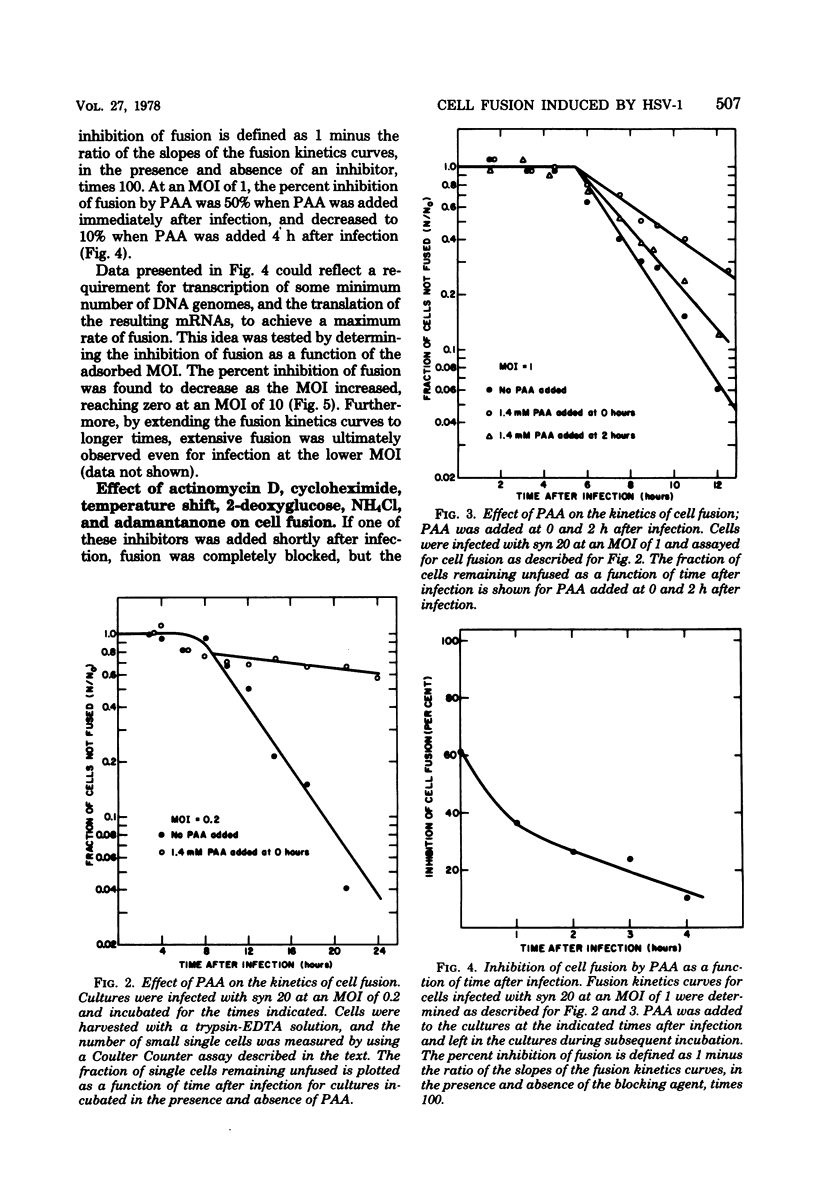
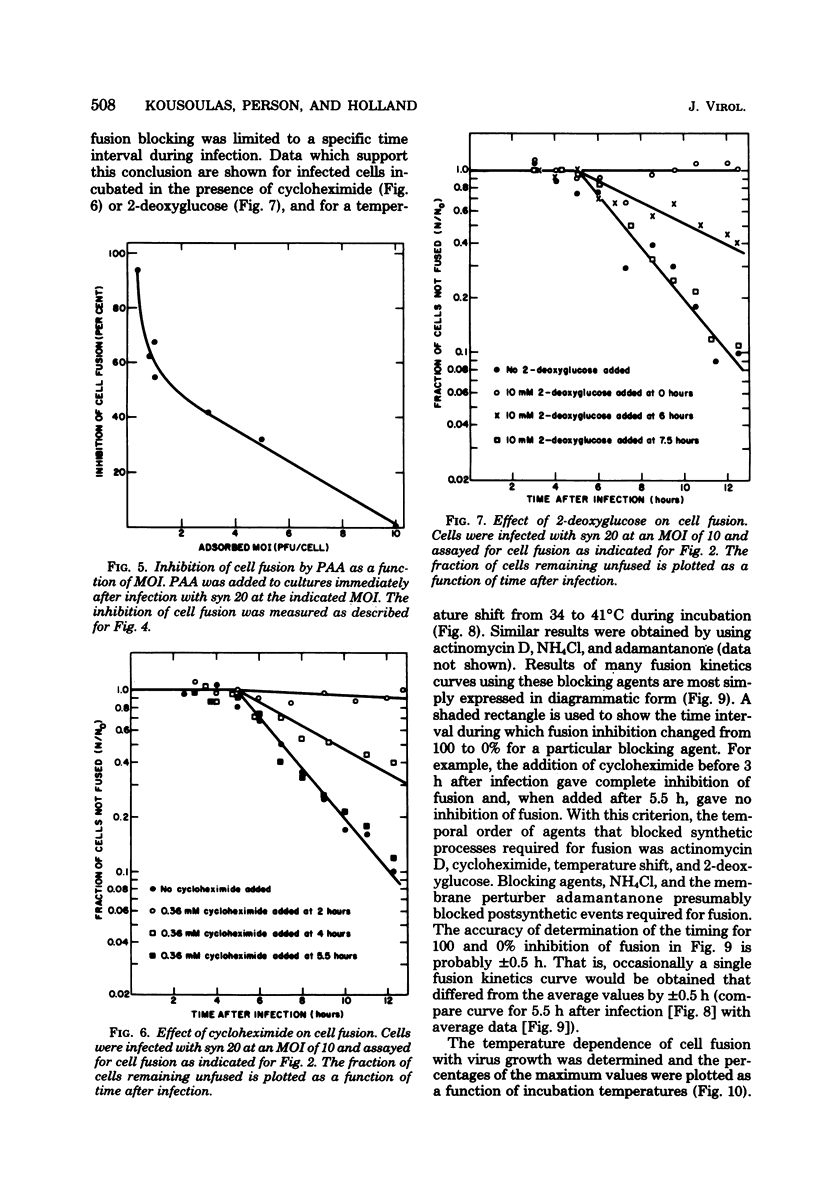
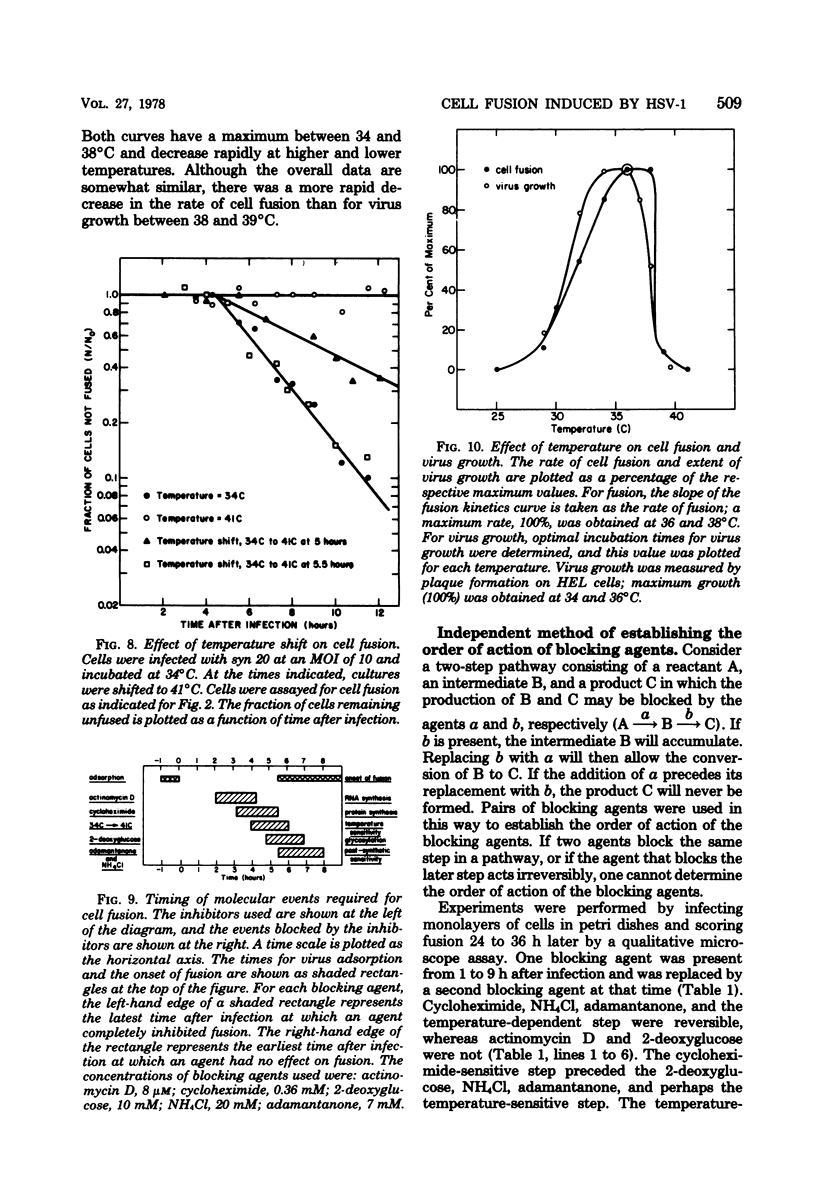
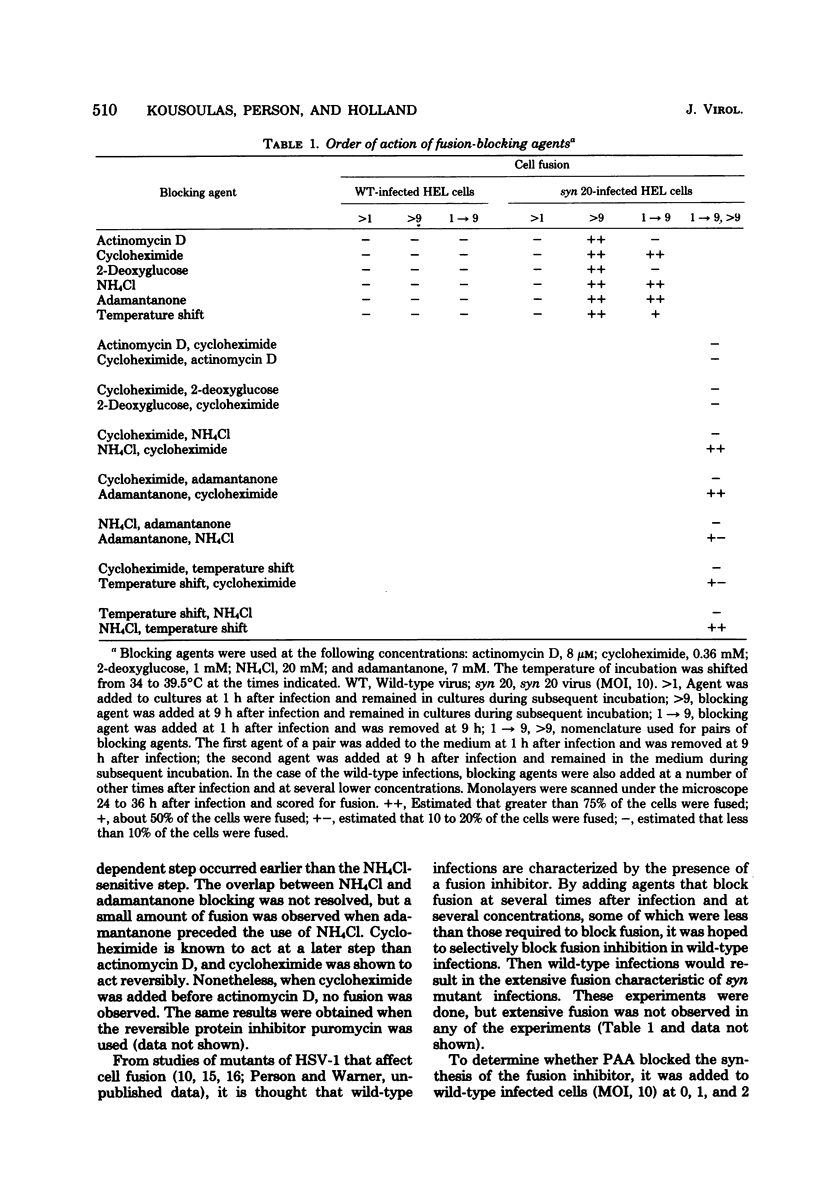
The timing of some of the molecular events that are required for cell fusion was investigated. Cell fusion was produced by a mutant of herpes simplex virus type 1 that causes extensive cell fusion during infection. The timing of molecular events required for fusion was established by the use of blocking agents. Phosphonoacetic acid blocks viral DNA synthesis; actinomycin D blocks RNA synthesis; cycloheximide blocks protein synthesis; 2-deoxyglucose blocks glycosylation of glycoproteins; high temperature, NH4Cl, and adamantanone block unknown steps required for cell fusion. For cells infected at a low multiplicity of infection, phosphonoacetic acid decreased the rate but not the final amount of fusion, but at a multiplicity of infection of 10 it had no effect on the rate of cell fusion. RNA synthesis was required for fusion until 4 h after infection, protein synthesis until 5.5 h after infection, and glycosylation until 7 h after infection. The temperature-dependent step occurred before 6 h after infection, whereas NH4Cl and adamantanone acted at steps that occurred until 8 h after infection. Cycloheximide, temperature, NH4Cl, and adamantanone acted reversibly; actinomycin D and 2-deoxyglucose acted irreversibly. The same order of action of the inhibitors was also determined by using pairs of inhibitors sequentially. These experiments also indicated that the fusion factor was not an α-polypeptide. Virus growth and cell fusion were both found to be highly dependent on temperature in the range of 30 to 40°C. Wild-type infections are apparently characterized by the presence of a fusion factor and a fusion inhibitor. The fusion-blocking agents were added to wild-type-infected cells under a variety of conditions in an attempt to selectively block the production of the fusion inhibitor molecule and thereby cause extensive cell fusion. However, fusion was not observed in any of these experiments.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Courtney R. J., Steiner S. M., Benyesh-Melnick M. Effects of 2-deoxy-D-glucose on herpes simplex virus replication. Virology. 1973 Apr;52(2):447–455. doi: 10.1016/0042-6822(73)90340-1. [DOI] [PubMed] [Google Scholar]
- Cupp J., Klymkowski M., Sands J., Keith A., Snipes W. Effect of lipid alkyl chain perturbations on the assembly of bacteriophage PM2. Biochim Biophys Acta. 1975 May 6;389(2):345–357. doi: 10.1016/0005-2736(75)90327-2. [DOI] [PubMed] [Google Scholar]
- FRANKLIN R. M. THE INHIBITION OF RIBONUCLEIC ACID SYNTHESIS IN MAMMALIAN CELLS BY ACTINOMYCIN D. Biochim Biophys Acta. 1963 Aug 20;72:555–565. [PubMed] [Google Scholar]
- Falke D., Peterknecht W. DNS-, RNS- und Proteinsynthese und ihre Relation zur Riesenzellbildung in vitro nach infektion mit Herpesvirus hominis. Arch Gesamte Virusforsch. 1968;24(3):267–287. [PubMed] [Google Scholar]
- Holland T. C., Person S. Ammonium chloride inhibits cell fusion induced by syn mutants of herpes simplex virus type 1. J Virol. 1977 Jul;23(1):213–215. doi: 10.1128/jvi.23.1.213-215.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974 Jul;14(1):8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller J. M. The expression of the syn- gene of herpes simplex virus type 1. II. Requirements for macromolecular synthesis. Virology. 1976 Jul 15;72(2):402–409. doi: 10.1016/0042-6822(76)90169-0. [DOI] [PubMed] [Google Scholar]
- Knowles R. W., Person S. Effects of 2-deoxyglucose, glucosamine, and mannose on cell fusion and the glycoproteins of herpes simplex virus. J Virol. 1976 May;18(2):644–651. doi: 10.1128/jvi.18.2.644-651.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manservigi R., Spear P. G., Buchan A. Cell fusion induced by herpes simplex virus is promoted and suppressed by different viral glycoproteins. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3913–3917. doi: 10.1073/pnas.74.9.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J. C., Robishaw E. E., Overby L. R. Inhibition of DNA polymerase from herpes simplex virus-infected wi-38 cells by phosphonoacetic Acid. J Virol. 1975 May;15(5):1281–1283. doi: 10.1128/jvi.15.5.1281-1283.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Person S., Knowles R. W., Read G. S., Warner S. C., Bond V. C. Kinetics of cell fusion induced by a syncytia-producing mutant of herpes simplex virus type I. J Virol. 1975 Jan;17(1):183–190. doi: 10.1128/jvi.17.1.183-190.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REICH E., FRANKLIN R. M., SHATKIN A. J., TATUMEL Action of actinomycin D on animal cells and viruses. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1238–1245. doi: 10.1073/pnas.48.7.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhlig M. A., Person S. Alterations of neutral glycolipids in cells infected with syncytium-producing mutants of herpes simplex virus type 1. J Virol. 1977 Nov;24(2):602–608. doi: 10.1128/jvi.24.2.602-608.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J. G. Selective inhibition of herpesvirus DNA synthesis at elevated temperature. Virology. 1966 Aug;29(4):570–579. doi: 10.1016/0042-6822(66)90280-7. [DOI] [PubMed] [Google Scholar]


