Abstract
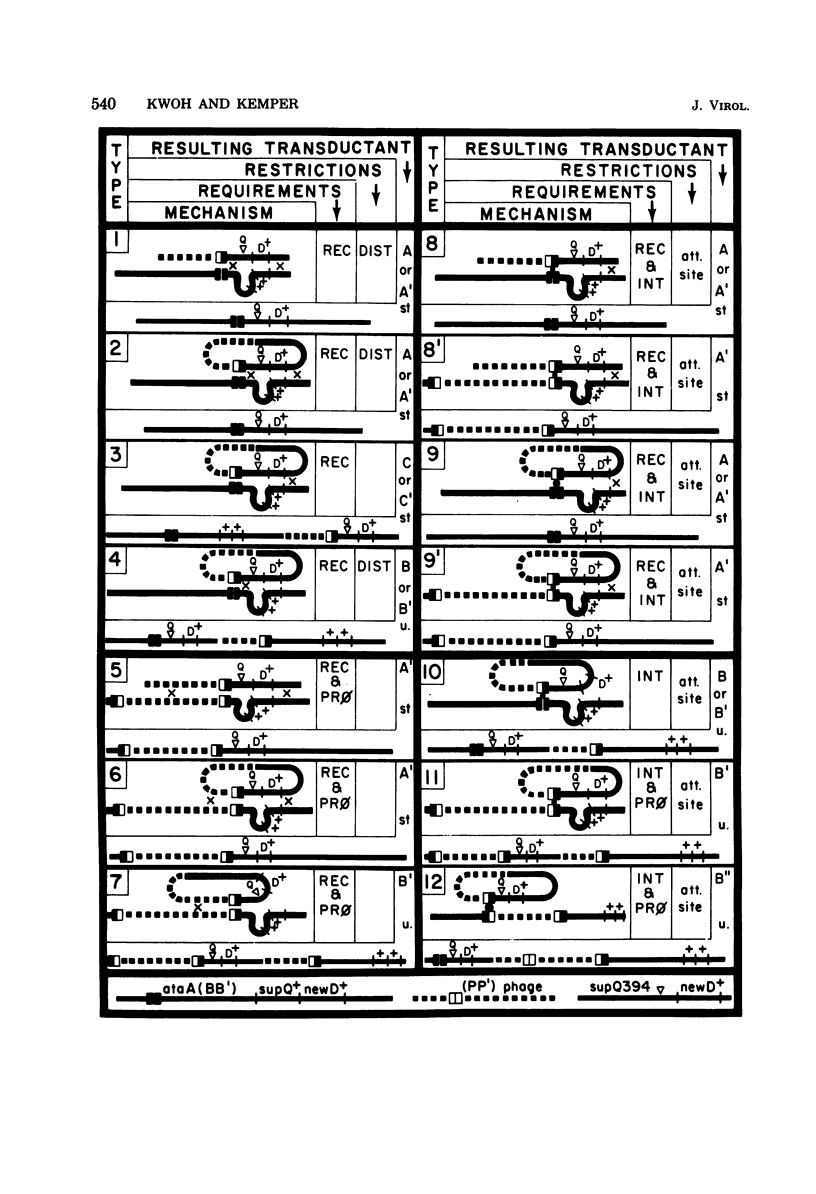
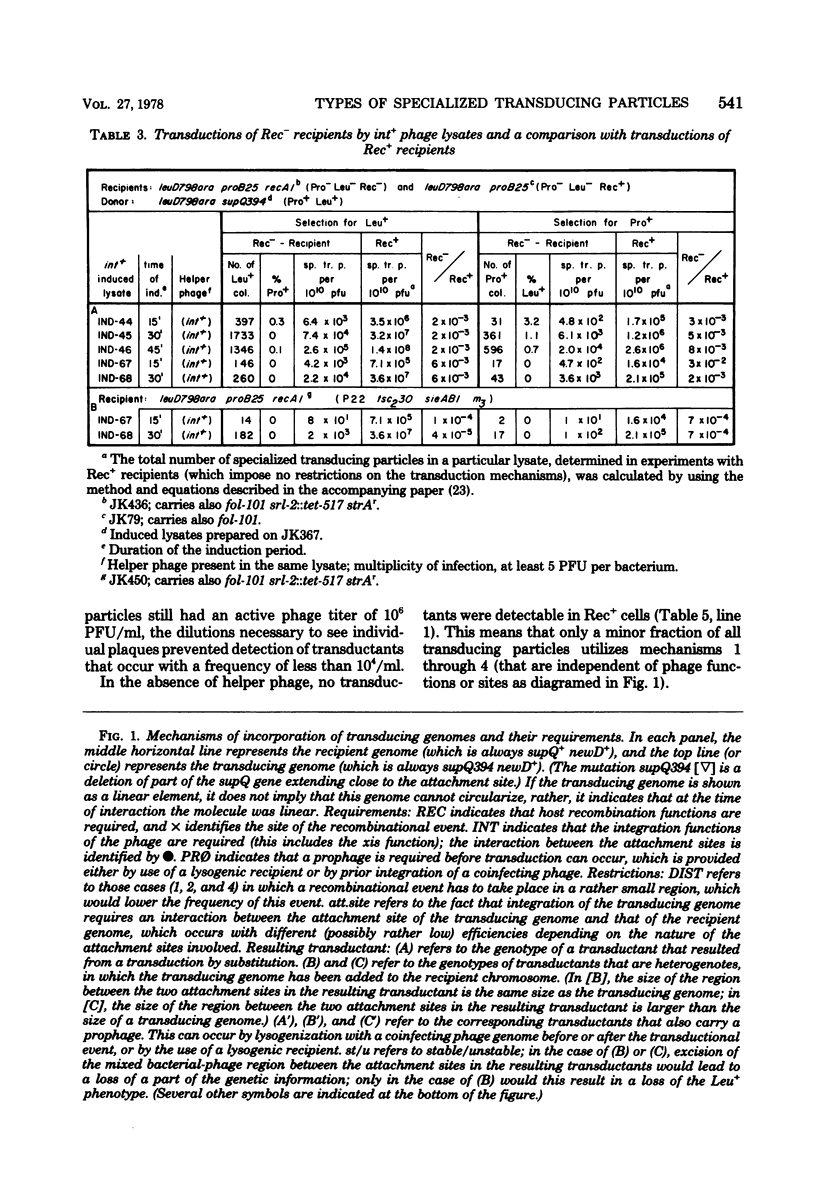
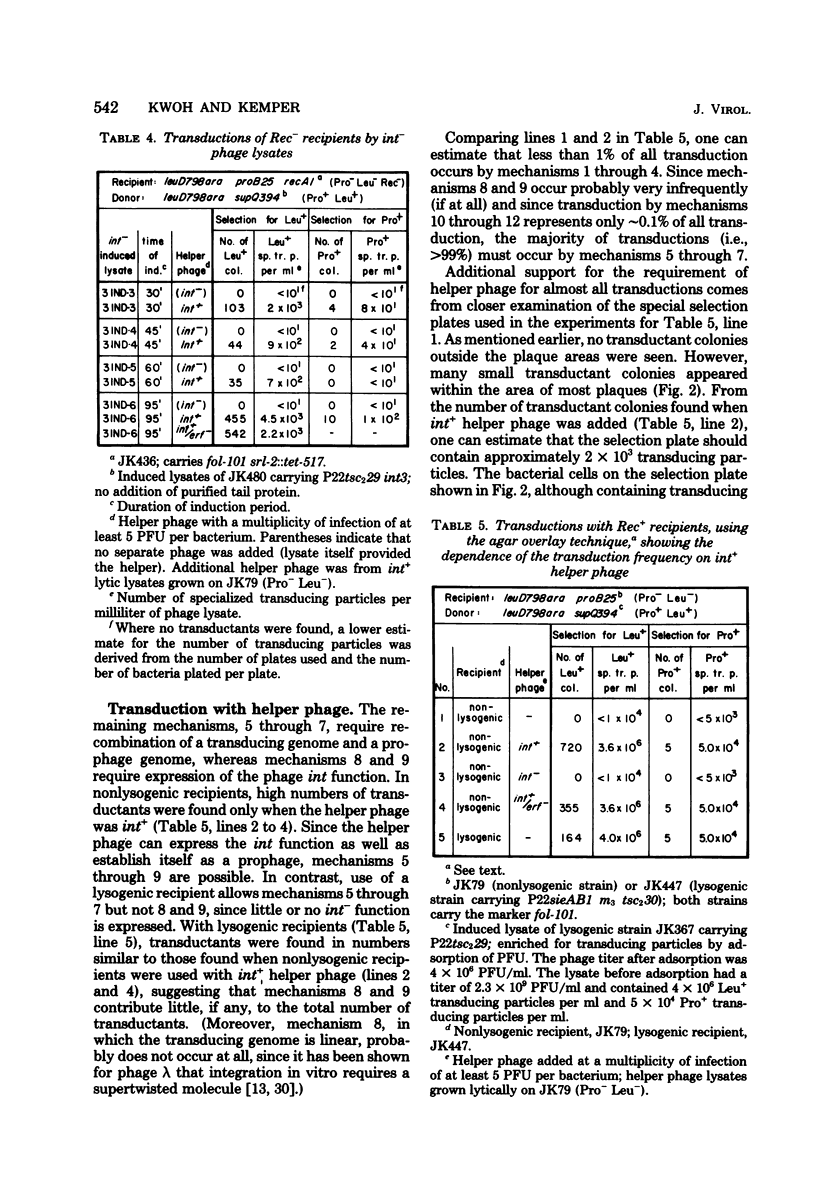
The temperate bacteriophage P22 mediates both generalized and specialized transduction in Salmonella typhimurium. Specialized transduction by phage P22 is different from, and less restricted than, the well characterized specialized transduction by phage lambda, due to differences in the phage DNA packaging mechanism. Phage lysates produced by induction of lysogenic strains contain very high frequencies of supQ newD- and proA,B-specialized transducing particles (10(-2)/PFU and 10(-3)/PFU, respectively), most of which are produced by independent aberrant excision events of various types. In a model, 12 different modes of transduction mechanisms were characterized by: (i) the structure of the specialized transducing genomes after injection into a new host cell, i.e., linear or circular, and (ii) the requirements for the transduction process, i.e., host recombination functions, phage integration functions, or presence of a prophage. By using different recipient strains and phage helper strains, it was possible to show that most specialized transducing particles (ca. 99%) contain linear genomes that cannot circularize upon injection into a new host cell and that require the presence of an integrated prophage as a site for a recombinational event to give rise to a transductant. Only 0.1% of all specialized transducing particles were shown to transduce by integration, suggesting that transducing genomes containing terminally redundant ends represent only a minor fraction of all transducing particles that are produced. However, it should be pointed out that the frequency (approximately 10(-5)/PFU) of these specialized transducing genomes that can circularize upon injection into a new host cell is as high as or even higher than the frequency of specialized transducing particles of phage lambda. The remaining approximately 1% of all specialized transducing particles can transduce by any one of the other mechanisms described.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Botstein D., Chan R. K., Waddell C. H. Genetics of bacteriophage P22. II. Gene order and gene function. Virology. 1972 Jul;49(1):268–282. doi: 10.1016/s0042-6822(72)80028-x. [DOI] [PubMed] [Google Scholar]
- Botstein D., Matz M. J. A recombination function essential to the growth of bacteriophage P22. J Mol Biol. 1970 Dec 28;54(3):417–440. doi: 10.1016/0022-2836(70)90119-1. [DOI] [PubMed] [Google Scholar]
- Botstein D., Waddell C. H., King J. Mechanism of head assembly and DNA encapsulation in Salmonella phage p22. I. Genes, proteins, structures and DNA maturation. J Mol Biol. 1973 Nov 15;80(4):669–695. doi: 10.1016/0022-2836(73)90204-0. [DOI] [PubMed] [Google Scholar]
- Botstein K., Lew K. K., Jarvik V., Swanson C. A. Role of antirepressor in the bipartite control of repression and immunity by bacteriophage P22. J Mol Biol. 1975 Feb 5;91(4):439–462. doi: 10.1016/0022-2836(75)90271-5. [DOI] [PubMed] [Google Scholar]
- Chan R. K., Botstein D. Specialized transduction by bacteriophage P22 in Salmonella typhimurium: genetic and physical structure of the transducing genomes and the prophage attachment site. Genetics. 1976 Jul;83(3 PT2):433–458. [PMC free article] [PubMed] [Google Scholar]
- Chan R. K., Botstein D., Watanabe T., Ogata Y. Specialized transduction of tetracycline resistance by phage P22 in Salmonella typhimurium. II. Properties of a high-frequency-transducing lysate. Virology. 1972 Dec;50(3):883–898. doi: 10.1016/0042-6822(72)90442-4. [DOI] [PubMed] [Google Scholar]
- Chelala C. A., Margolin P. Effects of deletions on cotransduction linkage in Salmonella typhimurium: evidence that bacterial chromosome deletions affect the formation of transducing DNA fragments. Mol Gen Genet. 1974;131(2):97–112. doi: 10.1007/BF00266146. [DOI] [PubMed] [Google Scholar]
- Drexler H. Specialized transduction of the biotin region of Escherichia coli by phage T1. Mol Gen Genet. 1977 Mar 28;152(1):59–63. doi: 10.1007/BF00264940. [DOI] [PubMed] [Google Scholar]
- Ebel-Tsipis J., Botstein D. Superinfection exclusion by P22 prophage in lysogens of Salmonella typhimurium. 1. Exclusion of generalized transducing particles. Virology. 1971 Sep;45(3):629–637. doi: 10.1016/0042-6822(71)90177-2. [DOI] [PubMed] [Google Scholar]
- Emmons S. W. Bacteriophage lambda derivatives carrying two copies of the cohesive end site. J Mol Biol. 1974 Mar 15;83(4):511–525. doi: 10.1016/0022-2836(74)90511-7. [DOI] [PubMed] [Google Scholar]
- Freifelder D., Chud L., Levine E. E. Requirement for maturation of Escherichia coli bacteriophage lambda. J Mol Biol. 1974 Mar 15;83(4):503–509. doi: 10.1016/0022-2836(74)90510-5. [DOI] [PubMed] [Google Scholar]
- Gellert M., Mizuuchi K., O'Dea M. H., Nash H. A. DNA gyrase: an enzyme that introduces superhelical turns into DNA. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3872–3876. doi: 10.1073/pnas.73.11.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratia J. P. Coliphage phi gamma, a novel type of specialized transducer. Mol Gen Genet. 1973 Aug 10;124(2):157–166. doi: 10.1007/BF00265148. [DOI] [PubMed] [Google Scholar]
- Greenstein M., Skalka A. Replication of bacteriophage lambda DNA: in vivo studies of the interaction between the viral gamma protein and the host recBC DNAase. J Mol Biol. 1975 Oct 5;97(4):543–549. doi: 10.1016/s0022-2836(75)80058-1. [DOI] [PubMed] [Google Scholar]
- Hilliker S., Botstein D. An early regulatory gene of Salmonella phage P22 analogous to gene N of coliphage lambda. Virology. 1975 Dec;68(2):510–524. doi: 10.1016/0042-6822(75)90291-3. [DOI] [PubMed] [Google Scholar]
- Hoppe I., Roth J. Specialized transducing phages derived from salmonella phage P22. Genetics. 1974 Apr;76(4):633–654. doi: 10.1093/genetics/76.4.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel J. V., Anderson T. F., Levine M. in vitro MORPHOGENESIS OF PHAGE P22 FROM HEADS AND BASE-PLATE PARTS. Proc Natl Acad Sci U S A. 1967 Feb;57(2):284–291. doi: 10.1073/pnas.57.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel V., Rosen H., Levine M. Binding of bacteriophage P22 tail parts to cells. J Virol. 1972 Dec;10(6):1152–1158. doi: 10.1128/jvi.10.6.1152-1158.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessop A. P. Specialized transducing phages derived from phage P22 that carry the pro AB region of the host, Salmonella typhimurium: genetic evidence for their structure and mode of transduction. Genetics. 1976 Jul;83(3 PT2):459–475. [PMC free article] [PubMed] [Google Scholar]
- Kemper J. Evolution of a new gene substituting for the leuD gene of Salmonella typhimurium: characterization of supQ mutations. J Bacteriol. 1974 Sep;119(3):937–951. doi: 10.1128/jb.119.3.937-951.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper J. Gene order and co-transduction in the leu-ara-fol-pyrA region of the Salmonella typhimurium linkage map. J Bacteriol. 1974 Jan;117(1):94–99. doi: 10.1128/jb.117.1.94-99.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwoh D. Y., Kemper J. Bacteriophage P22-mediated specialized transduction in Salmonella typhimurium: high frequency of aberrant prophage excision. J Virol. 1978 Sep;27(3):519–534. doi: 10.1128/jvi.27.3.519-534.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINE M. Mutations in the temperate phage P22 and lysogeny in Salmonella. Virology. 1957 Feb;3(1):22–41. doi: 10.1016/0042-6822(57)90021-1. [DOI] [PubMed] [Google Scholar]
- MARGOLIN P. Genetic fine structure of the leucine operon in Salmonella. Genetics. 1963 Mar;48:441–457. doi: 10.1093/genetics/48.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake T, Demerec M. Proline Mutants of Salmonella Typhimurium. Genetics. 1960 Jun;45(6):755–762. doi: 10.1093/genetics/45.6.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuuchi K., Nash H. A. Restriction assay for integrative recombination of bacteriophage lambda DNA in vitro: requirement for closed circular DNA substrate. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3524–3528. doi: 10.1073/pnas.73.10.3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaki Y. Inactivation of the ATP-dependent DNase of Escherichia coli after infection with double-stranded DNA phages. J Virol. 1974 Dec;14(6):1611–1612. doi: 10.1128/jvi.14.6.1611-1612.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson K. E., Hartman P. E. Linkage map of Salmonella typhimurium, edition V. Microbiol Rev. 1978 Jun;42(2):471–519. doi: 10.1128/mr.42.2.471-519.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. O. Defective phage formation by lysogens of integration deficient phage P22 mutants. Virology. 1968 Feb;34(2):203–223. doi: 10.1016/0042-6822(68)90231-6. [DOI] [PubMed] [Google Scholar]
- Smith H. O., Levine M. A phage P22 gene controlling integration of prophage. Virology. 1967 Feb;31(2):207–216. doi: 10.1016/0042-6822(67)90164-x. [DOI] [PubMed] [Google Scholar]
- Sternberg N., Weisberg R. Packaging of prophage and host DNA by coliphage lambda. Nature. 1975 Jul 10;256(5513):97–103. doi: 10.1038/256097a0. [DOI] [PubMed] [Google Scholar]
- Susskind M. M., Botstein D., Wright A. Superinfection exclusion by P22 prophage in lysogens of Salmonella typhimurium. III. Failure of superinfecting phage DNA to enter sieA+ lysogens. Virology. 1974 Dec;62(2):350–366. doi: 10.1016/0042-6822(74)90398-5. [DOI] [PubMed] [Google Scholar]
- Susskind M. M., Wright A., Botstein D. Superinfection exclusion by P22 prophage in lysogens of Salmonella typhimurium. II. Genetic evidence for two exclusion systems. Virology. 1971 Sep;45(3):638–652. doi: 10.1016/0042-6822(71)90178-4. [DOI] [PubMed] [Google Scholar]
- Susskind M. M., Wright A., Botstein D. Superinfection exclusion by P22 prophage in lysogens of Salmonella typhimurium. IV. Genetics and physiology of sieB exclusion. Virology. 1974 Dec;62(2):367–384. doi: 10.1016/0042-6822(74)90399-7. [DOI] [PubMed] [Google Scholar]
- Syvanen M. Processing of bacteriophage lambda DNA during its assembly into heads. J Mol Biol. 1975 Jan 15;91(2):165–174. doi: 10.1016/0022-2836(75)90157-6. [DOI] [PubMed] [Google Scholar]
- Tye B. K., Chan R. K., Botstein D. Packaging of an oversize transducing genome by Salmonella phage P22. J Mol Biol. 1974 Jan 5;85(4):485–500. doi: 10.1016/0022-2836(74)90311-8. [DOI] [PubMed] [Google Scholar]
- Weaver S., Levine M. Recombinational circularization of Salmonella phage P22 DNA. Virology. 1977 Jan;76(1):29–38. doi: 10.1016/0042-6822(77)90278-1. [DOI] [PubMed] [Google Scholar]
- Weaver S., Levine M. Replication in situ and DNA encapsulation following induction of an excision-defective lysogen of Salmonella bacteriophage P22. J Mol Biol. 1978 Jan 25;118(3):389–411. doi: 10.1016/0022-2836(78)90235-8. [DOI] [PubMed] [Google Scholar]
- Weaver S., Levine M. The timing of erf-mediated recombination in replication, lysogenization, and the formation of recombinant progeny by Salmonella phage P22. Virology. 1977 Jan;76(1):19–28. doi: 10.1016/0042-6822(77)90277-x. [DOI] [PubMed] [Google Scholar]
- Wing J. P. Transduction by phage P22 in a recombination-deficient mutant of Salmonella typhimurium. Virology. 1968 Oct;36(2):271–276. doi: 10.1016/0042-6822(68)90144-x. [DOI] [PubMed] [Google Scholar]
- ZINDER N. D. Lysogenization and superinfection immunity in Salmonella. Virology. 1958 Apr;5(2):291–326. doi: 10.1016/0042-6822(58)90025-4. [DOI] [PubMed] [Google Scholar]




