Abstract
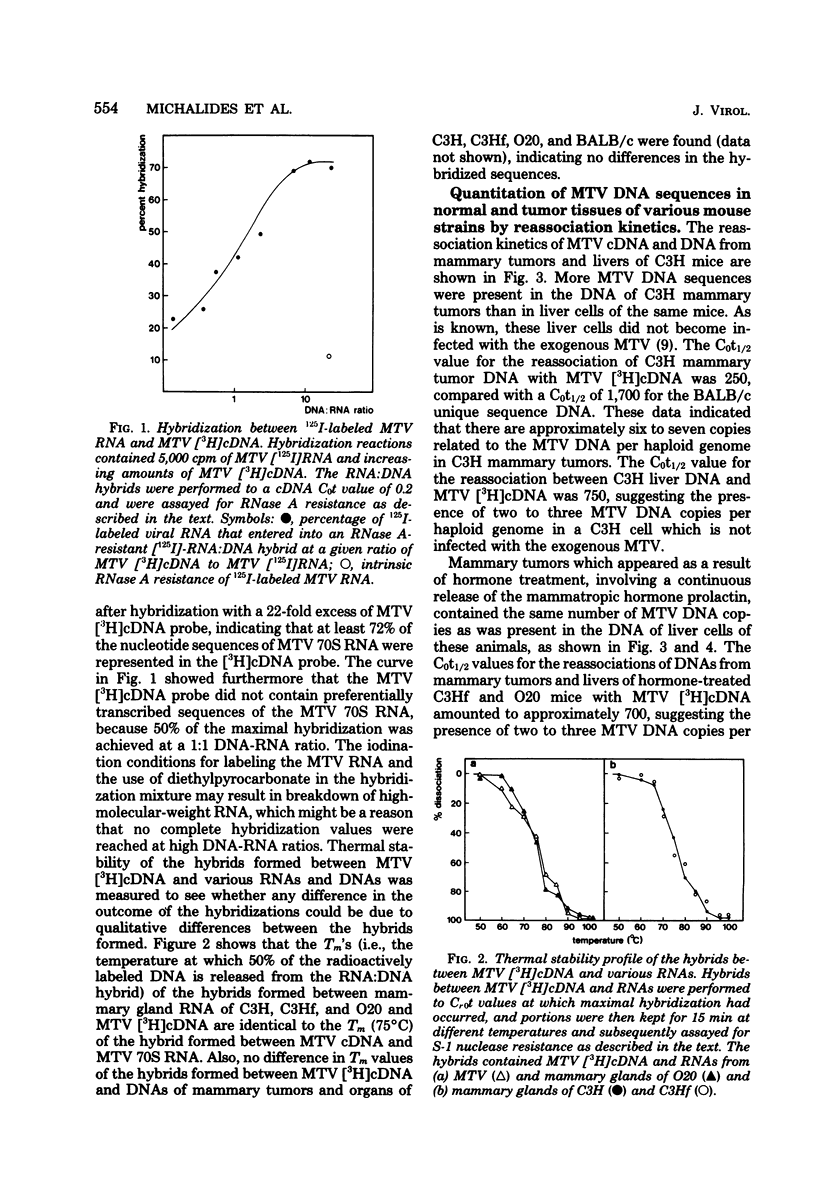
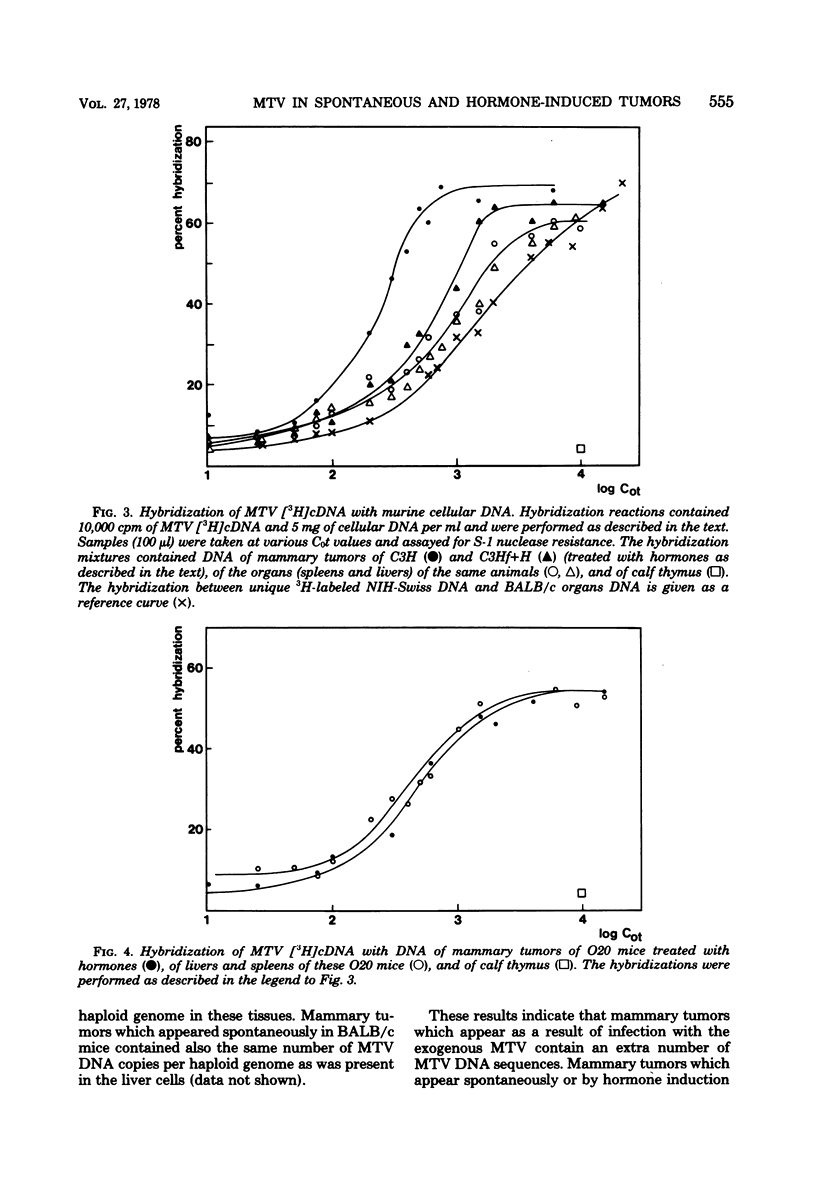
The involvement of the mouse mammary tumor virus (MTV) in spontaneous and hormone-induced mammary tumors in low-mammary-tumor mouse strains was studied by comparing the amounts of MTV RNA and MTV DNA sequences in mammary tumors and other tissues of mice with an without hormonal treatments. The following results were obtained. (i) Mammary tumors which appeared in C3H mice as a result of an infection with MTV contained more MTV DNA compared with noninfected organs; these mammary tumors also contained more MTV RNA than was present in lactating mammary gland cells. (ii) Hormonal stimulation by administration of excessive amounts of prolactin via hypophyseal isografts in C3Hf and O20 mice resulted in an increased expression of MTV RNA in the mammary glands. This elevated level of MTV RNA expression was, however, not maintained in the hormone-induced mammary tumors. (iii) Spontaneous mammary tumors in BALB/c mice contained similar levels of MTV DNA and MTV RNA sequences as were found in other cells of these animals.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentvelzen P., Daams J. H., Hageman P., Calafat J., Timmermans A. Interactions between viral and genetic factors in the origin of mammary tumors in mice. J Natl Cancer Inst. 1972 Apr;48(4):1089–1094. [PubMed] [Google Scholar]
- Bentvelzen P. The biology of the mouse mammary tumor virus. Int Rev Exp Pathol. 1972;11:259–297. [PubMed] [Google Scholar]
- Benveniste R. E., Scolnick E. M. RNA in mammalian sarcoma virus transformed nonproducer cells homologous to murine leukemia virus RNA. Virology. 1973 Feb;51(2):370–382. doi: 10.1016/0042-6822(73)90436-4. [DOI] [PubMed] [Google Scholar]
- Birnstiel M. L., Sells B. H., Purdom I. F. Kinetic complexity of RNA molecules. J Mol Biol. 1972 Jan 14;63(1):21–39. doi: 10.1016/0022-2836(72)90519-0. [DOI] [PubMed] [Google Scholar]
- Boot L. M., Röpcke G. Studies on hypophyseal isografts in mice. I. Biologic aspects. Cancer Res. 1966 Jul;26(7):1492–1496. [PubMed] [Google Scholar]
- Britten R. J., Graham D. E., Neufeld B. R. Analysis of repeating DNA sequences by reassociation. Methods Enzymol. 1974;29:363–418. doi: 10.1016/0076-6879(74)29033-5. [DOI] [PubMed] [Google Scholar]
- Drohan W., Kettmann R., Colcher D., Schlom J. Isolation of the mouse mammary tumor virus sequences not transmitted as germinal provirus in the C3H and RIII mouse strains. J Virol. 1977 Mar;21(3):986–995. doi: 10.1128/jvi.21.3.986-995.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine D. L., Plowman J. K., Kelley S. P., Arthur L. O., Hillman E. A. Enhanced production of mouse mammary tumor virus in dexamethasone-treated, 5-iododeoxyuridine-stimulated mammary tumor cell cultures. J Natl Cancer Inst. 1974 Jun;52(6):1881–1886. doi: 10.1093/jnci/52.6.1881. [DOI] [PubMed] [Google Scholar]
- Frankel A. E., Fischinger P. J. Nucleotide sequences in mouse DNA and RNA specific for Moloney sarcoma virus. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3705–3709. doi: 10.1073/pnas.73.10.3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas M. Transient virus expression during murine leukemia induction by X-irradiation. J Natl Cancer Inst. 1977 Feb;58(2):251–257. doi: 10.1093/jnci/58.2.251. [DOI] [PubMed] [Google Scholar]
- MUHLBOCK O., BOOT L. M. Induction of mammary cancer in mice without the mammary tumor agent by isografts of hypophyses. Cancer Res. 1959 May;19(4):402–412. [PubMed] [Google Scholar]
- Michalides R., Schlom J. Relationship in nucleic acid sequences between mouse mammary tumor virus variants. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4635–4639. doi: 10.1073/pnas.72.11.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalides R., Vlahakis G., Schlom J. A biochemical approach to the study of the transmission of mouse mammary tumor viruses in mouse strains RIII and C3H. Int J Cancer. 1976 Jul 15;18(1):105–115. doi: 10.1002/ijc.2910180114. [DOI] [PubMed] [Google Scholar]
- Michalides R., van Deemter L., Nuss R. R., van Nie R. Identification of the Mtv-2 gene responsible for the early appearance of mammary tumors in the GR mouse by nucleic acid hybridization. Proc Natl Acad Sci U S A. 1978 May;75(5):2368–2372. doi: 10.1073/pnas.75.5.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris V. L., Medeiros E., Ringold G. M., Bishop J. M., Varmus H. E. Comparison of mouse mammary tumor virus-specific DNA in inbred, wild and Asian mice, and in tumors and normal organs from inbred mice. J Mol Biol. 1977 Jul;114(1):73–91. doi: 10.1016/0022-2836(77)90284-4. [DOI] [PubMed] [Google Scholar]
- Mühlbock O. Note on a new inbred mouse-strain GR-A. Eur J Cancer. 1965 Oct;1(2):123–124. doi: 10.1016/0014-2964(65)90003-4. [DOI] [PubMed] [Google Scholar]
- Parks W. P., Ransom J. C., Young H. A., Scolnick E. M. Mammary tumor virus induction by glucocorticoids. Characterization of specific transcriptional regulation. J Biol Chem. 1975 May 10;250(9):3330–3336. [PubMed] [Google Scholar]
- Prensky W. The radioiodination of RNA and DNA to high specific activities. Methods Cell Biol. 1976;13:121–152. doi: 10.1016/s0091-679x(08)61800-2. [DOI] [PubMed] [Google Scholar]
- Ringold G. M., Yamamoto K. R., Tomkins G. M., Bishop M., Varmus H. E. Dexamethasone-mediated induction of mouse mammary tumor virus RNA: a system for studying glucocorticoid action. Cell. 1975 Nov;6(3):299–305. doi: 10.1016/0092-8674(75)90181-6. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stehelin D., Guntaka R. V., Varmus H. E., Bishop J. M. Purification of DNA complementary to nucleotide sequences required for neoplastic transformation of fibroblasts by avian sarcoma viruses. J Mol Biol. 1976 Mar 5;101(3):349–365. doi: 10.1016/0022-2836(76)90152-2. [DOI] [PubMed] [Google Scholar]
- Svec J., Links J. Mouse mammary tumor virus production stimulated by hormones and polyamines in cells grown in semi-synthetic in vitro conditions. Int J Cancer. 1977 Feb 15;19(2):249–257. doi: 10.1002/ijc.2910190215. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Illmensee R., Summers J. Efficeint transcription of RNA into DNA by avian sarcoma virus polymerase. Biochim Biophys Acta. 1976 Sep 6;442(3):324–330. doi: 10.1016/0005-2787(76)90307-5. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Quintrell N., Medeiros E., Bishop J. M., Nowinski R. C., Sarkar N. H. Transcription of mouse mammary tumor virus genes in tissues from high and low tumor incidence mouse strains. J Mol Biol. 1973 Oct 5;79(4):663–679. doi: 10.1016/0022-2836(73)90070-3. [DOI] [PubMed] [Google Scholar]
- van Nie R., Verstraeten A. A. Studies of genetic transmission of mammary tumour virus by C3Hf mice. Int J Cancer. 1975 Dec 15;16(6):922–931. doi: 10.1002/ijc.2910160606. [DOI] [PubMed] [Google Scholar]



