Abstract
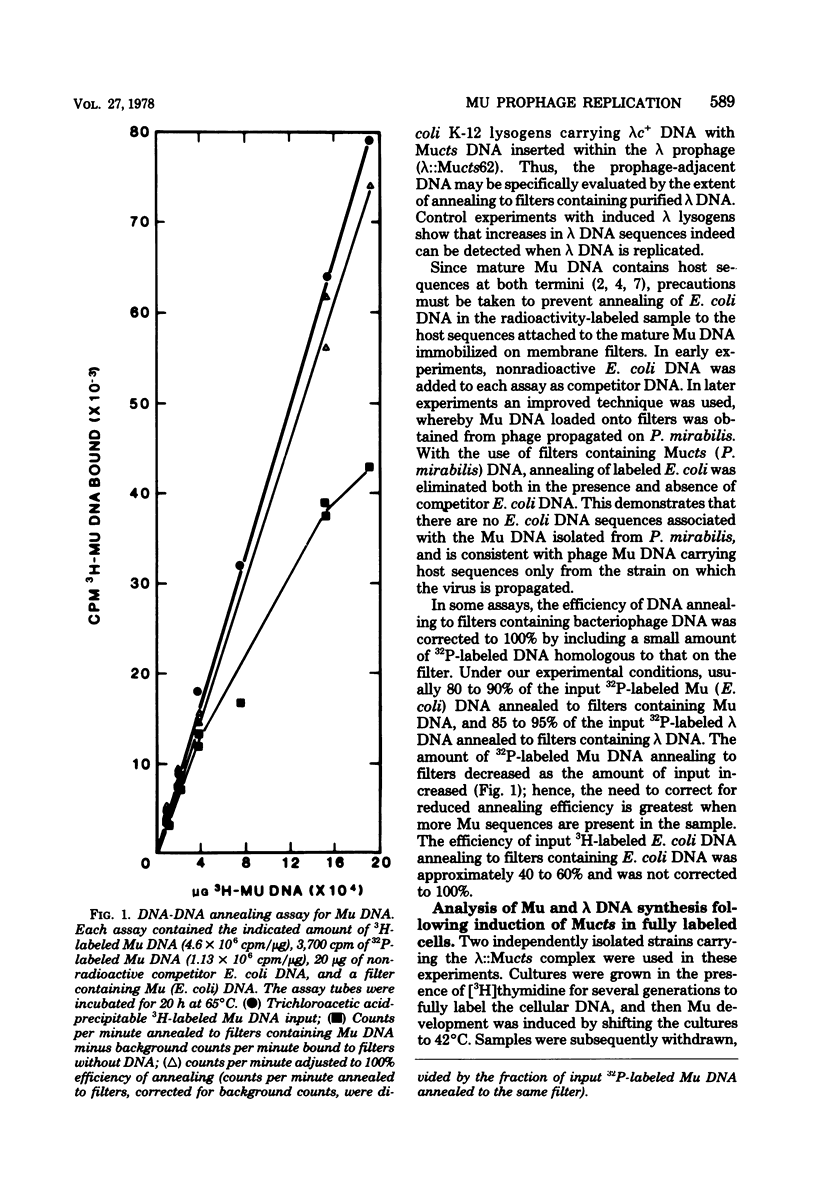
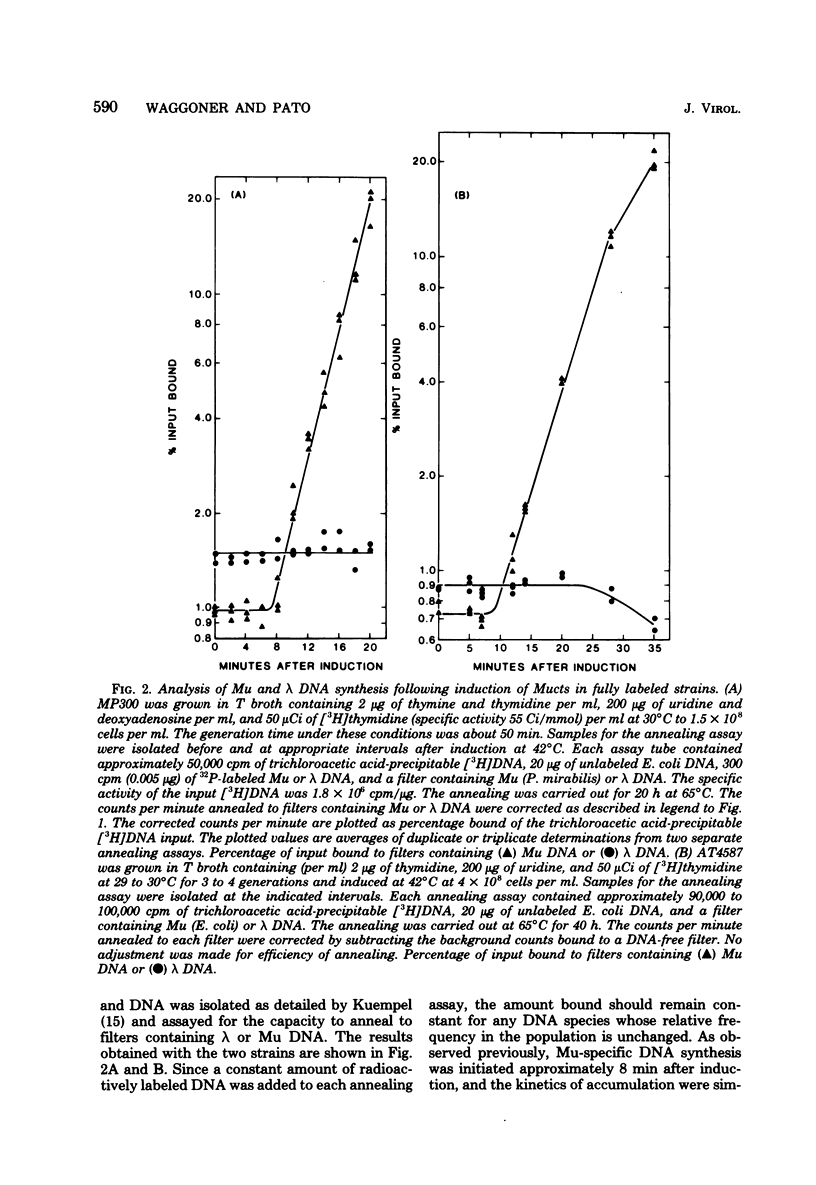
To determine whether the early replication of Mu prophage DNA proceeds beyond the termini of the prophage into hose DNA, the amounts of both Mu DNA and the prophage-adjacent host DNA sequences were measured using a DNA-DNA annealing assay after induction of the Mu vegetative cycle. Whereas Mu-specific DNA synthesis began 6 to 8 min after induction, no amplification of the adjacent DNA sequences was observed. These data suggest that early Mu-induced DNA synthesis is constrained within the boundaries of the Mu prophage. Since prophage Mu DNA does not undergo a prophage lambda-like excision from its original site after induction (E. Ljungquist and A. I. Bukhari, Proc. Natl. Acad. Sci. U.S.A. 74:3143--3147, 1977), we propose the existence of a control mechanism which excludes prophage-adjacent sequences from the initial mu prophage replication. The frequencies of the Mu prophage-adjacent DNA sequences, relative to other Escherichia coli genes, were not observed to change after the onset of Mu-specific DNA replication. This suggests that these regions remain associated with the host chromosome and continue to be replicated by the chromosomal replication fork. Therefore, we conclude that both the Mu prophage and adjacent host sequences are maintained in the host chromosome, rather than on an extrachromosomal form containing Mu and host DNA.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allet B., Bukhari A. I. Analysis of bacteriophage mu and lambda-mu hybrid DNAs by specific endonucleases. J Mol Biol. 1975 Mar 15;92(4):529–540. doi: 10.1016/0022-2836(75)90307-1. [DOI] [PubMed] [Google Scholar]
- Bade E. G. Asymmetric transcription of bacteriophage Mu-1. J Virol. 1972 Dec;10(6):1205–1207. doi: 10.1128/jvi.10.6.1205-1207.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukhari A. I. Bacteriophage mu as a transposition element. Annu Rev Genet. 1976;10:389–412. doi: 10.1146/annurev.ge.10.120176.002133. [DOI] [PubMed] [Google Scholar]
- Bukhari A. I., Froshauer S., Botchan M. Ends of bacteriophage mu DNA. Nature. 1976 Dec 9;264(5586):580–583. doi: 10.1038/264580a0. [DOI] [PubMed] [Google Scholar]
- Couturier M. The integration and excision of the bacteriophage Mu-1. Cell. 1976 Feb;7(2):155–163. doi: 10.1016/0092-8674(76)90015-5. [DOI] [PubMed] [Google Scholar]
- Daniell E., Kohne D. E., Abelson J. Characterization of the inhomogeneous DNA in virions of bacteriophage Mu by DNA reannealing kinetics. J Virol. 1975 Apr;15(4):739–743. doi: 10.1128/jvi.15.4.739-743.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Faelen M., Toussaint A. Bacteriophage Mu-1: a tool to transpose and to localize bacterial genes. J Mol Biol. 1976 Jul 5;104(3):525–539. doi: 10.1016/0022-2836(76)90118-2. [DOI] [PubMed] [Google Scholar]
- Freifelder D. Molecular weights of coliphages and coliphage DNA. IV. Molecular weights of DNA from bacteriophages T4, T5 and T7 and the general problem of determination of M. J Mol Biol. 1970 Dec 28;54(3):567–577. doi: 10.1016/0022-2836(70)90127-0. [DOI] [PubMed] [Google Scholar]
- Howe M. M., Bade E. G. Molecular biology of bacteriophage mu. Science. 1975 Nov 14;190(4215):624–632. doi: 10.1126/science.1103291. [DOI] [PubMed] [Google Scholar]
- Hsu M. T., Davidson N. Electron microscope heteroduplex study of the heterogeneity of Mu phage and prophage DNA. Virology. 1974 Mar;58(1):229–239. doi: 10.1016/0042-6822(74)90157-3. [DOI] [PubMed] [Google Scholar]
- Imae Y., Fukasawa T. Regional replication of the bacterial chromosome induced by derepression of prophage lambda. J Mol Biol. 1970 Dec 28;54(3):585–597. doi: 10.1016/0022-2836(70)90129-4. [DOI] [PubMed] [Google Scholar]
- Kuempel P. L. Deoxyribonucleic acid-deoxyribonucleic acid hybridization assay for replication origin deoxyribonucleic acid of Escherichia coli. J Bacteriol. 1972 Jun;110(3):917–925. doi: 10.1128/jb.110.3.917-925.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuempel P. L., Duerr S. A., Seeley N. R. Terminus region of the chromosome in Escherichia coli inhibits replication forks. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3927–3931. doi: 10.1073/pnas.74.9.3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C. J., Nathans D. Non-specific termination of simian virus 40 DNA replication. J Mol Biol. 1975 Sep 5;97(1):113–118. doi: 10.1016/s0022-2836(75)80026-x. [DOI] [PubMed] [Google Scholar]
- Levine M. Replication and lysogeny with phage P22 in Salmonella typhimurium. Curr Top Microbiol Immunol. 1972;58:135–156. doi: 10.1007/978-3-642-65357-5_4. [DOI] [PubMed] [Google Scholar]
- Lindqvist B. H. Vegetative DNA of temperate coliphage P2. Mol Gen Genet. 1971;110(2):178–196. doi: 10.1007/BF00332647. [DOI] [PubMed] [Google Scholar]
- Ljungquist E., Bukhari A. I. State of prophage Mu DNA upon induction. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3143–3147. doi: 10.1073/pnas.74.8.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louarn J., Patte J., Louarn J. M. Evidence for a fixed termination site of chromosome replication in Escherichia coli K12. J Mol Biol. 1977 Sep 25;115(3):295–314. doi: 10.1016/0022-2836(77)90156-5. [DOI] [PubMed] [Google Scholar]
- Lovett M. A., Sparks R. B., Helinski D. R. Bidirectional replication of plasmid R6K DNA in Escherichia coli; correspondence between origin of replication and position of single-strand break in relaxed complex. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2905–2909. doi: 10.1073/pnas.72.8.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzaki T., Bukhari A. I. Events following prophage Mu induction. J Bacteriol. 1975 May;122(2):437–442. doi: 10.1128/jb.122.2.437-442.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder W., Bade E. G., Delius H. Participation of Escherichia coli DNA in the replication of temperate bacteriophage Mu1. Virology. 1974 Aug;60(2):534–542. doi: 10.1016/0042-6822(74)90347-x. [DOI] [PubMed] [Google Scholar]
- Schröder W., van de Putte P. Genetic study of prophage excision with a temperature inducible mutant of Mu-1. Mol Gen Genet. 1974 May 21;130(2):99–104. doi: 10.1007/BF00269081. [DOI] [PubMed] [Google Scholar]
- TAYLOR A. L. BACTERIOPHAGE-INDUCED MUTATION IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1963 Dec;50:1043–1051. doi: 10.1073/pnas.50.6.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toussaint A., Faelen M. Connecting two unrelated DNA sequences with a Mu dimer. Nat New Biol. 1973 Mar 7;242(114):1–4. doi: 10.1038/newbio242001a0. [DOI] [PubMed] [Google Scholar]
- Van Brunt J., Waggoner B. T., Pato M. L. Re-examination of F plasmid replication in a dnaC mutant of Escherichia coli. Mol Gen Genet. 1977 Feb 15;150(3):285–292. doi: 10.1007/BF00268127. [DOI] [PubMed] [Google Scholar]
- Waggoner B. T., González N. S., Taylor A. L. Isolation of heterogeneous circular DNA from induced lysogens of bacteriophage Mu-1. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1255–1259. doi: 10.1073/pnas.71.4.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijffelman C., Gassler M., Stevens W. F., van de Putte P. On the control of transcription of bacteriophage Mu. Mol Gen Genet. 1974;131(2):85–96. doi: 10.1007/BF00266145. [DOI] [PubMed] [Google Scholar]
- Wijffelman C., Lotterman B. Kinetics of Mu DNA synthesis. Mol Gen Genet. 1977 Mar 7;151(2):169–174. doi: 10.1007/BF00338691. [DOI] [PubMed] [Google Scholar]



