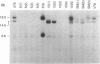
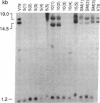
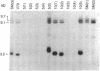
Abstract
We have shown previously a good correlation between etoposide-induced sister chromatid exchanges (SCE) and cytotoxicity. A semisynthetic derivative of podophyllotoxin, etoposide is also called Vepesid (Bristol; code designation VP-16-213, abbreviated VP-16). Since SCE represent DNA recombinational events, we hypothesized that VP-16-induced SCE might result in nonhomologous recombination in which segments of DNA were either deleted or added, leading to an alteration of gene sequences responsible for essential cell proteins. Alterations of such essential genes and consequent interference with formation of their products could consequently lead to cell death. To evaluate whether VP-16 treatment caused sufficient levels of DNA sequence alterations to interfere with gene product formation, we isolated hypoxanthine (guanine) phosphoribosyltransferase (HPRT)-deficient mutants from Chinese hamster V79 cells grown in the presence or absence of VP-16. DNA from 3 spontaneous mutants and 10 VP-16-induced mutants was analyzed by Southern blot hybridization to a full-length hamster HPRT cDNA probe. Most of the VP-16-induced mutants showed partial deletions and/or rearrangements of the HPRT gene. In contrast, spontaneous mutants showed negligible deletions or rearrangements. These results provide strong support for our hypothesis that deletion of genetic sequences may constitute an important component of the mechanism of VP-16-induced cell death.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brennand J., Konecki D. S., Caskey C. T. Expression of human and Chinese hamster hypoxanthine-guanine phosphoribosyltransferase cDNA recombinants in cultured Lesch-Nyhan and Chinese hamster fibroblasts. J Biol Chem. 1983 Aug 25;258(16):9593–9596. [PubMed] [Google Scholar]
- Brown R., Thacker J. The nature of mutants induced by ionising radiation in cultured hamster cells. I. Isolation and initial characterisation of spontaneous, ionising radiation-induced, and ethyl methanesulphonate-induced mutants resistant to 6-thioguanine. Mutat Res. 1984 Nov;129(2):269–281. doi: 10.1016/0027-5107(84)90160-x. [DOI] [PubMed] [Google Scholar]
- Chatterjee S., Trivedi D., Petzold S. J., Berger N. A. Mechanism of epipodophyllotoxin-induced cell death in poly(adenosine diphosphate-ribose) synthesis-deficient V79 Chinese hamster cell lines. Cancer Res. 1990 May 1;50(9):2713–2718. [PubMed] [Google Scholar]
- Chen G. L., Yang L., Rowe T. C., Halligan B. D., Tewey K. M., Liu L. F. Nonintercalative antitumor drugs interfere with the breakage-reunion reaction of mammalian DNA topoisomerase II. J Biol Chem. 1984 Nov 10;259(21):13560–13566. [PubMed] [Google Scholar]
- Chow K. C., Ross W. E. Topoisomerase-specific drug sensitivity in relation to cell cycle progression. Mol Cell Biol. 1987 Sep;7(9):3119–3123. doi: 10.1128/mcb.7.9.3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewinko B., Barlogie B. Survival and cycle-progression delay of human lymphoma cells in vitro exposed to VP-16-213. Cancer Treat Rep. 1976 Sep;60(9):1295–1306. [PubMed] [Google Scholar]
- Gellert M. DNA topoisomerases. Annu Rev Biochem. 1981;50:879–910. doi: 10.1146/annurev.bi.50.070181.004311. [DOI] [PubMed] [Google Scholar]
- Holm C., Covey J. M., Kerrigan D., Pommier Y. Differential requirement of DNA replication for the cytotoxicity of DNA topoisomerase I and II inhibitors in Chinese hamster DC3F cells. Cancer Res. 1989 Nov 15;49(22):6365–6368. [PubMed] [Google Scholar]
- Konecki D. S., Brennand J., Fuscoe J. C., Caskey C. T., Chinault A. C. Hypoxanthine-guanine phosphoribosyltransferase genes of mouse and Chinese hamster: construction and sequence analysis of cDNA recombinants. Nucleic Acids Res. 1982 Nov 11;10(21):6763–6775. doi: 10.1093/nar/10.21.6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long B. H., Musial S. T., Brattain M. G. DNA breakage in human lung carcinoma cells and nuclei that are naturally sensitive or resistant to etoposide and teniposide. Cancer Res. 1986 Aug;46(8):3809–3816. [PubMed] [Google Scholar]
- Markovits J., Pommier Y., Kerrigan D., Covey J. M., Tilchen E. J., Kohn K. W. Topoisomerase II-mediated DNA breaks and cytotoxicity in relation to cell proliferation and the cell cycle in NIH 3T3 fibroblasts and L1210 leukemia cells. Cancer Res. 1987 Apr 15;47(8):2050–2055. [PubMed] [Google Scholar]
- Pommier Y., Kerrigan D., Covey J. M., Kao-Shan C. S., Whang-Peng J. Sister chromatid exchanges, chromosomal aberrations, and cytotoxicity produced by antitumor topoisomerase II inhibitors in sensitive (DC3F) and resistant (DC3F/9-OHE) Chinese hamster cells. Cancer Res. 1988 Feb 1;48(3):512–516. [PubMed] [Google Scholar]
- Pommier Y., Schwartz R. E., Zwelling L. A., Kerrigan D., Mattern M. R., Charcosset J. Y., Jacquemin-Sablon A., Kohn K. W. Reduced formation of protein-associated DNA strand breaks in Chinese hamster cells resistant to topoisomerase II inhibitors. Cancer Res. 1986 Feb;46(2):611–616. [PubMed] [Google Scholar]
- Pommier Y., Zwelling L. A., Kao-Shan C. S., Whang-Peng J., Bradley M. O. Correlations between intercalator-induced DNA strand breaks and sister chromatid exchanges, mutations, and cytotoxicity in Chinese hamster cells. Cancer Res. 1985 Jul;45(7):3143–3149. [PubMed] [Google Scholar]
- Ross W. E. DNA topoisomerases as targets for cancer therapy. Biochem Pharmacol. 1985 Dec 15;34(24):4191–4195. doi: 10.1016/0006-2952(85)90273-4. [DOI] [PubMed] [Google Scholar]
- Ross W., Rowe T., Glisson B., Yalowich J., Liu L. Role of topoisomerase II in mediating epipodophyllotoxin-induced DNA cleavage. Cancer Res. 1984 Dec;44(12 Pt 1):5857–5860. [PubMed] [Google Scholar]
- Singh B., Gupta R. S. Mutagenic responses of thirteen anticancer drugs on mutation induction at multiple genetic loci and on sister chromatid exchanges in Chinese hamster ovary cells. Cancer Res. 1983 Feb;43(2):577–584. [PubMed] [Google Scholar]
- Spiridonidis C. A., Chatterjee S., Petzold S. J., Berger N. A. Topoisomerase II-dependent and -independent mechanisms of etoposide resistance in Chinese hamster cell lines. Cancer Res. 1989 Feb 1;49(3):644–650. [PubMed] [Google Scholar]
- Thacker J. The nature of mutants induced by ionising radiation in cultured hamster cells. III. Molecular characterization of HPRT-deficient mutants induced by gamma-rays or alpha-particles showing that the majority have deletions of all or part of the hprt gene. Mutat Res. 1986 May;160(3):267–275. doi: 10.1016/0027-5107(86)90137-5. [DOI] [PubMed] [Google Scholar]
- Wang J. C. DNA topoisomerases. Annu Rev Biochem. 1985;54:665–697. doi: 10.1146/annurev.bi.54.070185.003313. [DOI] [PubMed] [Google Scholar]
- Wang J. C. Recent studies of DNA topoisomerases. Biochim Biophys Acta. 1987 Jun 6;909(1):1–9. doi: 10.1016/0167-4781(87)90040-6. [DOI] [PubMed] [Google Scholar]
- Zwelling L. A. DNA topoisomerase II as a target of antineoplastic drug therapy. Cancer Metastasis Rev. 1985;4(4):263–276. doi: 10.1007/BF00048092. [DOI] [PubMed] [Google Scholar]