Abstract
The relative initiation rates for encephalomyocarditis virus mRNA and host mRNA's in infected cells were measured using two independent techniques. In both cases the results showed that viral mRNA initiates at a much higher rate than host mRNA'S. This difference was observed midway in the infectious cycle, well before virus-induced cytopathic effects (leakage of low-molecular-weight metabolites, failure to exclude trypan blue) were apparent. These results confirm that encephalomyocarditis viral mRNA is a more efficient initiator than host mRNA's in vivo, as has previously been demonstrated in in vitro experiments.
Full text
PDF




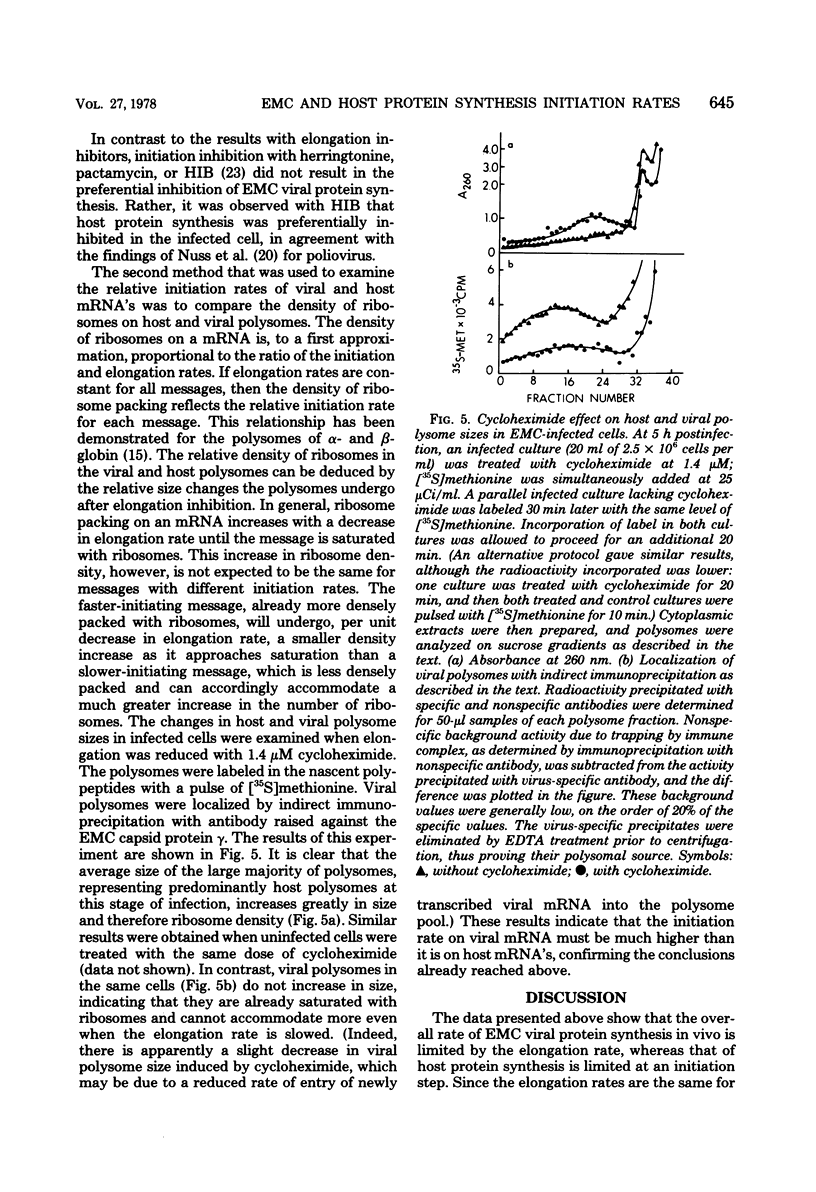


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abreu S. L., Lucas-Lenard J. Cellular protein synthesis shutoff by mengovirus: translation of nonviral and viral mRNA's in extracts from uninfected and infected Ehrlich ascites tumor cells. J Virol. 1976 Apr;18(1):182–194. doi: 10.1128/jvi.18.1.182-194.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair G. E., Dahl H. H., Truelsen E., Lelong J. C. Functional identity of a mouse ascites and a rabbit reticulocyte initiation factor required for natural mRNA translation. Nature. 1977 Feb 17;265(5595):651–653. doi: 10.1038/265651a0. [DOI] [PubMed] [Google Scholar]
- Carrasco L., Smith A. E. Sodium ions and the shut-off of host cell protein synthesis by picornaviruses. Nature. 1976 Dec 23;264(5588):807–809. doi: 10.1038/264807a0. [DOI] [PubMed] [Google Scholar]
- David A. E. Control of vesicular stomatitis virus protein synthesis. Virology. 1976 May;71(1):217–229. doi: 10.1016/0042-6822(76)90107-0. [DOI] [PubMed] [Google Scholar]
- Egberts E., Hackett P. B., Traub P. Alteration of the intracellular energetic and ionic conditions by mengovirus infection of Ehrlich ascites tumor cells and its influence on protein synthesis in the midphase of infection. J Virol. 1977 Jun;22(3):591–597. doi: 10.1128/jvi.22.3.591-597.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanegan J. B., Petterson R. F., Ambros V., Hewlett N. J., Baltimore D. Covalent linkage of a protein to a defined nucleotide sequence at the 5'-terminus of virion and replicative intermediate RNAs of poliovirus. Proc Natl Acad Sci U S A. 1977 Mar;74(3):961–965. doi: 10.1073/pnas.74.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golini F., Thach S. S., Birge C. H., Safer B., Merrick W. C., Thach R. E. Competition between cellular and viral mRNAs in vitro is regulated by a messenger discriminatory initiation factor. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3040–3044. doi: 10.1073/pnas.73.9.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett P. B., Egberts E., Traub P. Selective translation of mengovirus RNA over Host mRNA in homologous, fractionated, cell-free translational systems from Ehrlich-ascites-tumor cells. Eur J Biochem. 1978 Feb;83(2):353–361. doi: 10.1111/j.1432-1033.1978.tb12101.x. [DOI] [PubMed] [Google Scholar]
- Helentjaris T., Ehrenfeld E. Control of protein synthesis in extracts from poliovirus-infected cells. I. mRNA discrimination by crude initiation factors. J Virol. 1978 May;26(2):510–521. doi: 10.1128/jvi.26.2.510-521.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewlett M. J., Rose J. K., Baltimore D. 5'-terminal structure of poliovirus polyribosomal RNA is pUp. Proc Natl Acad Sci U S A. 1976 Feb;73(2):327–330. doi: 10.1073/pnas.73.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr I. M., Martin E. M. Simple method for the isolation of encephalomyocarditis virus ribonucleic acid. J Virol. 1972 Mar;9(3):559–561. doi: 10.1128/jvi.9.3.559-561.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence C., Thach R. E. Encephalomyocarditis virus infection of mouse plasmacytoma cells. I. Inhibition of cellular protein synthesis. J Virol. 1974 Sep;14(3):598–610. doi: 10.1128/jvi.14.3.598-610.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. F., Nomoto A., Detjen B. M., Wimmer E. A protein covalently linked to poliovirus genome RNA. Proc Natl Acad Sci U S A. 1977 Jan;74(1):59–63. doi: 10.1073/pnas.74.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. F. Alpha and beta globin messenger ribonucleic acid. Different amounts and rates of initiation of translation. J Biol Chem. 1971 Dec 10;246(23):7131–7138. [PubMed] [Google Scholar]
- Lodish H. F., Froshauer S. Rates of initiation of protein synthesis by two purified species of vesicular stomatitis virus messenger RNA. J Biol Chem. 1977 Dec 25;252(24):8804–8811. [PubMed] [Google Scholar]
- Lodish H. F. Translational control of protein synthesis. Annu Rev Biochem. 1976;45:39–72. doi: 10.1146/annurev.bi.45.070176.000351. [DOI] [PubMed] [Google Scholar]
- Nomoto A., Lee Y. F., Wimmer E. The 5' end of poliovirus mRNA is not capped with m7G(5')ppp(5')Np. Proc Natl Acad Sci U S A. 1976 Feb;73(2):375–380. doi: 10.1073/pnas.73.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuss D. L., Oppermann H., Koch G. Selective blockage of initiation of host protein synthesis in RNA-virus-infected cells. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1258–1262. doi: 10.1073/pnas.72.4.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman S., Hirsch M., Penman S. Utilization of messenger in adenovirus-2-infected cells at normal and elevated temperatures. Nat New Biol. 1972 Aug 2;238(83):143–144. doi: 10.1038/newbio238143a0. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Trachsel H., Leong K., Baltimore D. Inhibition of translation by poliovirus: inactivation of a specific initiation factor. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2732–2736. doi: 10.1073/pnas.75.6.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart A. G., Lloyd M., Arnstein H. R. Maintenance of the ratio of alpha and beta globin synthesis in rabbit reticulocytes. Eur J Biochem. 1977 Nov 1;80(2):453–459. doi: 10.1111/j.1432-1033.1977.tb11900.x. [DOI] [PubMed] [Google Scholar]
- Svitkin Y. V., Agol V. I. Complete translation of encephalomyocarditis virus RNA and faithful cleavage of virus-specific proteins in a cell-free system from Krebs-2 cells. FEBS Lett. 1978 Mar 1;87(1):7–11. doi: 10.1016/0014-5793(78)80121-5. [DOI] [PubMed] [Google Scholar]
- Yau P. M., Godefroy-Colburn T., Birge C. H., Ramabhadran T. V., Thach R. E. Specificity of interferon action in protein synthesis. J Virol. 1978 Sep;27(3):648–658. doi: 10.1128/jvi.27.3.648-658.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]