Abstract
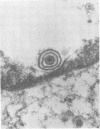
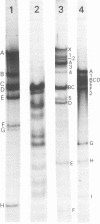
Human cells transformed by cytomegalovirus and transplanted to athymic nude mice yielded a cytopathic virus, Hershey Medical Center virus, following prolonged in vitro passage of the tumor cells. The virus is a double-enveloped herpesvirus, is sensitive to ether, and is inhibited by iododeoxyuridine. No significant antigenic relationship to herpes simplex virus was detected using herpes simplex virus-immune sera in neutralization and immunofluorescence tests, but indirect immunofluorescence tests revealed cytomegalovirus-related antigenicity. Further immunological tests revealed that Hershey Medical Center virus is antigenically indistinguishable from infectious bovine rhinotracheitis virus. Thus, it appears that Hershey Medical Center virus is infectious bovine rhinotracheitis virus, which presumably appeared in the cell culture as a contaminant from fetal calf serum.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht T., Rapp F. Malignant transformation of hamster embryo fibroblasts following exposure to ultraviolet-irradiated human cytomegalovirus. Virology. 1973 Sep;55(1):53–61. doi: 10.1016/s0042-6822(73)81007-4. [DOI] [PubMed] [Google Scholar]
- BURGI E., HERSHEY A. D. Sedimentation rate as a measure of molecular weight of DNA. Biophys J. 1963 Jul;3:309–321. doi: 10.1016/s0006-3495(63)86823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doller E., Duff R., Rapp F. Resistance of hamster cells transformed by herpes simplex virus type 2 to superinfection by herpes simplex viruses. Intervirology. 1973;1(3):154–167. doi: 10.1159/000148842. [DOI] [PubMed] [Google Scholar]
- Figueroa M., Zambrana A. El herpes genital en Honduras y su relación con el carcinoma del cervix uterino. Rev Latinoam Microbiol. 1976 Apr-Jun;18(2):111–116. [PubMed] [Google Scholar]
- Freifelder D. Molecular weights of coliphages and coliphage DNA. IV. Molecular weights of DNA from bacteriophages T4, T5 and T7 and the general problem of determination of M. J Mol Biol. 1970 Dec 28;54(3):567–577. doi: 10.1016/0022-2836(70)90127-0. [DOI] [PubMed] [Google Scholar]
- Geder K. M., Lausch R., O'Neill F., Rapp F. Oncogenic transformation of human embryo lung cells by human cytomegalovirus. Science. 1976 Jun 11;192(4244):1134–1137. doi: 10.1126/science.179143. [DOI] [PubMed] [Google Scholar]
- Geder L., Kreider J., Rapp F. Human cells transformed in vitro by human cytomegalovirus: tumorigenicity in athymic nude mice. J Natl Cancer Inst. 1977 Apr;58(4):1003–1009. doi: 10.1093/jnci/58.4.1003. [DOI] [PubMed] [Google Scholar]
- Geder L., Rapp F. Evidence for nuclear antigens in cytomegalovirus-transformed human cells. Nature. 1977 Jan 13;265(5590):184–186. doi: 10.1038/265184a0. [DOI] [PubMed] [Google Scholar]
- HYman R. W., Oakes J. E., Kudler L. In vitro repair of the preexisting nicks and gaps in herpes simplex virus DNA. Virology. 1977 Jan;76(1):286–294. doi: 10.1016/0042-6822(77)90303-8. [DOI] [PubMed] [Google Scholar]
- Hayward G. S., Frenkel N., Roizman B. Anatomy of herpes simplex virus DNA: strain differences and heterogeneity in the locations of restriction endonuclease cleavage sites. Proc Natl Acad Sci U S A. 1975 May;72(5):1768–1772. doi: 10.1073/pnas.72.5.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward G. S., Jacob R. J., Wadsworth S. C., Roizman B. Anatomy of herpes simplex virus DNA: evidence for four populations of molecules that differ in the relative orientations of their long and short components. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4243–4247. doi: 10.1073/pnas.72.11.4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hudson J. B., Misra V., Mosmann T. R. Properties of the multicapsid virions of murine cytomegalovirus. Virology. 1976 Jul 1;72(1):224–234. doi: 10.1016/0042-6822(76)90325-1. [DOI] [PubMed] [Google Scholar]
- Iltis J. P., Oakes J. E., Hyman R. W., Rapp F. Comparison of the DNAs of varicella-zoster viruses isolated from clinical cases of varicella and herpes zoster. Virology. 1977 Oct 15;82(2):345–352. doi: 10.1016/0042-6822(77)90009-5. [DOI] [PubMed] [Google Scholar]
- Kieff E. D., Bachenheimer S. L., Roizman B. Size, composition, and structure of the deoxyribonucleic acid of herpes simplex virus subtypes 1 and 2. J Virol. 1971 Aug;8(2):125–132. doi: 10.1128/jvi.8.2.125-132.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick B. A., Huang E. S., Pagano J. S. Analysis of cytomegalovirus genomes with restriction endonucleases Hin D III and EcoR-1. J Virol. 1976 Jun;18(3):1095–1105. doi: 10.1128/jvi.18.3.1095-1105.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. R., Hudson J. B. Some properties of the genome of murine cytomegalovirus (MCV). Virology. 1973 Jul;54(1):135–149. doi: 10.1016/0042-6822(73)90123-2. [DOI] [PubMed] [Google Scholar]
- Oakes J. E., Hyman R. W., Rapp F. Genome location of polyadenylated transcripts of herpes simplex virus type 1 and type 2 DNA. Virology. 1976 Nov;75(1):145–154. doi: 10.1016/0042-6822(76)90013-1. [DOI] [PubMed] [Google Scholar]
- Oakes J. E., Iltis J. P., Hyman R. W., Rapp F. Analysis by restriction enzyme cleavage of human varicella-zoster virus DNAs. Virology. 1977 Oct 15;82(2):353–361. doi: 10.1016/0042-6822(77)90010-1. [DOI] [PubMed] [Google Scholar]
- Pater M. M., Hyman R. W., Rapp F. Isolation of herpes simplex virus DNA from the "hirt supernatant". Virology. 1976 Dec;75(2):481–483. doi: 10.1016/0042-6822(76)90046-5. [DOI] [PubMed] [Google Scholar]
- Plummer G., Goodheart C. R., Henson D., Bowling C. P. A comparative study of the DNA density and behavior in tissue cultures of fourteen different herpesviruses. Virology. 1969 Sep;39(1):134–137. doi: 10.1016/0042-6822(69)90355-9. [DOI] [PubMed] [Google Scholar]
- Rapp F., Geder L., Murasko D., Lausch R., Ladda R., Huang E. S., Webber M. M. Long-term persistence of cytomegalovirus genome in cultured human cells of prostatic origin. J Virol. 1975 Oct;16(4):982–990. doi: 10.1128/jvi.16.4.982-990.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Skare J., Summers W. P., Summers W. C. Structure and function of herpesvirus genomes. I. comparison of five HSV-1 and two HSV-2 strains by cleavage their DNA with eco R I restriction endonuclease. J Virol. 1975 Apr;15(4):726–732. doi: 10.1128/jvi.15.4.726-732.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkie N. M. The synthesis and substructure of herpesvirus DNA: the distribution of alkali-labile single strand interruptions in HSV-1 DNA. J Gen Virol. 1973 Dec;21(3):453–467. doi: 10.1099/0022-1317-21-3-453. [DOI] [PubMed] [Google Scholar]