Abstract
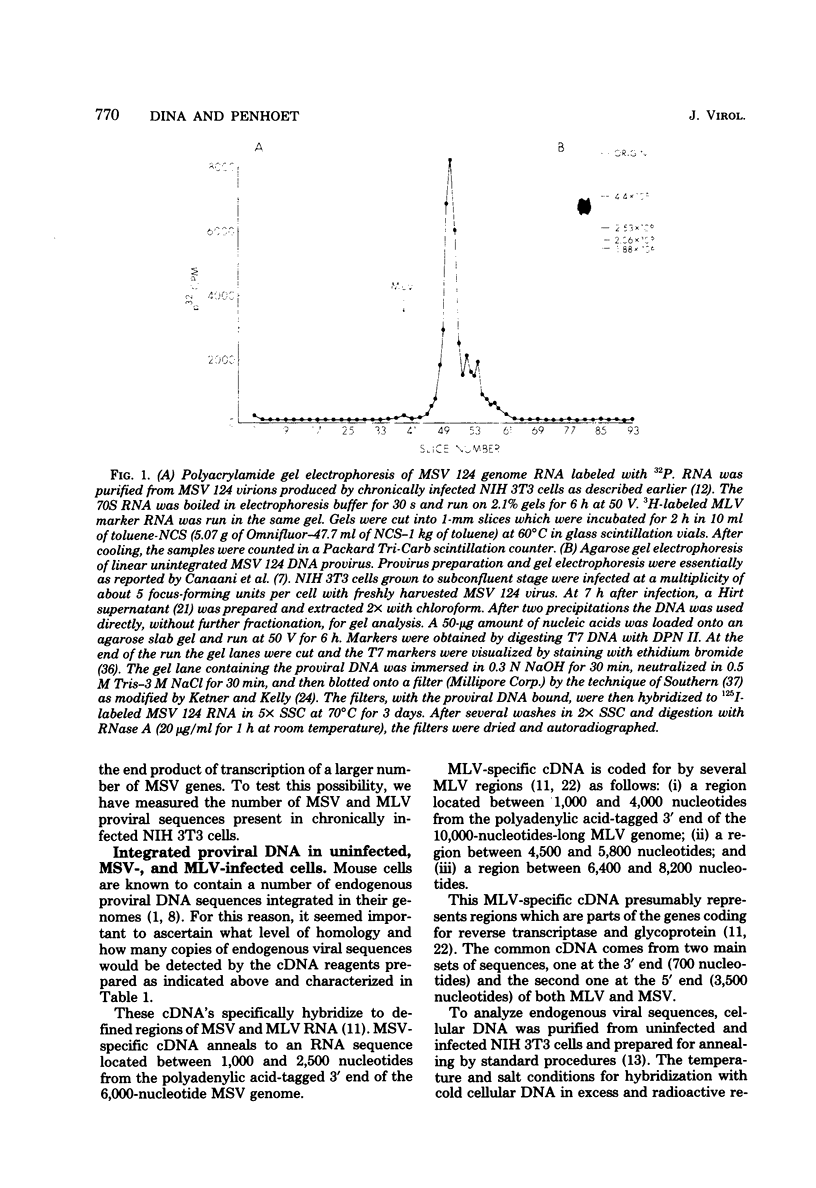
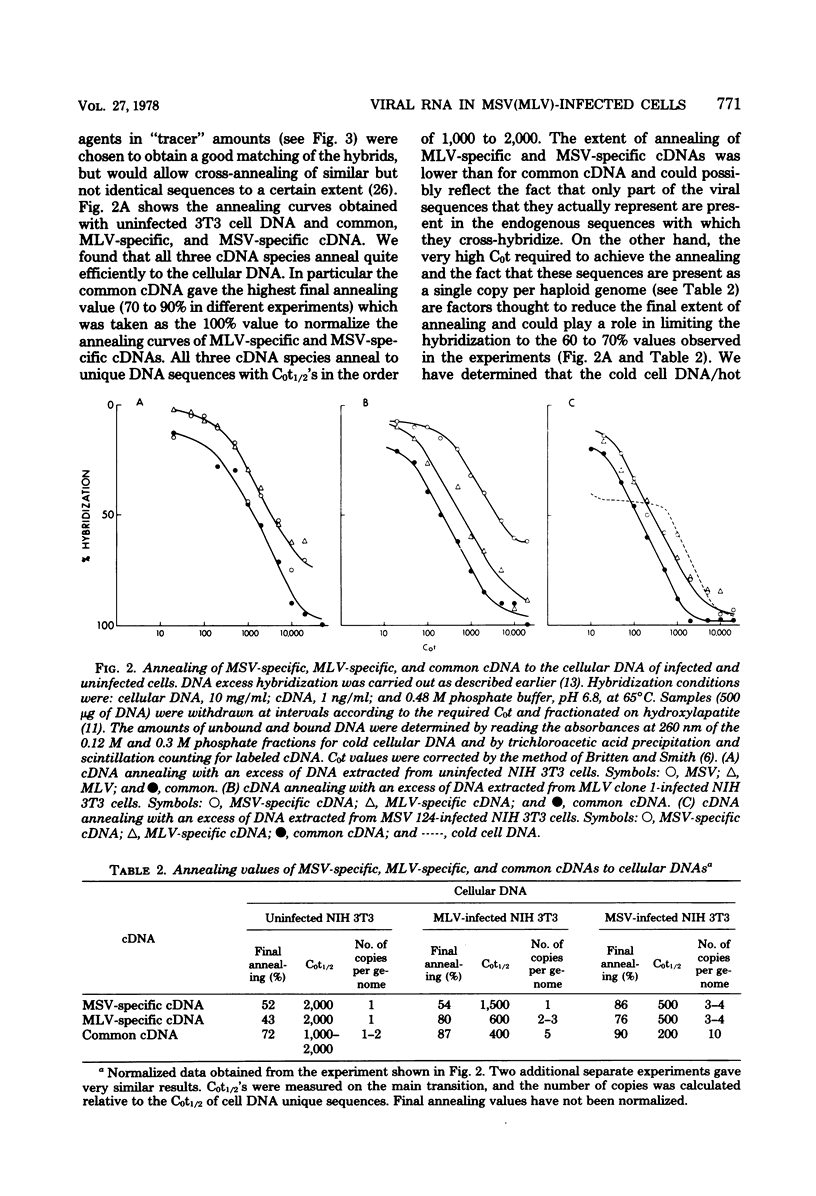
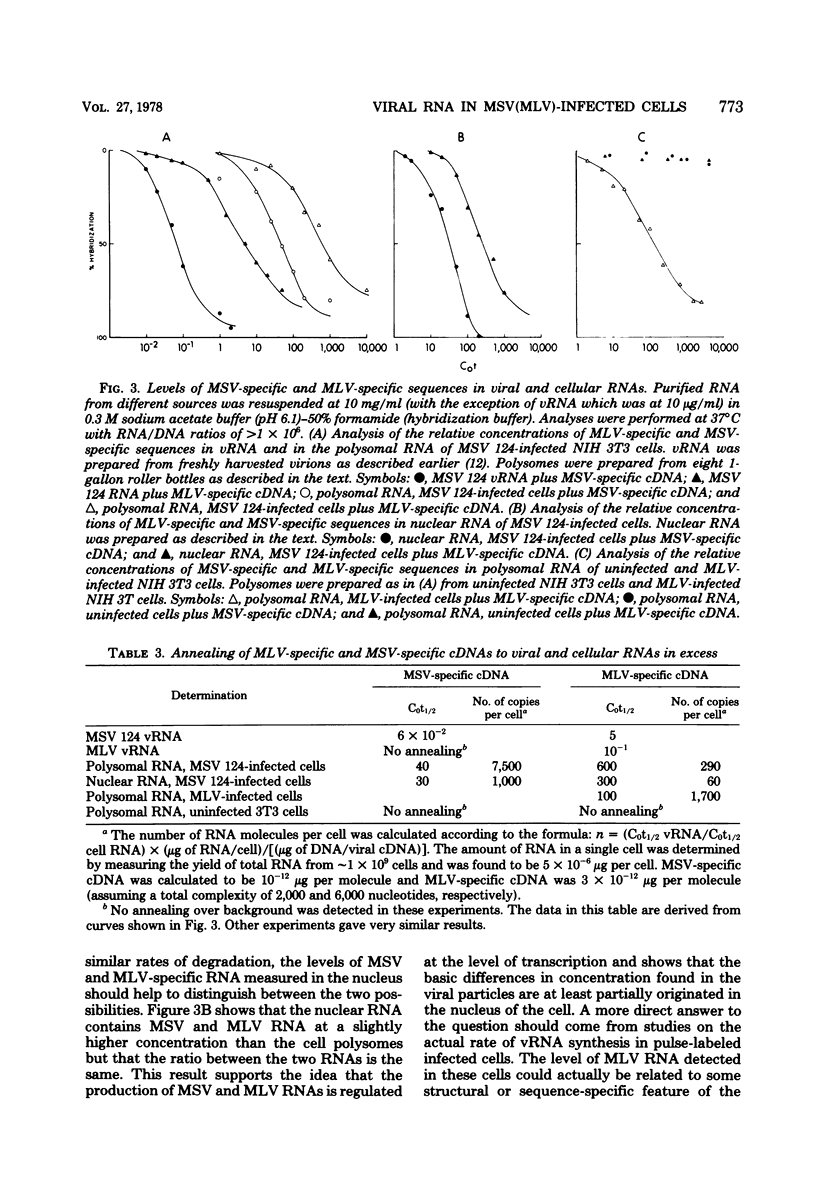
NIH 3T3 cells infected with Moloney murine sarcoma virus (murine leukemia virus) produce virions which contain about 99% murine sarcoma virus RNA and 1% murine leukemia virus RNA. This report describes experiments which measured intracellular concentrations of proviral DNA and RNA transcripts for each of the viruses. We found that three to four copies of proviral DNA from each virus were integrated into the cellular DNA. Measurements of RNA specific for each of the genomes by hybridization to specific cDNA reagents revealed a 10- to 15-fold difference in concentration in both nuclear and polysomal RNA fractions, with murine sarcoma virus RNA predominating in both cases. Unless there are major differences in stability between the two viral RNAs, our results suggest that transcriptional control is responsible for much of the difference in final levels of virus synthesis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Stephenson J. R. Differential cellular regulation of three distinct classes of type C RNA viruses endogenous to mouse cells. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):1129–1137. doi: 10.1101/sqb.1974.039.01.129. [DOI] [PubMed] [Google Scholar]
- Ball J., McCarter J. A., Sunderland S. M. Evidence for helper independent murine sarcoma virus. I. Segregation of replication-defective and transformation-defective viruses. Virology. 1973 Nov;56(1):268–284. doi: 10.1016/0042-6822(73)90305-x. [DOI] [PubMed] [Google Scholar]
- Beemon K. L., Faras A. J., Hasse A. T., Duesberg P. H., Maisel J. E. Genomic complexities of murine leukemia and sarcoma, reticuloendotheliosis, and visna viruses. J Virol. 1976 Feb;17(2):525–537. doi: 10.1128/jvi.17.2.525-537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettiger D. Virogenic nontransformed cells isolated following infection of normal rat kidney cells with B77 strain Rous sarcoma virus. Cell. 1974 Sep;3(1):71–76. doi: 10.1016/0092-8674(74)90042-7. [DOI] [PubMed] [Google Scholar]
- Canaani E., Duesberg P., Dina D. Cleavage map of linear mouse sarcoma virus DNA. Proc Natl Acad Sci U S A. 1977 Jan;74(1):29–33. doi: 10.1073/pnas.74.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Lowy D. R., Teich N. M., Levine A. S., Rowe W. P. Qualitative and quantitative studies of AKR-type murine leukemia virus sequences in mouse DNA. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):1085–1101. doi: 10.1101/sqb.1974.039.01.124. [DOI] [PubMed] [Google Scholar]
- Deng C. T., Boettiger D., Macpherson I., Varmus H. E. The persistence and expression of virus-specific DNA in revertants of Rous sarcoma virus-transformed BHK-21 cells. Virology. 1974 Dec;62(2):512–521. doi: 10.1016/0042-6822(74)90411-5. [DOI] [PubMed] [Google Scholar]
- Deng C. T., Stehelin D., Bishop J. M., Varmus H. E. Characteristics of virus-specific RNA in avian sarcoma virus-transformed BHK-21 cells and revertants. Virology. 1977 Jan;76(1):313–330. doi: 10.1016/0042-6822(77)90305-1. [DOI] [PubMed] [Google Scholar]
- Dina D., Beemon K., Duesberg P. The 30S Moloney sarcoma virus RNA contains leukemia virus nucleotide sequences. Cell. 1976 Oct;9(2):299–309. doi: 10.1016/0092-8674(76)90120-3. [DOI] [PubMed] [Google Scholar]
- Dina D., Beemon K. Relationship between Moloney murine leukemia and sarcoma virus RNAs: purification and hybridization map of complementary DNAs from defined regions of Moloney murine sarcoma virus 124. J Virol. 1977 Sep;23(3):524–532. doi: 10.1128/jvi.23.3.524-532.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dina D., Meza I., Crippa M. Relative positions of the "repetitive", "unique" and poly(A) fragments of mRNA. Nature. 1974 Apr 5;248(448):486–490. doi: 10.1038/248486a0. [DOI] [PubMed] [Google Scholar]
- Fan H., Mueller-Lantzsch N. RNA metabolism of murine leukemia virus. III. Identification and quantitation of endogenous virus-specific mRNA in the uninfected BALB/c cell line JLS-V9. J Virol. 1976 May;18(2):401–410. doi: 10.1128/jvi.18.2.401-410.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H., Paskind M. Measurement of the sequence complexity of cloned Moloney murine leukemia virus 60 to 70S RNA: evidence for a haploid genome. J Virol. 1974 Sep;14(3):421–429. doi: 10.1128/jvi.14.3.421-429.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel A. E., Fischinger P. J. Nucleotide sequences in mouse DNA and RNA specific for Moloney sarcoma virus. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3705–3709. doi: 10.1073/pnas.73.10.3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel A. E., Neubauer R. L., Fischinger P. J. Fractionation of DNA nucleotide transcripts from Moloney sarcoma virus and isolation of sarcoma virus-specific complementary DNA. J Virol. 1976 May;18(2):481–490. doi: 10.1128/jvi.18.2.481-490.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelb L. D., Milstien J. B., Martin M. A., Aaronson S. A. Characterization of murine leukaemia virus-specific DNA present in normal mouse cells. Nat New Biol. 1973 Jul 18;244(133):76–79. doi: 10.1038/newbio244076a0. [DOI] [PubMed] [Google Scholar]
- Georgiev G. P., Ryskov A. P., Coutelle C., Mantieva V. L., Avakyan E. R. On the structure of transcriptional unit in mammalian cells. Biochim Biophys Acta. 1972 Jan 31;259(2):259–283. doi: 10.1016/0005-2787(72)90066-4. [DOI] [PubMed] [Google Scholar]
- Hayward W. S., Hanafusa H. Independent regulation of endogenous and exogenous avian RNA tumor virus genes. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2259–2263. doi: 10.1073/pnas.73.7.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hu S., Davidson N. A heteroduplex study of the sequence relationships between the RNAs of M-MSV and M-MLV. Cell. 1977 Mar;10(3):469–477. doi: 10.1016/0092-8674(77)90034-4. [DOI] [PubMed] [Google Scholar]
- Jacquet M., Groner Y., Monroy G., Hurwitz J. The in vitro synthesis of avian myeloblastosis viral RNA sequences. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3045–3049. doi: 10.1073/pnas.71.8.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketner G., Kelly T. J., Jr Integrated simian virus 40 sequences in transformed cell DNA: analysis using restriction endonucleases. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1102–1106. doi: 10.1073/pnas.73.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury A. T., Hanafusa H. Synethesis and integration of viral DNA in chicken cells at different time after infection with various multiplicities of avian oncornavirus. J Virol. 1976 May;18(2):383–400. doi: 10.1128/jvi.18.2.383-400.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisel J., Dina D., Duesberg P. Murine sarcoma viruses: the helper-independence reported for a Moloney variant is unconfirmed; distinct strains differ in the size of their RNAs. Virology. 1977 Jan;76(1):295–312. doi: 10.1016/0042-6822(77)90304-x. [DOI] [PubMed] [Google Scholar]
- McCarthy B. J., Church R. B. The specificity of molecular hybridization reactions. Annu Rev Biochem. 1970;39:131–150. doi: 10.1146/annurev.bi.39.070170.001023. [DOI] [PubMed] [Google Scholar]
- Parks W. P., Ransom J. C., Young H. A., Scolnick E. M. Mammary tumor virus induction by glucocorticoids. Characterization of specific transcriptional regulation. J Biol Chem. 1975 May 10;250(9):3330–3336. [PubMed] [Google Scholar]
- Parks W. P., Scolnick E. M., Kozikowski E. H. Dexamethasone stimulation of murine mammary tumor virus expression: a tissue culture source of virus. Science. 1974 Apr 12;184(4133):158–160. doi: 10.1126/science.184.4133.158. [DOI] [PubMed] [Google Scholar]
- Parks W. P., Scolnick E. M. Murine mammary tumor cell clones with varying degrees of virus expression. Virology. 1973 Sep;55(1):163–173. doi: 10.1016/s0042-6822(73)81018-9. [DOI] [PubMed] [Google Scholar]
- Ringold G. M., Yamamoto K. R., Tomkins G. M., Bishop M., Varmus H. E. Dexamethasone-mediated induction of mouse mammary tumor virus RNA: a system for studying glucocorticoid action. Cell. 1975 Nov;6(3):299–305. doi: 10.1016/0092-8674(75)90181-6. [DOI] [PubMed] [Google Scholar]
- Ringold G., Lasfargues E. Y., Bishop J. M., Varmus H. E. Production of mouse mammary tumor virus by cultured cells in the absence and presence of hormones: assay by molecular hybridization. Virology. 1975 May;65(1):135–147. doi: 10.1016/0042-6822(75)90014-8. [DOI] [PubMed] [Google Scholar]
- Rymo L., Parsons J. T., Coffin J. M., Weissmann C. In vitro synthesis of Rous sarcoma virus-specific RNA is catalyzed by a DNA-dependent RNA polymerase. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2782–2786. doi: 10.1073/pnas.71.7.2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E. M., Howk R. S., Anisowicz A., Peebles P. T., Scher C. D., Parks W. P. Separation of sarcoma virus-specific and leukemia virus-specific genetic sequences of Moloney sarcoma virus. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4650–4654. doi: 10.1073/pnas.72.11.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E. M., Young H. A., Parks W. P. Biochemical and physiological mechanisms in glucocorticoid hormone induction of mouse mammary tumor virus. Virology. 1976 Jan;69(1):148–156. doi: 10.1016/0042-6822(76)90202-6. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]




