Abstract
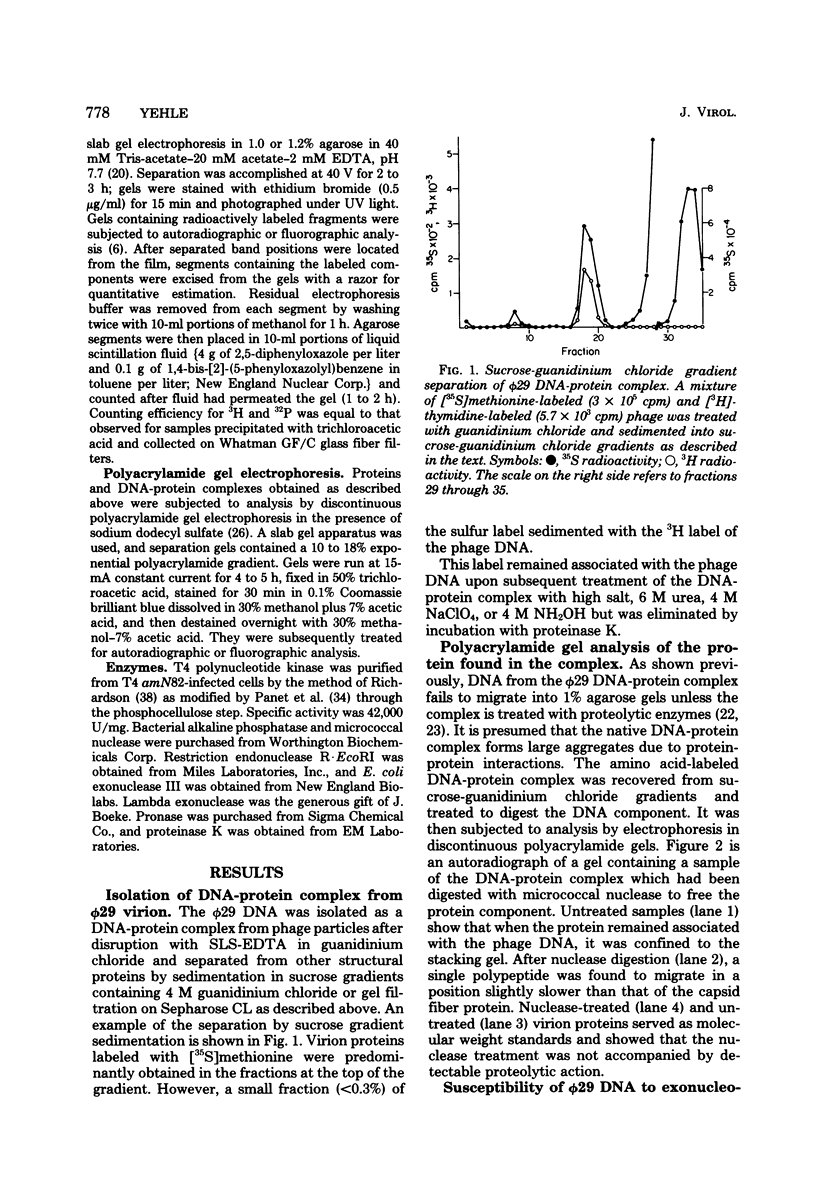
A DNA-protein complex was isolated from Bacillus subtilis bacteriophage phi29 by sucrose gradient sedimentation or gel filtration in the presence of agents known to break noncovalent bonds. A 28,000-dalton protein was released from this complex by subsequent hydrolysis of the DNA. The DNA-protein complex was examined for its susceptibility to enzymes which act upon the 5' and 3' termini of DNA molecules. It was susceptible to exonucleolytic degradation from the 3' termini by exonuclease III but not from the 5' termini by lambda exonuclease. Attempts to label radioactively the 5' termini by phosphorylation with T4 polynucleotide kinase were unsuccessful despite prior treatment with alkaline phosphatase or phosphatase treatment of denatured DNA. Removal of the majority of the bound protein by proteolytic digestion did not increase susceptibility. These results suggest that the linked protein is covalently attached to the 5' termini of phi29 DNA.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. L., Hickman D. D., Reilly B. E. Structure of Bacillus subtilis bacteriophage phi 29 and the length of phi 29 deoxyribonucleic acid. J Bacteriol. 1966 May;91(5):2081–2089. doi: 10.1128/jb.91.5.2081-2089.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. L., Mosharrafa E. T. Physical and biological properties of phage phi 29 deoxyribonucleic acid. J Virol. 1968 Oct;2(10):1185–1190. doi: 10.1128/jvi.2.10.1185-1190.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. L., Reilly B. E. Analysis of bacteriophage phi 29 gene function: protein synthesis in suppressor-sensitive mutant infection of Bacillus subtilis. J Virol. 1974 Jan;13(1):211–221. doi: 10.1128/jvi.13.1.211-221.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariga H., Shimojo H. Initiation and termination sites of adenovirus 12 DNA replication. Virology. 1977 May 15;78(2):415–424. doi: 10.1016/0042-6822(77)90118-0. [DOI] [PubMed] [Google Scholar]
- Blair D. G., Helinski D. R. Relaxation complexes of plasmid DNA and protein. I. Strand-specific association of protein and DNA in the relaxed complexes of plasmids ColE1 and ColE2. J Biol Chem. 1975 Nov 25;250(22):8785–8789. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Carrascosa J. L., Camacho A., Moreno F., Jiménez F., Mellado R. P., Viñuela E., Salas M. Bacillus subtilis phage phi29. Characterization of gene products and functions. Eur J Biochem. 1976 Jul 1;66(2):229–241. doi: 10.1111/j.1432-1033.1976.tb10512.x. [DOI] [PubMed] [Google Scholar]
- Carrascosa J. L., Viñuela E., Salas M. Proteins induced in Bacillus subtilis infected with bacteriophage phi 29. Virology. 1973 Nov;56(1):291–299. [PubMed] [Google Scholar]
- Carusi E. A. Evidence for blocked 5'-termini in human adenovirus DNA. Virology. 1977 Jan;76(1):380–394. doi: 10.1016/0042-6822(77)90310-5. [DOI] [PubMed] [Google Scholar]
- Cavalier-Smith T. Palindromic base sequences and replication of eukaryote chromosome ends. Nature. 1974 Aug 9;250(5466):467–470. doi: 10.1038/250467a0. [DOI] [PubMed] [Google Scholar]
- Depew R. E., Liu L. F., Wang J. C. Interaction between DNA and Escherichia coli protein omega. Formation of a complex between single-stranded DNA and omega protein. J Biol Chem. 1978 Jan 25;253(2):511–518. [PubMed] [Google Scholar]
- Flanegan J. B., Petterson R. F., Ambros V., Hewlett N. J., Baltimore D. Covalent linkage of a protein to a defined nucleotide sequence at the 5'-terminus of virion and replicative intermediate RNAs of poliovirus. Proc Natl Acad Sci U S A. 1977 Mar;74(3):961–965. doi: 10.1073/pnas.74.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M., Mizuuchi K., O'Dea M. H., Itoh T., Tomizawa J. I. Nalidixic acid resistance: a second genetic character involved in DNA gyrase activity. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4772–4776. doi: 10.1073/pnas.74.11.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiney D. G., Helinski D. R. Relaxation complexes of poasmid DNA and protein. III. Association of protein with the 5' terminus of the broken DNA strand in the relaxed complex of plasmid ColE1. J Biol Chem. 1975 Nov 25;250(22):8796–8803. [PubMed] [Google Scholar]
- Hagen E. W., Reilly B. E., Tosi M. E., Anderson D. L. Analysis of gene function of bacteriophage phi 29 of Bacillus subtilis: identification of cistrons essential for viral assembly. J Virol. 1976 Aug;19(2):501–517. doi: 10.1128/jvi.19.2.501-517.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding N. E., Ito J. DNA Replication of bacteriophage phi29: isolation of a DNA-protein complex from Bacillus subtilis cells infected with wild-type and with a suppressor-sensitive mutant. Virology. 1976 Sep;73(2):389–401. doi: 10.1016/0042-6822(76)90400-1. [DOI] [PubMed] [Google Scholar]
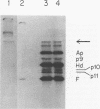
- Harding N. E., Ito J., David G. S. Identification of the protein firmly bound to the ends of bacteriophage phi 29 DNA. Virology. 1978 Feb;84(2):279–292. doi: 10.1016/0042-6822(78)90248-9. [DOI] [PubMed] [Google Scholar]
- Hawley L. A., Reilly B. E., Hagen E. W., Anderson D. L. Viral protein synthesis in bacteriophage phi 29-infected Bacillus subtilis. J Virol. 1973 Nov;12(5):1149–1159. doi: 10.1128/jvi.12.5.1149-1159.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa H. Transfecting deoxyribonucleic acid of Bacillus bacteriophage phi 29 that is protease sensitive. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1555–1559. doi: 10.1073/pnas.69.6.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi K., Vovis G. F., Enea V., Zinder N. D. Cleavage map of bacteriophage f1: location of the Escherichia coli B-specific modification sites. J Mol Biol. 1975 Jun 25;95(2):147–165. doi: 10.1016/0022-2836(75)90388-5. [DOI] [PubMed] [Google Scholar]
- Horwitz M. S. Bidirectional replication of adenovirus type 2 DNA. J Virol. 1976 Apr;18(1):307–315. doi: 10.1128/jvi.18.1.307-315.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inciarte M. R., Lázaro J. M., Salas M., Vińuela E. Physical map of bacteriophage phi29 DNA. Virology. 1976 Oct 15;74(2):314–323. [PubMed] [Google Scholar]
- Ito J., Kawamura F., Yanofsky S. Analysis of phi 29 and phi 15 genomes by bacterial restriction endonucleases, EcoR1 and Hpal. Virology. 1976 Mar;70(1):37–51. doi: 10.1016/0042-6822(76)90234-8. [DOI] [PubMed] [Google Scholar]
- Ivarie R. D., Pène J. J. DNA replication in bacteriophage ø29: the requirement of a viral-specfic product for association of ø29 DNA with the cell membrane of Bacillus amyloliquefaciens. Virology. 1973 Apr;52(2):351–362. doi: 10.1016/0042-6822(73)90330-9. [DOI] [PubMed] [Google Scholar]
- Kasamatsu H., Wu M. Structure of a nicked DNA-protein complex isolated from simian virus 40: covalent attachment of the protein to DNA and nick specificity. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1945–1949. doi: 10.1073/pnas.73.6.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee Y. F., Nomoto A., Detjen B. M., Wimmer E. A protein covalently linked to poliovirus genome RNA. Proc Natl Acad Sci U S A. 1977 Jan;74(1):59–63. doi: 10.1073/pnas.74.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W. An exonuclease induced by bacteriophage lambda. II. Nature of the enzymatic reaction. J Biol Chem. 1967 Feb 25;242(4):679–686. [PubMed] [Google Scholar]
- Little J. W., Lehman I. R., Kaiser A. D. An exonuclease induced by bacteriophage lambda. I. Preparation of the crystalline enzyme. J Biol Chem. 1967 Feb 25;242(4):672–678. [PubMed] [Google Scholar]
- Lovett M. A., Helinski D. R. Relaxation complexes of plasmid DNA and protein. II. Characterization of the proteins associated with the unrelaxed and relaxed complexes of plasmid ColE1. J Biol Chem. 1975 Nov 25;250(22):8790–8795. [PubMed] [Google Scholar]
- McGuire J. C., Pène J. J., Barrow-Carraway J. Gene expression during the development of bacteriophage phi 29. 3. Analysis of viral-specific protein synthesis with suppressible mutants. J Virol. 1974 Mar;13(3):690–698. doi: 10.1128/jvi.13.3.690-698.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuire J. C., Gilpatrick M. W., Pène J. J. DNA replication of bacteriophage phi29. Effect of two viral genes on the association of phage chromosomes with the host cell membrane. Virology. 1977 May 1;78(1):234–240. doi: 10.1016/0042-6822(77)90094-0. [DOI] [PubMed] [Google Scholar]
- Ortin J., Viñuela E., Salas M., Vasquez C. DNA-protein complex in circular DNA from phage phi-29. Nat New Biol. 1971 Dec 29;234(52):275–277. doi: 10.1038/newbio234275a0. [DOI] [PubMed] [Google Scholar]
- Panet A., van de Sande J. H., Loewen P. C., Khorana H. G., Raae A. J., Lillehaug J. R., Kleppe K. Physical characterization and simultaneous purification of bacteriophage T4 induced polynucleotide kinase, polynucleotide ligase, and deoxyribonucleic acid polymerase. Biochemistry. 1973 Dec 4;12(25):5045–5050. doi: 10.1021/bi00749a003. [DOI] [PubMed] [Google Scholar]
- REILLY B. E., SPIZIZEN J. BACTERIOPHAGE DEOXYRIBONUCLEATE INFECTION OF COMPETENT BACILLUS SUBTILIS. J Bacteriol. 1965 Mar;89:782–790. doi: 10.1128/jb.89.3.782-790.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHARDSON C. C., LEHMAN I. R., KORNBERG A. A DEOXYRIBONUCLEIC ACID PHOSPHATASE-EXONUCLEASE FROM ESCHERICHIA COLI. II. CHARACTERIZATION OF THE EXONUCLEASE ACTIVITY. J Biol Chem. 1964 Jan;239:251–258. [PubMed] [Google Scholar]
- Reilly B. E., Nelson R. A., Anderson D. L. Morphogenesis of bacteriophage phi 29 of Bacillus subtilis: mapping and functional analysis of the head fiber gene. J Virol. 1977 Oct;24(1):363–377. doi: 10.1128/jvi.24.1.363-377.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekosh D. M., Russell W. C., Bellet A. J., Robinson A. J. Identification of a protein linked to the ends of adenovirus DNA. Cell. 1977 Jun;11(2):283–295. doi: 10.1016/0092-8674(77)90045-9. [DOI] [PubMed] [Google Scholar]
- Richardson C. C. Phosphorylation of nucleic acid by an enzyme from T4 bacteriophage-infected Escherichia coli. Proc Natl Acad Sci U S A. 1965 Jul;54(1):158–165. doi: 10.1073/pnas.54.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A. J., Younghusband H. B., Bellett A. J. A circula DNA-protein complex from adenoviruses. Virology. 1973 Nov;56(1):54–69. doi: 10.1016/0042-6822(73)90287-0. [DOI] [PubMed] [Google Scholar]
- Salas M., Mellado R. P., Viñuela E. Characterization of a protein covalently linked to the 5' termini of the DNA of Bacillus subtilis phage phi29. J Mol Biol. 1978 Feb 25;119(2):269–291. doi: 10.1016/0022-2836(78)90438-2. [DOI] [PubMed] [Google Scholar]
- Schachtele C. F., Oman R. W., Anderson D. L. Effect of elevated temperature on deoxyribonucleic acid synthesis in bacteriophage phi-29-infected Bacillus amyloliquefaciens. J Virol. 1970 Oct;6(4):430–437. doi: 10.1128/jvi.6.4.430-437.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtele C. F., Reilly B. E., De Sain C. V., Whittington M. O., Anderson D. L. Selective replication of bacteriophage phi29 deoxyribonucleic acid in 6-(p-hydroxyphenylazo)-uracil-treated Bacillus subtilis. J Virol. 1973 Jan;11(1):153–155. doi: 10.1128/jvi.11.1.153-155.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabarova Z. A. Synthetic nucleotide-peptides. Prog Nucleic Acid Res Mol Biol. 1970;10:145–182. doi: 10.1016/s0079-6603(08)60564-4. [DOI] [PubMed] [Google Scholar]
- Shatkin A. J. Capping of eucaryotic mRNAs. Cell. 1976 Dec;9(4 Pt 2):645–653. doi: 10.1016/0092-8674(76)90128-8. [DOI] [PubMed] [Google Scholar]
- Subramanian K. N., Dhar R., Weissman S. M. Nucleotide sequence of a fragment of SV40 DNA that contains the origin of DNA replication and specifies the 5' ends of "early" and "late" viral RNA. III. Construction of the total sequence of EcoRII-G fragment of SV40 DNA. J Biol Chem. 1977 Jan 10;252(1):355–367. [PubMed] [Google Scholar]
- Sugino A., Hirose S., Okazaki R. RNA-linked nascent DNA fragments in Escherichia coli. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1863–1867. doi: 10.1073/pnas.69.7.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino A., Peebles C. L., Kreuzer K. N., Cozzarelli N. R. Mechanism of action of nalidixic acid: purification of Escherichia coli nalA gene product and its relationship to DNA gyrase and a novel nicking-closing enzyme. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4767–4771. doi: 10.1073/pnas.74.11.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussenbach J. S., Kuijk M. G. Studies on the mechanism of replication of adenovirus DNA. V. The location of termini of replication. Virology. 1977 Mar;77(1):149–157. doi: 10.1016/0042-6822(77)90414-7. [DOI] [PubMed] [Google Scholar]
- Talavera A., Salas M., Viñuela E. Temperature-sensitive mutants affected in DNA synthesis in phage phi29 of Bacillus subtilis. Eur J Biochem. 1972 Dec 4;31(2):367–371. doi: 10.1111/j.1432-1033.1972.tb02542.x. [DOI] [PubMed] [Google Scholar]
- Watson J. D. Origin of concatemeric T7 DNA. Nat New Biol. 1972 Oct 18;239(94):197–201. doi: 10.1038/newbio239197a0. [DOI] [PubMed] [Google Scholar]
- Weingärtner B., Winnacker E. L., Tolun A., Pettersson U. Two complementary strand-specific termination sites for adenovirus DNA replication. Cell. 1976 Oct;9(2):259–268. doi: 10.1016/0092-8674(76)90117-3. [DOI] [PubMed] [Google Scholar]
- Wu M., Roberts R. J., Davidson N. Structure of the inverted terminal repetition of adenovirus type 2 DNA. J Virol. 1977 Feb;21(2):766–777. doi: 10.1128/jvi.21.2.766-777.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]
- Yanofsky S., Kawamura F., Ito J. Thermolabile transfecting DNA from temperature-sensitive mutant of phage phi29. Nature. 1976 Jan 1;259(5538):60–63. doi: 10.1038/259060a0. [DOI] [PubMed] [Google Scholar]