Abstract
Oilseeds are crucial for the nutritional security of the global population. The conventional technology used for oil extraction from oilseeds is by solvent extraction. In solvent extraction, n-hexane is used as a solvent for its attributes such as simple recovery, non-polar nature, low latent heat of vaporization (330 kJ/kg) and high selectivity to solvents. However, usage of hexane as a solvent has lead to several repercussions such as air pollution, toxicity and harmfulness that prompted to look for alternative options. To circumvent the problem, green solvents could be a promising approach to replace solvent extraction. In this review, green solvents and technology like aqueous assisted enzyme extraction are better solution for oil extraction from oilseeds. Enzyme mediated extraction is eco-friendly, can obtain higher yields, cost-effective and aids in obtaining co-products without any damage. Enzyme technology has great potential for oil extraction in oilseed industry. Similarly, green solvents such as terpenes and ionic liquids have tremendous solvent properties that enable to extract the oil in eco-friendly manner. These green solvents and technologies are considered green owing to the attributes of energy reduction, eco-friendliness, non-toxicity and non-harmfulness. Hence, the review is mainly focussed on the prospects and challenges of green solvents and technology as the best option to replace the conventional methods without compromising the quality of the extracted products.
Keywords: Aqueous enzyme assisted extraction (AEAE), Green solvents, Ionic liquids, Terpenes
Background
Conventional oil extraction from oilseeds has been performed by hydraulic pressing, expeller pressing and solvent extraction (SE) [1]. Among these methods, solvent extraction has been widely adapted for economical and practical concerns. Before performing solvent extraction the oilseeds are processed (flaked, cracked, ground or pressed) to suit for the enhanced oil recovery by solvent extraction. In SE process, the oilseeds are washed with hexane, thereafter the hexane is separated from oil by evaporation and distillation [2]. Hexane has been widely used for oil extraction because of easy oil recovery, narrow boiling point (63–69 °C) and excellent solubilizing ability [3].
In contrary, while in extraction and recovery processes, hexane is released into the environment that react with the pollutants to form ozone and photo chemicals [4]. Moreover, several studies revealed that hexane affects neural system when inhaled by humans because of solubility in neural lipids. Toxicity has been observed in piglets fed with de-fatted meal containing residual hexane which was left over after the process [5]. Therefore, health perspective, safety and environment concerns have triggered to look for a substitute to n-hexane without compromising the yield of oil. Hence, green solvents coupled with technology are a viable alternative for oil extraction.
Green solvents and technology are aimed to develop an environment friendly process with simultaneous reduction of pollutants [6, 7] for oil extraction. Hence, green technology such as aqueous enzymatic extraction (AEE) coupled with green solvents have huge potential to replace n-hexane without any compromise in oil recovery from the process. In addition, the opportunities and challenges of AEE have been given comprehensively to understand the merits and de-merits of the technology.
Oil extractions by green solvents (GS)
Green solvents are derived either from naturally (water and CO2) or agricultural residues (terpenes) or petroleum sources, which have good solubilizing properties like conventional solvents. Recent advances on ‘green’ approaches have great impetus in oil industry because of green solvents i.e., terpenes (d-limonene, p-cymene and α-pinene). Terpenes are isoprene units (C5H8) derived chiefly from agriculture sources. For example, d-limonene is derived from citrus peels and employed in many applications. Similarly, p-cymene and α-pinene are derived from tree oils and pine forests respectively. Interestingly, these solvents have good Hansen solubility properties (HSP) to dissolve the like molecules. To determine the behavior of given solvent, Hansen has proposed three properties which is also called Hansen properties based on the energy of dispersive (δd), dipolar (δd) and hydrogen bond forces (δh), between the molecules [8]. In a study, the terpenes were found to possess the characteristics of n-hexane that substantiate the capability to dissolve the like molecules (Fig. 1). Moreover, terpenes are not only safer due to higher flash point, but also have slightly higher dissociating power due to slight differences in the dielectric constant in comparison with n-hexane [9].
Fig. 1.
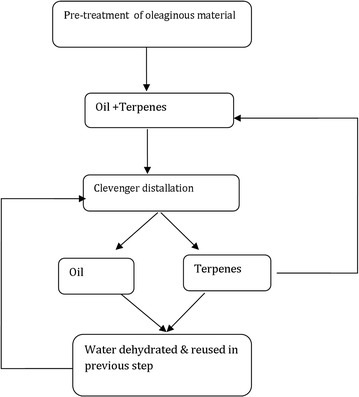
Schematic diagram of oil extraction from oilseeds using terpenes as solvent.
Ionic liquids
Ionic liquids are non-aqueous salt solution that comprise both anions and cations which can be maintained in a liquid state at moderate temperatures (0–140 °C) [10, 11]. Ionic liquids are considered as green solvents or green ‘designer’ solvents for their manifold applications in petroleum and oil industry. Ionic liquids are eco-friendly in nature as these do not have the detectable vapor pressure, as a result, no pollution. In addition, these are non-flammable, and remain in liquid state for wide range of temperatures [12]. As these solvents possess both the ions and versatile physico-chemical characteristics, these have allowed to design a suitable solvent with specific conductivity, hydrophobicity, polarity, and solubility based on the nature of solute for efficient recovery [13]. Interestingly, because of these properties about 600 molecular solvents were employed in various processes [14].
Ionic liquids were used as solvent for extraction, catalysis and synthesis of various compounds. These can also be used as a co-solvent for enzyme, medium for several reactions, biphasic system separations etc., [15]. However, studies on application of ionic liquids for oil extraction are scanty and needs to substantiate the technical and economical viability. Ma et al. [16] studied the extraction of essential oils using ionic liquids from Schisandra chinensis Baill fruit and projected that the ionic liquid coupled with microwave have reduced time, energy and eco-friendly [16]. In other study, the ionic liquid was used as a co-solvent for bio-oil extraction in a single step from microalgae [17]. However, a meta-analysis study reported that the IL’s should be chosen carefully and need to understand their adverse effects [18]. Although, this method is promising but it needs more studies to substantiate the hypothesis of oil extraction from ionic liquids. Another promising green solvent such as switchable solvent has showed potential for oil extraction from soy bean flakes [19]. In addition, super critical fluid, deep eutectic solvents, natural deep eutectic solvents and supramolecular solvents are gaining wide interest and there is a need to study their applicability in oil extraction [11, 20].
Green techniques for oil extraction from oilseeds
Aqueous enzymatic extraction (AEE)
Aqueous extraction involves water as a medium to extract the oil from oilseeds. It is well known that the lipid molecules are amphipathic in nature and the water soluble components diffuse into water which culminates into emulsion formation [21]. The emulsified oil in water can be de-emulsified by changing the temperature or deploying enzymes. Hence, in the process of AEE, enzymes are involved which segregate the desired extracted constituent without any damage. Recent investigations have unraveled the tremendous potential of AEE [22]. Moreover, this process is environmental-friendly, safer, healthier, simultaneous oil and protein extraction can be done without compromising the quality. In addition, it is cost-effective as consumption of solvent is reduced and is effective in removal of anti-nutritional factors, toxins and avoid degumming process [23–25]. These several merits make AEE a promising green technique not only for oilseed processing but also to extract the desired compound. The differences between solvent extraction (SE) and enzyme assisted extraction are given in Table 1.
Table 1.
Comparison of solvent extraction (SE) and aqueous assisted enzymatic (AAE) methods
| Parameter | Solvent extraction | Aqueous assisted enzymatic |
|---|---|---|
| Nature of the process | Non-environment friendly | Environment friendly |
| Toxic | Non-toxic | |
| Solvents used | n-Hexane | Green solvents |
| Energy efficiency | Energy demanding process due to consumption of oil | Less energy demand process |
| Co-product quality | Poor quality due to operational conditions at higher temperature and pressure | Food quality grade due to mild operational conditions |
| Degumming | It is essential because of phospholipids | Not required Aqueous medium dissolves the phospholipids |
| Others | Ineffective process in removal of toxins and anti-nutritional factors | Highly efficient in removal of toxins and anti-nutritional factors |
| Limitations | Limitations are cited above | An additional de-emulsification step is required. High cost for enzyme production |
To know the role of enzymes on seed, the basic understanding of the architecture of crop oilseeds is indispensable. Oil seed cotyledon consists of discrete lipid and protein bodies which contains oil and protein respectively. In the cotyledons, proteins occupy a major proportion of 60–70% ranging in size from 2 to 20 µm in various oilseeds (Fig. 2) . Lipid bodies are the lipid reserves in fruits as well as in oilseeds. Their size varies from one species to another with an average range of 1–2 µm for most of the oilseeds. Microscopic structure of peanuts and soybean oilseeds depicts that the lipids are embedded with protein like cytoskeleton and the gaps are packed with lipids and cytoskeleton. These internal discrete cell organelles are surrounded by cell wall that is composed of cellulose, hemicelluloses, lignin and pectin.
Fig. 2.
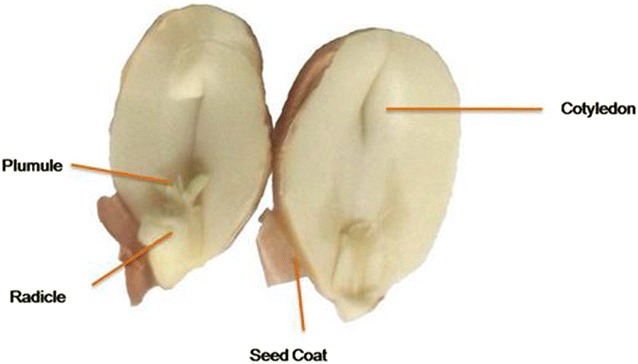
Diagram depicting the parts of groundnut oilseed
Selection of enzymes for oil extraction
Several factors are essential for the maximum recovery of oil from oilseeds. Application of enzymes either alone or in concoction can be determined based on the structure of oilseed, enzyme composition, type of enzyme, experimental conditions. For instance, heat treated soy bean flour separately treated with cellulase, pectinase, hemicellulase and protease (Alcalase 2.4 L from Bacillus licheniformis) enzymes, respectively. Among them, protease resulted higher yield (Alcalase 2.4 L) than rest of the enzymes [26]. Similarly, in extruded soybean flakes, protease treatment resulted higher yield of oil (96.0%) than phospholipase (73.4%) treatment [27]. Furthermore, when extruded soybean oil was treated with cellulase alone and with a mixture of cellulase and protease, no significant augmentation of soybean oil yields (68%) was observed. However, when the same oleaginous material was treated with protease it resulted in 88% of soybean oil [28]. It clearly elucidates that the hydrolysis of protein (which is in major proportion) in soybean by protease has succored the release of oil.
Similarly, rapeseed predominant with pectin in the cell wall was treated by pectinase that resulted 85.9% increase in oil yield [29]. On the other hand, some other research findings revealed that the application of enzyme mixtures have shown a better performance than individual enzymes presumably due to synergism [30]. For example, mixture of enzymes such as polygalacturonase, α-amylase and protease showed higher oil yield (80%) in coconut [31]. In contrary, soybean treated with combination of alcalase 2.4 L and viscozyme (a mixture of enzyme), no considerable increase in oil yield was observed [32]. The difference in activities of viscozyme can be attributed due to experimental conditions and the nature of oilseeds.
Consequently, these findings envisage for prior understanding of the architecture of targeted oilseed and selection of influential parameters to choose the best combination of enzymes. Hence, to achieve higher yields and recovery of co-products judicious use of enzymes is pre-requisite step. For optimization of the process, response surface methodology or genetic algorithm or any statistical methods could be employed to maximize the process by fixing the influential factors [14]. Several studies on application of enzymes either alone or in combination on different oilseeds for oil extraction have been presented in Table 2.
Table 2.
Oil yield by enzymatic extraction method
| Material | Enzymes applied | Oil yield (%) | References |
|---|---|---|---|
| Palm fruit | Pectinase/cellulase/tannase Tannase |
35.90 12.70 |
[42] |
| Peanut (grounded) | Viscozyme L | 13.10 | [56] |
| Peanut (grounded) | Alcalase | 42.86 | [43] |
| Protizyme™ | 24.43 | [43] | |
| Canola seeds (grounded) | Multifect CX 13L | 09.50 | [56] |
| Soybean flakes (extruded) | Multifect Neutral™ | 20.00 | [50] |
| Rapeseed slurry | Pectinase | 38.10 | [28, 57] |
| Rapeseed slurry | Pectinase/cellulase/b-glucanase (4:1:1) | 43.80 | [28] |
| Soybean flakes (extruded) | Protease | 13.40 | [50] |
| Moringa oleifera seeds (grounded) | Neutrase 0.8 L/Termamyl | 12.83 | [49] |
Factors affecting enzyme mediated oil extraction
Aqueous enzymatic extraction (AEE) efficiency depends on several factors. In order to develop a viable process for oil extraction from oilseeds, factors responsible for the maximization have to be known to maintain the optimum conditions.
Pre-treatment (grinding) of oleaginous materials
It is necessary to reduce the size of oleaginous materials (seeds/fruits) either by grinding or flaking to gain much access by enzymes. Grinding ruptures the cell constituents and releases the oil. In case of grinding, factors such as structural and chemical constituents of oilseed, initial moisture content are to be determined to make judicious choice either for wet or dry grinding [33]. Generally, oleaginous material with high moisture content can ground in wet condition, whereas for low moisture content oilseeds like rapeseed, peanut and soybean, drying is necessary. For example, grinding of coconut (high moisture content) in wet condition not only resulted higher oil yield but also alleviated drying step [34].
Oilseeds particle size
Generally, lower particle size favors higher yield but scrawny seeds coupled with oleaginous material when treated with solvents may lose their microporosity that may result into unfavorable extraction due to non-uniform distribution. For instance, different particle sizes of linseed kernels improved the efficiency of oil extraction whereas with the same substrate showed inadequate oil recovery due to lack of enzymes access [30]. In addition, Rosenthe et al. [26] reported an increase of 31% yield when the particle size reduced from 400 to 100 µm [27].
pH
Efficiency of oil extraction by enzyme depends mainly on pH factor. The extraction efficiency can be maximized at an optimum pH since each enzyme has a specific optimum value. Care should be taken to extract at far from the isoelectric point. Because, at specific isoelectric point of an enzyme, the protein is insoluble that might hamper the objective of oil extraction. For instance, a low yield of oil was observed in soybean, rapeseed, peanut and sunflower due to low solubility of protein at isoelectric point [35, 36]. To corroborate further, flaxseed oil yield was higher when treated with mixture of enzymes (cellulase, hemicellulase and pectinase, at a ratio of 1:1:1) at pH 4.5–5.0 than treatment with individual enzymes [32]. These studies envisage to maintain the pH at optimum level and to carry out the process far from the isoelectric point.
Temperature
Temperature is another important factor for optimization of enzyme activity. Generally, enzyme activities are effective at or below 45 °C and increase in temperature results in denaturation of protein; as a result, it reduce the oil release from oilseeds [36]. Temperature has to be determined as per the quality of oilseed and fatty acids. For example, the congenial temperature for olive oil extraction is 30 °C and for linseed it is 34 °C, respectively. In a study conducted on peanut, the maximum yield was obtained at 40 °C, however, upon reduction of temperature to 37 °C resulted reduced yield [37]. Therefore, it is vital to optimize the temperature range as per the desired quality and the nature of the seed.
Enzyme concentration/substrate ratio
Generally, the increase in concentration of enzyme leads to more interaction with substrate that consequently degrades the peptide bonds [38]. Increase in enzyme concentration until saturation of substrate active sites lead to more degradation of desired product and enhanced oil recovery. Additionally, increase beyond saturation levels may set off flavors, bitterness and carmelization of sugars which may hinder the oil extraction process [36, 39]. In addition, the cost of the enzyme (economics of the process) and quality of the oil are some other factors to consider before determination of the enzyme concentration [40].
Oil:water ratio
Enzyme activity needs water or moisture content for several functions like diffusion, mobility of enzymes and hydrolytic reactions [41]. If an oleaginous material possesses low moisture content it leads to formation of thick suspension [29]. As a result, the enzyme action can be inhibited. On the other hand, if the oilseed contain higher moisture content it may dilute the enzyme and substrate concentrations which may feeble the reaction [42]. Hence, in order to have profound enzymatic reaction on the target, optimization of moisture content is inevitable.
Shaking regime
Shaking or agitation regime helps in disruption of mechanical barriers (cell wall) and also perform uniform mixing of all contents in the reaction mixture [43]. Oil extraction from Moringa oleifera has been done at agitation speed of 50, 80 and 120 rpm, respectively. At 120 rpm, the oil droplets (bigger in size) were accumulated at the surface which has an advantage of easy separation [41]. In contrary, agitation is an energy driven process that may incur more cost on the process. In addition, it forms a stable emulsion that is cumbersome to separate [42].
Challenges of green solvents and aqueous enzyme oil extraction (AEE)
Unprecedently, green solvents such as terpenes, IL’s and switchable solvents have huge potential to replace conventional solvent systems. Terpenes are gaining wide interest but scalability of the process is limited due to its high heat of vaporization, boiling point, density and viscosity. The problem could be avoided by extracting the solvents (terpenes) at low temperature and pressure using Clevenger apparatus. Generally, the bio-solvents are to be extracted by Clevenger apparatus at about 97–98 °C at atmospheric pressure. For instance, Sean et al. [44] have studied the quality of rice bran oil extraction with hexane and d-limonene solvents. The bio-solvent d-limonene is equivalent in terms of quality to that of hexane process [44]. Li et al. [21] has done similar studies of oil extraction from rapeseed. Hexane, ethanol, butanol, isopropanol, d-limonene, p-cymene and α-pinene were used to extract the oil from the rapeseed. Among the solvents, p-cymene obtained higher oil yield than the other solvents. The major oil components are free fatty acids (FFA), diglyceride (DAG), monoglyceride (MAG) and triglycerides, respectively. In p-cymene, the triglyceride content was low but high in free fatty acids, diglyceride and monoglyceride contents, respectively [21–44]. The result observed can be explained due to more polarity of the terpenes than hexane. Hence, it is intriguing that the terpenes can be a viable option to replace hexane and deploying this green solvent would ensure a cleaner environment, safer handling and non-toxicity.
Although aqueous enzyme oil extraction has huge potential, application of this technology is still hampered due to the factors such as high cost for enzyme production and downstream processing, long incubation time and unavoidable added step (de-emulsification) in the process. Nevertheless, due to the wide applications of AEE, commercial enzyme production has been expedited and as of now the enzyme production has become cheaper [38, 45]. Similarly, the downstream processing costs could be minimized by adapting suitable technologies than the conventional process. For instance, expanded bed affinity chromatography resulted 89% green fluorescent protein (GFP) with 2.7-purification fold using Ni2+ Streamline™, whereas Ni2+ alginate gave 91% of GFP recovery with 3.1-fold purification in a single step [46, 47]. Unlike chromatographic techniques, membrane technology has been employed to purify protein (penicillin acylase) from the cell lysate in a single step. Further, the specific enzyme activity has been confirmed by SDS-PAGE [48]. Moreover, several other techniques such as perfusion chromatography, affinity precipitation may be applied to make the process simpler with concomitant reduction in price [49, 50].
Another strategy for reducing the cost is enzyme immobilization, through which many cycles can be performed for oil extraction. The application of extracted cream emulsion, which possesses enzyme activity even after extraction, will certainly be a viable approach to reduce the cost. Cream emulsion is obtained in the process of AEE. Initially, the oleaginous material was pre-treated, extracted by solvent and separation leads to formation of oil and skim emulsion. It is reported that Protex 6L possessed 100% of activity in the fractions after extraction of oil [51]. Similarly, after extraction of oil from soybean around 84.7% of activity was observed in aqueous phases [52]. Apart from the above measures, AEE process saves energy by alleviating the necessity of solvent (used for stripping), process monitoring (in SE volatile compound emission has to be controlled) and simultaneous oil and protein recovery may compensate the challenges in the implementation of AEE [53–56].
Conclusion
In the course of time, green solvents and technologies are in great demand because of environmental, health and energy issues. It is inevitable to develop a novel green technology for the oil extraction from various oilseeds. As each oilseed comprises of different architecture, the process needs to look for suitability of technology in economical and technical ways. In this review, green solvents coupled with AEE (green technology) not only ensure oil quality and protein extraction but also eco-friendly. In addition, they could reduce downstream processing steps. Furthermore, green solvents are effective in consumption of solvent, reduction of downstream processing steps (reclamation of solvent) without causing any effect to other desired products. AEE coupled with green solvents could be economical, eco-friendly and safer. Adoption of green technology and solvents is the need of an hour, as these are promising approaches for oil extraction towards environmental safety. However, further research findings should substantiate the viability of these approaches for the oil extraction from oilseeds.
Authors’ contributions
SPJK has conceived the idea and authored paper on green solvents. KSK and KVR have authored part of the manuscript over enzyme technologies. SRP edited the text and took part in design of the paper. RB conceived the idea and edited the manuscript meticulously. DKA edited the text and took part in design of the paper. All authors read and approved the final manuscript.
Acknowledgements
Authors’ acknowledge Mr. Ram Nayan Yadav for his help in taking the photograph of the figure.
Competing interests
The authors declare that they have no competing interests.
Funding
The authors thank the ICAR for financial support.
Abbreviations
- AEE
aqueous enzyme oil extraction
- DAG
diglyceride
- FFA
free fatty acids
- GS
green solvents
- HSP
Hansen solubility properties
- MAG
monoglyceride
Contributor Information
S. P. Jeevan Kumar, Phone: +91-547-2530325, Email: jeevaniitkgp@gmail.com.
S. Rajendra Prasad, Email: srprasad1989@yahoo.co.in.
Rintu Banerjee, Email: rb@iitkgp.ac.in.
Dinesh K. Agarwal, Email: agarwaldk4@gmail.com
Kalyani S. Kulkarni, Email: kalyaniaau@gmail.com
K. V. Ramesh, Email: ramesh.iari@gmail.com
References
- 1.Kalia VC, Rashmi LS, Gupta MN. Using enzymes for oil recovery from edible seeds. J Sci Ind Res. 2001;60:298–310. [Google Scholar]
- 2.Serrato AG. Extraction of oil from soya bean. J Am Oil Chem Soc. 1981;3:157–159. doi: 10.1007/BF02582327. [DOI] [Google Scholar]
- 3.Liu SX, Mamidipally PK. Quality comparison of rice bran oil extracted with d-limonene and hexane. Cereal Chem. 2005;82:209–215. doi: 10.1094/CC-82-0209. [DOI] [Google Scholar]
- 4.Hanmoungjai P, Pyle L, Niranjan K. Extraction of rice bran oil using aqueous media. J Chem Technol Biotechnol. 2000;75:348–352. doi: 10.1002/(SICI)1097-4660(200005)75:5<348::AID-JCTB233>3.0.CO;2-P. [DOI] [Google Scholar]
- 5.Toxicological review of n-hexane: in support of summary information on the integrated risk information system (IRIS). U.S. Environmental Protection Agency (2005), Washington, DC, (EPA/635/R-03/012)
- 6.Wan PJ, Hron RJ, Dowd MK, Kuk MS, Conkerton EJ. Alternative hydrocarbon solvents for cottonseed extraction: plant trials. J Am Oil Chem Soc. 1995;72(6):661–664. doi: 10.1007/BF02635651. [DOI] [Google Scholar]
- 7.Anastas P, Warner J. Theory and practice. New York: Oxford University Press; 1998. [Google Scholar]
- 8.Tanzi CD, Vian MA, Ginies C, Elmaataoui M, Chemat F. Terpenes as green solvents for extraction of oil from microalgae. Molecules. 2012;17:8196–8205. doi: 10.3390/molecules17078196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matthieu V, Vr Tomaoa, Ginies C, Visinoni F, Chemat F. Green procedure with a green solvent for fats and oils’ determination microwave-integrated Soxhlet using limonene followed by microwave Clevenger distillation. J Chromatogr A. 2008;1196–1197:147–152. doi: 10.1016/j.chroma.2008.04.035. [DOI] [PubMed] [Google Scholar]
- 10.Kumar RR, Rao PH, Arumugam M. Lipid extraction methods from microalgae: a comprehensive review. Front Energy Res. 2015 [Google Scholar]
- 11.Jessop PG, Stanley RR, Brown RA, Eckert CA, Liotta CL, Ngob TT, Pollet Pamela. Neoteric solvents for asymmetric hydrogenation: supercritical fluids, ionic liquids, and expanded ionic liquids. Green Chem. 2003;5:123–128. doi: 10.1039/b211894g. [DOI] [Google Scholar]
- 12.Swapnil DA. Ionic liquids (a review): the green solvents for petroleum and hydrocarbon industries. Res J Chem Sci. 2012;2(8):80–85. [Google Scholar]
- 13.Cooney M, Young G, Nagle N. Extraction of bio-oils from microalgae. Sep Purif Rev. 2009;38:291–325. doi: 10.1080/15422110903327919. [DOI] [Google Scholar]
- 14.Carmichael AJ, Haddlettn DM, Bon SAF, Seddon KR. Selective sulfur removal from fuels using ionic liquids at room temperature. Chem Commun. 2000;4:1237–1238. doi: 10.1039/b003335i. [DOI] [Google Scholar]
- 15.Salem ASH, Hamid HS. Removal of sulfur compounds from naphtha solutions using solid adsorbents. Chem Eng Technol. 1997;20:342. doi: 10.1002/ceat.270200511. [DOI] [Google Scholar]
- 16.Ma C, Liu T, Yang L, Zu Y, Chen X, Zhang L, Zhang Y, Zhao C. Ionic liquid-based microwave-assisted extraction of essential oil and biphenyl cyclooctene lignans from Schisandra chinensis Baill fruits. J Chromatogr A. 2011;1218:8573–8580. doi: 10.1016/j.chroma.2011.09.075. [DOI] [PubMed] [Google Scholar]
- 17.Cooney MJ, Young GY (2009) Methods and compositions for extraction and transesterification of biomass components. In Patent Application Full Text and Image Database 2009: U.S.P.T. Office, Ed. University of Hawaii: United States
- 18.Heckenbach ME, Romero FN, Green MD, Halden RU. Meta-analysis of ionic liquid literature and toxicology. Chemosphere. 2016;150:266–274. doi: 10.1016/j.chemosphere.2016.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phan L, Brown H, White J, Hodgsonc A, Jessop PG. Soybean oil extraction and separation using switchable or expanded solvents. Green Chem. 2009;11:53–59. doi: 10.1039/B810423A. [DOI] [Google Scholar]
- 20.Jeevan Kumar SP, Kumar GV, Dash A, Scholz P, Banerjee R. Sustainable green solvents and techniques for lipid extraction from microalgae: a review. Algal Res. 2017;21:138–147. doi: 10.1016/j.algal.2016.11.014. [DOI] [Google Scholar]
- 21.Li Y, Fine F, Fabiano-Tixier A, Abert-Vian M, Carre P, Pages X, Chemat F. Evaluation of alternative solvents for improvement of oil extraction from rapeseeds. C R Chim. 2014;17:242–251. doi: 10.1016/j.crci.2013.09.002. [DOI] [Google Scholar]
- 22.Olivier B, Elisabeth B. Extraction from oleaginous seeds using supercritical CO2: experimental design and products quality. J Food Eng. 2009;92(4):396–402. doi: 10.1016/j.jfoodeng.2008.12.007. [DOI] [Google Scholar]
- 23.Latif S, Anwar F, Hussain AI, Shahid M. Aqueous enzymatic process for oil and protein extraction from Moringa oleifera seed. Eur J Lipid Sci Technol. 2011;113:1012–1018. doi: 10.1002/ejlt.201000525. [DOI] [Google Scholar]
- 24.Chabrand RM, Glatz CE. Destabilization of the emulsion formed during the enzyme-assisted aqueous extraction of oil from soybean flour. Enzyme Microb Technol. 2009;45:28–35. doi: 10.1016/j.enzmictec.2009.03.008. [DOI] [Google Scholar]
- 25.Yang L, Jiang L, Sui X, Wang S. Optimization of the aqueous enzymatic extraction of pie kernel oil by response surface methodology. Procedia Eng. 2011;15:4641–4652. doi: 10.1016/j.proeng.2011.08.872. [DOI] [Google Scholar]
- 26.Wu J, Johnson LA, Jung S. Demulsification of oil-rich emulsion from enzyme-assisted aqueous extraction of extruded soybean flakes. Bioresour Technol. 2009;100:527–533. doi: 10.1016/j.biortech.2008.05.057. [DOI] [PubMed] [Google Scholar]
- 27.Rosenthal A, Pyle DL, Niranjan K, Gilmour S, Trinca L. Combined effect of operational variables and enzyme activity on aqueous enzymatic extraction of oil & protein from soybean. Enzyme Microb Technol. 2001;28:499–509. doi: 10.1016/S0141-0229(00)00351-3. [DOI] [PubMed] [Google Scholar]
- 28.Jung S, Maurer D, Johnson LA. Factors affecting emulsion stability and quality of oil recovered from enzyme assisted aqueous extraction of soybeans. Bioresour Technol. 2009;100:5340–5347. doi: 10.1016/j.biortech.2009.03.087. [DOI] [PubMed] [Google Scholar]
- 29.Lamsal BP, Murphy PA, Johnson LA. Flaking and extrusion as mechanical treatments for enzyme-assisted aqueous extraction of oil from soybeans. J Am Oil Chem Soc. 2006;83(11):973–979. doi: 10.1007/s11746-006-5055-5. [DOI] [Google Scholar]
- 30.Zhang SB, Wang Z, Xu SY. Optimization of the aqueous enzymatic extraction of rapeseed oil and protein hydrolysates. J Am Oil Chem Soc. 2007;84:97–105. doi: 10.1007/s11746-006-1004-6. [DOI] [Google Scholar]
- 31.Passos CP, Yilmaz S, Silva CM, Coimbra MA. Enhancement of grape seed oil extraction using a cell wall degrading enzyme cocktail. Food Chem. 2009;115:48–53. doi: 10.1016/j.foodchem.2008.11.064. [DOI] [Google Scholar]
- 32.Duarte Costa HMP, Sameiro MEM. Quality of olive oil extracted using enzyme preparation. Bol Inst do Azeite Prod Oleaginose. 1978;6:25–38. [Google Scholar]
- 33.Rovaris AA, Dias CO, da Cunha IP, Scaff RMC, de Francisco A, Petkowicz CLO. Chemical composition of solid waste & effect of enzymatic oil extraction on the microstructure of soybean (Glycine max) Ind Crop Prod. 2012;36:405–414. doi: 10.1016/j.indcrop.2011.10.001. [DOI] [Google Scholar]
- 34.Kumar SPJ, Banerjee R. Optimization of lipid enriched biomass production from oleaginous fungus using response surface methodology. Indian J Exp Biol. 2013;51:979–983. [PubMed] [Google Scholar]
- 35.Cater CM, Rhee KC, Hagenrnaier RO, Mattil KF. Aqueous extraction—an alternative oilseed milling process. J Am Oil Chem Soc. 1974;51(4):137. doi: 10.1007/BF02639723. [DOI] [Google Scholar]
- 36.Hagenrnaier R. Aqueous processing of full-fat sunflower seeds: yields of oil and protein. J Am Oil Chem Soc. 1974;51(10):470. doi: 10.1007/BF02635157. [DOI] [Google Scholar]
- 37.Lanzani A, Petrini MC, Cozzoli O, Gallavresi P, Carola C, Jacini G. On the use of enzymes for vegetable-oil extraction, a preliminary report. Riv Ital Sostanze Grasse. 1975;11:226–229. [Google Scholar]
- 38.Lawhon JT, Manak LJ, Rhee KC, Lusas EW. Production of oil and protein food products from raw peanuts by aqueous extraction and ultrafiltration. J Food Sci. 1981;46:391–395. doi: 10.1111/j.1365-2621.1981.tb04868.x. [DOI] [Google Scholar]
- 39.Rhee KC, Cater CM, Mattil KF. Simultaneous recovery of protein and oil from raw peanuts in an aqueous system. J Food Sci. 1972;37:90–93. doi: 10.1111/j.1365-2621.1972.tb03393.x. [DOI] [Google Scholar]
- 40.Zuniga ME, Soto C, Mora A. Enzymic pre-treatment of Guevina avellana mol oil extraction by pressing. Process Biochem. 2003;39:51–57. doi: 10.1016/S0032-9592(02)00286-8. [DOI] [Google Scholar]
- 41.Sharma A, Khare SK, Gupta MN. Enzyme-assisted aqueous extraction of peanut oil. J Am Oil Chem Soc. 2003;79(3):215–218. doi: 10.1007/s11746-002-0463-0. [DOI] [Google Scholar]
- 42.Teixeira CB, Macedo GA, Macedo JA, da Silva LHM, Rodrigues AMC. Simultaneous extraction of oil and antioxidant compounds from oil palm fruit (Elaeis guineensis) by an aqueous enzymatic process. Bioresour Technol. 2013;129:575–581. doi: 10.1016/j.biortech.2012.11.057. [DOI] [PubMed] [Google Scholar]
- 43.Jiang L, Hua D, Wang Z, Xu S. Aqueous enzymatic extraction of peanut oil and protein hydrolysates. Food Bioprod Process. 2010;88:233–238. doi: 10.1016/j.fbp.2009.08.002. [DOI] [Google Scholar]
- 44.Sean XL, Mamidipally PK. Quality comparison of rice bran oil extracted with d-limonene and hexane. Cereal Chem. 2005;82(2):209–215. doi: 10.1094/CC-82-0209. [DOI] [Google Scholar]
- 45.Long JJ, Fu YJ, Zu YG, Li J, Wang W, Gu CB. Ultrasound-assisted extraction of flaxseed oil using immobilized enzymes. Bioresour Technol. 2011;102:9991–9996. doi: 10.1016/j.biortech.2011.07.104. [DOI] [PubMed] [Google Scholar]
- 46.Yusoff MM, Gordon MH, Niranjan K. Aqueous enzyme assisted oil extraction from oilseeds and emulsion de-emulsifying methods: a review. Trends Food Sci Technol. 2015;41:60–82. doi: 10.1016/j.tifs.2014.09.003. [DOI] [Google Scholar]
- 47.Dalal S, Raghava S, Gupta MN. Single-step purification of recombinant green fluorescent protein on expanded beds of immobilized metal affinity chromatography media. Biochem Eng J. 2008;42(3):301–307. doi: 10.1016/j.bej.2008.07.010. [DOI] [Google Scholar]
- 48.Suck K, Walter J, Menzel F, Tappe A, Kasper C, Naumann C, Zeidler R, Scheper T. Fast and efficient protein purification using membrane adsorber systems. J Biotechnol. 2006;121:361–367. doi: 10.1016/j.jbiotec.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 49.Hilbrig F, Freitag R. Protein purification by affinity precipitation. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;790(1–2):79–90. doi: 10.1016/S1570-0232(03)00081-3. [DOI] [PubMed] [Google Scholar]
- 50.Lamsal BP, Johnson LA. Separating oil from aqueous extraction fractions of soybean. J Am Oil Chem Soc. 2007;84:785–792. doi: 10.1007/s11746-007-1090-0. [DOI] [Google Scholar]
- 51.Sineiro J, Domínguez H, Núñez MJ, Lema JM. Ethanolic extraction of sunflower oil in a pulsing extractor. J Am Oil Chem Soc. 1998;75:753–754. doi: 10.1007/s11746-998-0220-7. [DOI] [Google Scholar]
- 52.Abdulkarim SM, Lai OM, Muhammad SKS, Long K, Ghazali HM. Use of enzymes to enhance oil recovery during aqueous extraction of Moringa oleifera seed oil. J Food Lipids. 2006;13:113–130. doi: 10.1111/j.1745-4522.2006.00038.x. [DOI] [Google Scholar]
- 53.Nyam KL, Tan CP, Lai OM, Long K, Che Man YB. Physicochemical properties and bioactive compounds of selected seed oils. LWT Food Sci Technol. 2009;42:1396–1403. doi: 10.1016/j.lwt.2009.03.006. [DOI] [Google Scholar]
- 54.Roy I, Sharma S, Gupta MN. Smart biocatalysts: design and applications. In: Scheper T, editor. Biochem Eng J. Berlin: Springer; 2004. pp. 159–189. [DOI] [PubMed] [Google Scholar]
- 55.Soto C, Concha J, Zuniga ME. Antioxidant content of oil and defatted meal obtained from borage seeds by an enzymatic-aided cold pressing process. Process Biochem. 2008;43(6):696–699. doi: 10.1016/j.procbio.2008.02.006. [DOI] [Google Scholar]
- 56.Latif S, Diosady LL, Anwar F. Enzyme-assisted aqueous extraction of oil and protein from canola (Brassica napus L.) seeds. Eur J Lipid Sci Technol. 2008;110:887–892. doi: 10.1002/ejlt.200700319. [DOI] [Google Scholar]
- 57.Virot M, Tomao V, Ginies C, Visinoni F, Chemat F. Microwave-integrated extraction of total fats and oils. J Chromatogr A. 2008;4(1196–1197):57–64. doi: 10.1016/j.chroma.2008.05.023. [DOI] [PubMed] [Google Scholar]


