Abstract
Background
Budd-Chiari syndrome (BCS) is a rare disease resulting from obstruction of the hepatic venous outflow tract that typically presents with abdominal pain, jaundice and ascites without frank liver failure. However, BCS may also evolve more rapidly to acute liver failure (ALF-BCS).
Aims
To describe the clinical features, treatment and outcomes of ALF due to BCS and compare our results with those in the published literature.
Methods
Twenty of the 2,300 patients enrolled in the Acute Liver Failure Study Group (ALFSG) registry since 1998, presented with a clinical diagnosis of BCS. An additional 19 cases of ALF-BCS in the English-language literature were reviewed and compared to the ALFSG cases.
Results
Most ALF-BCS patients were white (84%) and female (84%) in their fourth decade. A hypercoagulable state was noted in 63% of cases. BCS was diagnosed by Doppler ultrasonography or abdominal CT in all cases. Liver biopsies (6) all had evidence of severe peri-central necrosis. Treatments used included most commonly anticoagulation (71%), but also transjugular intrahepatic portosystemic shunt (TIPS) (37%) and orthotopic liver transplantation (37%). In-hospital mortality was approximately 60%.
Conclusions
Budd-Chiari syndrome is a rare cause of ALF and mandates prompt diagnosis and management for successful outcomes. Once the diagnosis is confirmed, prompt anticoagulation is recommended in conjunction with evaluation for malignancy or thrombophilic disorder. Mortality may have improved in recent years with use of TIPS and/or OLT compared to prior published reports.
INTRODUCTION
Budd-Chiari Syndrome (BCS) is a rare clinical condition resulting from obstruction of the hepatic venous outflow tract anywhere from the hepatic venules to the right atrium, including the small and large hepatic veins (HVs) and the inferior vena cava (IVC) (1). Most cases of BCS in the Western world result from venous thrombosis that leads to diffuse obliteration of the veins, while in Asia the most common cause is IVC and/or HV obstruction due to membranous webs (2). Clinically, most patients have evidence of portosystemic collaterals with an enlarged caudate lobe at presentation due to incomplete occlusion of the 3 main hepatic veins. Most Western BCS patients have evidence of one or more underlying hypercoagulable states, most commonly polycythemia vera (PCV) (3).
Acute liver failure (ALF) is traditionally defined as coagulopathy and altered mental status occurring within 26 weeks of onset, although most cases evolve over 1–3 weeks. Most cases of BCS present in sub-acute fashion, that is, over several weeks, not days due to partial occlusion of one or more of the hepatic veins with increased return of intrahepatic blood via small perforating veins in the caudate lobe that drain directly into the vena cava or portosystemic collaterals that divert portal flow away from the liver. (1). When the 3 main hepatic veins draining the liver simultaneously clot, there can be diffuse and severe intrahepatic ischemia with massive necrosis and resultant ALF and inadequate time for collaterals to develop. ALF is the rarest presentation of BCS, and BCS is one of the rarest etiologies of ALF (4). BCS, in all of its presentations, is an orphan disease, with only a handful of clinical trials dedicated to its study (5). The pathophysiology of ALF due to BCS likely differs from that of many other sub-acute causes of ALF, in that it has a very high mortality rate and relatively rapid clinical course; necessitating urgent determination and management of the underlying cause.
Historically, there have been multiple management options for BCS, including anticoagulation, thrombolysis, angioplasty, transjugular intrahepatic portosystemic shunt (TIPS), surgical portosystemic shunt, and orthotopic liver transplantation. For these reasons, it is worth seeking patterns in the clinical presentation and outcomes of ALF-BCS patients. However, ALF due to BCS (ALF-BCS) is very rare and has mainly been described in case reports. Although series of BCS patients have been published, nearly all of them studied all levels of severity of BCS together, without clearly defining the level of acuity or severity (6–8). The aim of this study is to describe the presenting laboratory and clinical features of 19 consecutive adults with ALF-BCS that were enrolled in the U.S. Acute Liver Failure Study Group (ALFSG) over a 15 year period. We reviewed the presenting features, management and short and long-term outcomes and compared them with the previously reported cases.
PATIENTS AND METHODS
Patients
To date, more than 2,344 patients have been enrolled in the ALFSG registry from 1998 to 2015, all meeting standard criteria of hepatic encephalopathy and coagulopathy (international normalized ratio (INR) ≥ 1.5) after an acute illness. Per institutional review board guidelines, informed consent was obtained from next of kin prior to enrollment since all patients are, by definition, encephalopathic at the time of enrollment. Demographics, history, ALF etiology (based on diagnostic criteria utilized at all participating sites), physical findings, lab values, imaging and biopsy reports, and hospital course are recorded for each patient at the time of admission to the study. Also recorded are outcome data (death, liver transplantation, or survival) 3 weeks after admission to the study and, if available, at 1 and 2 years following admission.
Literature Review
We searched for English-language case reports and series specifically devoted to BCS leading to ALF in the Medline database from January 1998 to December 2015 using various combinations of one or more of the following text keywords: “fulminant,” “Budd-Chiari,” and “acute liver failure.” The titles and (where available) abstracts of all search results were read. When a title or abstract suggested that the corresponding article described one or more ALF-BCS patients, the entire article was then read. Articles that were referenced within the main text of these articles as describing ALF-BCS patients were also reviewed. Only articles describing one or more ALF-BCS patients for whom the text explicitly made clear that our full definition of ALF was met were included in our analysis. Finally, we ensured that none of the published cases we found were also among the ones we found in the ALFSG database.
Analysis
Data are reported as medians and ranges. Comparisons between continuous variables were performed with Wilcoxon rank sum test and categorical variables were analyzed with the chi-squared test. All tests were two sided and p-values of less than 0.05 were considered statistically significant. Stata/SE 14.0 (StataCorp, College Station, TX) was used to perform all analyses.
RESULTS
Clinical Characteristics
From January 1, 1998 to December 1, 2015, 20 cases out of 2,344 enrolled in the ALFSG registry during the study period were determined to have ALF secondary to BCS. From these 20, we selected 19 that were considered truly acute. The excluded patient had hepatic vein obstruction from a metastatic adenocarcinoma that evolved over a prolonged period of time. Overall, the patients were young (median age 38; range: 19–59) and comprised of mostly Caucasian (84%) women (84%). Polycythemia vera was the most commonly identified thrombophilic factor (37%) and three patients were presumed to be hypercoagulable based on estrogen use and history of prior clots. One patient had a confirmed homozygous Factor V Leiden mutation (Table 1).
Table 1.
Patient Characteristics of Patients with ALF due to BCS and a Comparison of Survivors and non-Survivors
| Characteristic | Overall | Survivors | Non-Survivors | p-value |
|---|---|---|---|---|
| Age | 38 (19–59) | 44 (26–59) | 35 (19–55) | 0.43 |
| Caucasian | 16 (84%) | 6 (75%) | 10(91%) | 0.46 |
| Female | 16 (84%) | 10 (91%) | 6 (75%) | 0.35 |
| Patients with PCV | 7 (37%) | 5(63%) | 2(18%) | 0.06 |
| Encephalopathy onset day |
2 (−1 – 19) | 6 (1–19) | 2(−1–5) | 0.07 |
| WBC (1000/mm3) | 31 (13–104) | 28.3 (13–104) | 32 (16.4 – 80.7) | 0.32 |
| Total bilirubin peak (mg/dL) |
13.8 (3.3 – 29.5) | 7.9 (5.2–29.5) | 16 (3.3–22) | 0.56 |
| AST peak (IU/L) | 3705.5 (214–11865) | 1842 (214–3811) | 5255 (1211–11856) | 0.01 |
| ALT peak (IU/L) | 2937.5 (310–6230) | 1335 (310–3400) | 4317 (708–6230) | 0.05 |
| INR peak | 5.5 (3–18.6) | 4.9 (3.1–12) | 6 (3.0–18.6) | 0.59 |
| Cr peak (mg/dL) | 3 (1.1–15.8) | 2.6 (1.07–4.8) | 4 (1.6–15.8) | 0.04 |
| MELD score | 44 (29–63) | 44.5 (29–52) | 44 (35–63) | 0.96 |
| Kings College Criteria Met |
8/17 (47%) | 3/7 (43%) | 5/11 (45%) | 0.91 |
| Anticoagulated on admission |
12/17 (71%) | 7/7 (100%) | 5/12 (42%) | 0.03 |
| Received Liver Transplant |
7 (37%) | 4 (50%) | 3 (27%) | 0.31 |
| TIPS Alone | 7 (37%) | 3 (38%) | 4 (36%) | 0.91 |
| Year of Transplant | 2005 (1999–2015) | 2010 | 2002.5 | 0.04 |
All values are either number (%) or medians (range). PCV = polycythemia vera. WBC = White blood cell. Total serum bilirubin. INR = international normalized ratio. Cr = Serum creatinine. MELD = Model for End Stage Liver Disease
Diagnostic Studies
All patients underwent abdominal ultrasound (US) with Doppler analysis as part of their initial evaluation. Computed tomography (CT) or magnetic resonance imaging (MRI) were often used later in the hospital course to better define anatomy and/or extent of the clot burden. Notable findings on US or CT included presence of ascites, hepatomegaly, heterogeneous (or mottled on CT) hepatic parenchyma, caudate lobe hypertrophy, thickened gallbladder wall and splenomegaly. Doppler US findings included IVC compression, no flow in or non-visualization of the hepatic veins, and slow, hepatofugal or bidirectional flow in the portal or splenic veins. CT scans showed non-opacification of all of the hepatic veins and/or IVC compression at the level of the caudate lobe. MRI findings were analogous to those on CT. There were no patients who underwent venography without already having had evidence of venous occlusion on US, CT, or MRI. There were no venous collaterals seen by any modality. Likewise, there were no findings of diffuse liver atrophy or hepatic regenerative nodules as can be seen in chronic BCS patients with longstanding occlusion of one or more hepatic veins. For the most part, routine diagnostic studies failed to reveal a cause for the BCS such as an unsuspected intrahepatic tumor or disease of the vena cava such as a membranous web or congenital anomalies. Of the 19, 12 patients underwent formal work-up for coagulation abnormalities during their ALF hospitalization. Of interest, the AST:ALT ratio in this population exceeded 1 in 18/19 (~95%) of cases. Liver biopsy was used sparingly in this population: 6 patients underwent biopsy, all showing severe centri-lobular congestion and hepatocyte necrosis, without significant fibrosis.
Treatment and Outcomes
A summary of each case is provided in Table 2. Overall, anticoagulation was the first line of therapy with 71% of patients being treated on or shortly after admission with IV heparin. Patients who were anticoagulated were more likely to survive than those who were not. In fact, 100% of survivors were anticoagulated on or shortly after admission compared to 50% of patients who died (Table 1). Furthermore, complications of bleeding were uncommon in this population despite usage of anticoagulation. Only two clinically relevant episodes were captured; one associated with TIPS and the other with placement of an intracranial pressure monitor.
Table 2.
Course of Patients with Acute Liver Failure from Budd Chiari
| Patient | Year | Age | Diagnosis | Peak AST (U/L) |
Peak INR |
Peak Cr (mg/dL) |
Treatment Course | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | 1999 | 30 | OCPs/Hypercoaguable | 9960 | 3.08 | 6.4 | -Angioplasty/TIPS -No anticoagulation |
Died of cerebral edema, HD 5 |
| 2 | 2000 | 49 | Myelodyplastic Syndrome |
3840 | 3.1 | 3.0 | -Surgical shunt then transplant -No anticoagulation |
Died after transplant from primary non-function |
| 3 | 2000 | 19 | OCPs/Hypercoaguable | 6352 | 7.1 | 1.6 | -Anticoagulation -TIPS Day 3, transplant Day 6 -Suffered hepatic artery thrombosis and then re-transplanted |
Died HD 40 |
| 4 | 2000 | 42 | PCV | 214 | 1.2 | -Anticoagulation and TIPS | Survived | |
| 5 | 2001 | 53 | PCV | 5662 | 6.8 | 3.6 | -Transplant day 7 -No anticoagulation |
Died POD 11 |
| 6 | 2002 | 26 | PCV | 3811 | 12 | 2.6 | -TIPS (timing of anticoagulation unclear from chart) |
Survived |
| 7 | 2002 | 34 | PCV | 8786 | 18.6 | 4.8 | -Surgical shunt -No anticoagulation |
Died HD 21 |
| 8 | 2003 | 32 | PNH | 2471 | 3.45 | 4.4 | -TPA/anticoagulation -TIPS day 11 |
Died 4.5 months after admission |
| 9 | 2005 | 55 | CML | 1211 | 3.1 | 3.3 | -Anticoagulation and supportive care |
Died HD 28 |
| 10 | 2005 | 46 | F V Leiden | 4.8 | -Anticoagulation then transplant | Survived | ||
| 11 | 2006 | 21 | ? | 5255 | 3 | 1.9 | -Anticoagulation then TIPS | Bled after TIPS. Died day HD 11 |
| 12 | 2007 | 38 | ? | 1867 | 9 | 15.8 | -Thrombolysis | Died from cerebral edema |
| 13 | 2009 | 35 | Thrombocythemia | 5005 | 6 | 7.15 | -Anticoagulation and TIPS | Died HD 13 from intracerebral hemorrhage related to intracranial pressure monitor |
| 14 | 2010 | 47 | ? | 1842 | 3.1 | 3 | -Anticoagulation and transplant(day 5) |
Survived |
| 15 | 2010 | 59 | PCV | 922 | 7.1 | 1.89 | -Anticoagulation then transplant (day 11) |
Survived |
| 16 | 2011 | 28 | ? | 1038 | 3.9 | 1.07 | -Anticoagulation the TIPS | Survived |
| 17 | 2015 | 50 | PCV | 3600 | 8.2 | 2.63 | -Anticoagulation then transplant | Survived |
| 18 | 2015 | 49 | ? | 11856 | 6.6 | -Supportive care only, “too sick” | Died HD 2 | |
| 19 | 2015 | 35 | PCV | 2482 | 4.9 | 1.8 | Anticoagulation alone | Survived |
Various interventions were also employed in the management of BCS with varying success. The two patients who underwent surgical shunts both died. The one patient who underwent thrombolysis/angioplasty alone also died. The remaining cases of thrombolysis/angioplasty were done along with or followed by TIPS that was employed in eight patients. Seven patients underwent liver transplant; with two patient undergoing both TIPS and transplant. Patients were not offered transplant for the following reasons: considered too ill, improvement after TIPS or anticoagulation, history of medical non-adherence or malignancy.
Overall, 8 (42%) patients survived their inpatient hospitalization. All 8 were alive at the time of follow-up; median 4 months, range 1 to 94 months. Of all lab parameters examined, elevated peak AST, peak ALT and peak creatinine were associated with patient death. As previously mentioned, anticoagulation at the time of admission was a statistically significant predictor of survival. In comparing treatment modalities, there was no discernible survival benefit to TIPS or transplant in this small population. However, the majority of the survivors have been in the more modern era, with 7/8 survivors identified during the post-MELD era and 5 of those being enrolled after 2010. Though limited by the size of the patient population, year of transplant was also a statistically significant predictor of survival (Table 1).
Literature Review
We found 19 ALF-BCS cases published among 17 case reports/series that considered all forms of BCS (10–25). No case series specifically focused on ALF-BCS and all were published prior to 2007. The characteristics of the 19 patients from the literature are presented in Table 3.
Table 3.
Overall patient characteristics of 17 previously reported ALF-BCS cases in the literature.
| Characteristic | |
|---|---|
| Age | 38 (20–77) |
| Female | 9 (47%) |
| Oral contraceptive use | 2/14 (14%) |
| Patients with PCV | 7/17 (41%) |
| Symptom onset day | −7 (−30 – −1) |
| Signs and symptoms on hospital day 1 | |
| Abdominal pain | 16/16 (100%) |
| Ascites | 17/18 (94%) |
| Jaundice | 10/10 (100%) |
| Encephalopathy onset day | 2 (1–25) |
| Admission laboratory values | |
| WBC count (1000/mm3) | 19 (8.1–36) |
| TBili (mg/dL) | 2.8 (0.9–23) |
| AST (IU/L) | 557 (73–8686) |
| ALT (IU/L) | 512 (80–3185) |
| Serum albumin (g/dL) | 2.9 (2.5–3.3) |
| INR | 2.2 (1.6–4.8) |
| Cr (mg/dL) | 3 (0.5–11.5) |
| AST:ALT ratio > 1 | 7/12 (58%) |
| Anticoagulated at admission | 8/11 (73%) |
| Received liver transplant | 5 (26%) |
| Day of liver transplant | 28 (14–30) |
| Survival at 3 weeks | 11/19 (58%) |
| Survival during initial hospital stay | 7/19 (37%) |
| Survivors’ length of initial hospital stay (days) | 33 |
| Spontaneous survivors (without LT) at 3 weeks | 10/19 (53%) |
| Spontaneous survivors of initial hospitalization | 6/19 (32%) |
All values are either number (%) or medians (range). PCV = polycythemia vera. Hct = Serum hematocrit. WBC = White blood cell. TBili = Total serum bilirubin. INR = international normalized ratio. Cr = Serum creatinine.
The most common etiology determined in published series was PCV (41% of cases). As with the ALFSG cases, the published cases variably featured imaging with US, CT, MRI, and VEN. Findings were analogous to those described for the ALFSG cases, with the exception that two of the patients (both with PCV) had venous collaterals around the thrombi visualized on MRI, CT, or venography.
Table 3 lists features of the clinical presentations, treatments and outcomes of the patients from the literature, categorized by survival. None of the articles defined a three-week study period, but we deduced the outcomes at this time point in order to compare them to the ALFSG cases. The results were analogous to those for ALFSG cases. Six of the 19 patients received only supportive treatment with anticoagulation. Only two of these six survived the initial hospital stay and were alive at two months and one year, respectively. Two of 19 received supportive treatment alone, without anticoagulation; and three received supportive treatment, but with unknown anticoagulation status. All five of the latter died during the initial hospital stay. Only one patient each received thrombolysis (death on day 18 from gastrointestinal hemorrhage), angioplasty (death on day 11 from undocumented cause), and surgical shunts (in addition to TIPS; death on day 17 from MOF). Three of the four TIPS patients (75%) survived both the three-week study period and their initial hospital stays, and these three patients were also the only three published cases who received both TIPS and liver transplant. All five of the liver transplant patients (including the three who also received TIPS prior to liver transplant) survived at least a median of 3.5 (range 1–8) months. Complications were generally similar to the ones noted for the ALFSG cases, the most common being acute renal failure (6 patients; 35%).
Among the 19 literature cases reviewed, 8 (42%) died by the end of three weeks, 12 (63%) succumbed during their initial hospital stay, and 5 of the 17 patients (29%) for whom follow-up data were available survived at least a median of 4 (range 2–12) months. The most common cause of death among the seven patients whose causes of death were specified was multi-organ failure (43%).
DISCUSSION
Using the ALFSG study cohort, we have assembled the largest series of ALF from BCS reported in the literature. Overall, acute liver failure from BCS is an extremely rare condition; accounting for less than 1% of cases in the ALFSG registry. Even physicians at tertiary transplant centers are likely to see this once in their careers, making the lessons of this case series particularly valuable.
The ALFSG patients had similar demographics and etiologies when compared to previously published cases of ALF-BCS and to BCS patients in general (6, 7). Most were white females in their fourth to fifth decades, and the most common etiology was PCV. Most of the ALF-BCS patients in our series had one or more thrombophilic risk factors, as has been observed elsewhere for BCS in general.
The most common presenting symptoms and signs (abdominal pain, ascites, jaundice, lower-extremity edema, and malaise) in both the ALFSG and published cases were non-specific, although the presence of pain and ascites are atypical for most forms of ALF. Regardless of presentation, a patient presenting with ALF should undergo ultrasound examination with Doppler imaging. Ultrasound has become the mainstay of diagnosis and a positive sonogram should be adequate to initiate anticoagulation. Confirmatory studies such as CT or MRI provide additional imaging which may help the surgeon or interventional radiologist develop their treatment plan. Interestingly, lab values may provide an early clue to the diagnosis of ALF from BCS as the majority of these patients had an AST:ALT ratio greater than 1. In fact, this pattern of injury, which is less common in ALF cases due to acetaminophen toxicity and viral hepatitis (26, 27), was found in 95% of patients in this series. The peak AST and ALT were also some of the statistically significant predictors of survival in our series, providing a clear marker of the severity and/or acuity of the event. This is consistent with Rautou et al, who also found that peak ALT was associated with poor outcomes; supporting the idea that greater ischemia portends worse patient outcomes (28). Higher peak creatinine level was also observed to be a significant predictor of poor outcome. However, total bilirubin and peak INR were not. In fact, both MELD score and the Kings College criteria for non-acetaminophen liver failure were not predictive of survival in these patients. This may be due to the fact the patients are uniformly ill but early anticoagulation and TIPS may offer therapeutic benefit which are not available to other etiologies of acute liver failure. Therefore, these traditional scoring systems may not serve as an accurate predictor of survival and clinicians should focus on encephalopathy and overall clinical trends when deciding if a patient is improving.
The survival of ALF-BCS patients has historically been poor. During the initial hospital stay, the survival of both ALFSG and previously published cases was between 37–40%, even with the availability of liver transplantation. These survival rates are much lower than the five-year survival rates of BCS cases in general, which has been as high as 80% in recent cohorts, but is similar to other subacute presentations of ALF (3). Closer examination reveals a more positive outlook when you focus on the modern era. With improved surgical techniques, critical care, widely available high quality ultrasound, and recognition that anticoagulation is safe and even required in these patients; survival seems to have improved. Though our case series is too small to draw definitive conclusions, the majority of survivors occurred after 2010 and year of transplant was a statistically significant predictor of survival on univariate analysis.
Because of its rarity, ALF-BCS has seldom been examined carefully as a distinct entity. In the last decade, a stepwise algorithm, in order of increasing invasiveness, has emerged for BCS patients in general (30–33). Based on our review of this case series and the literature, we have proposed a similar algorithm for patients with ALF due to BCS (Figure 1). Despite the small sample size, there are some clear lessons that can be taken away from the present case series. Anticoagulation should be initiated as soon as the diagnosis of BCS is made. Surgical shunts have largely been replaced by TIPS (32, 34). TIPS/angioplasty can be pursued early on while the underlying cause of BCS is being determined. This is consistent with Garcia-Pagan who found TIPS to be an effective treatment for BCS in a large series which included 9 patients with acute liver failure (8).
Figure 1.
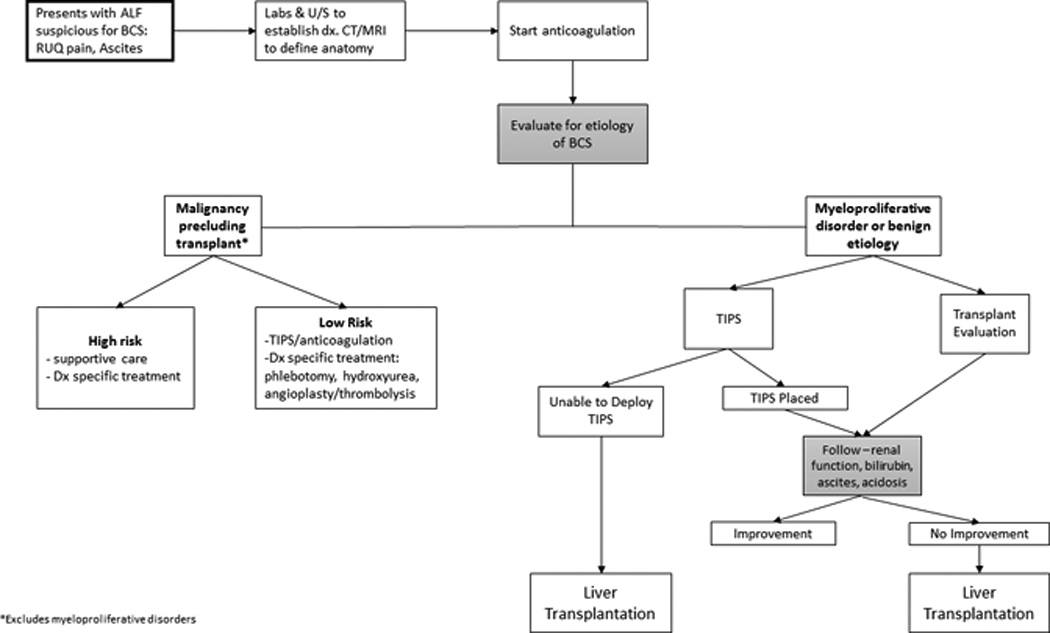
Proposed Algorithm for Management of Patients with Acute Liver Failure from Budd Chiari Syndrome
Should malignancy that precludes transplant be found, then care appropriate to the patient’s condition and prognosis can be implemented. A diagnosis of PCV or any myeloproliferative disorder does not preclude listing for transplantation and in these patients and evaluation of transplant candidacy should occur in parallel with anticoagulation and TIPS. Early initiation of anticoagulation and TIPS may then provide clinicians with a window of several days during which time assessment for clinical improvement can be made while the decision to list/transplant is undertaken. The INR may not be reliable depending on the method of anticoagulation; notably, both argatroban and coumadin affect prothrombin time, making it difficult to judge improvement in hepatic function based on the INR value. Should the patient improve, transplant can be avoided. However, if no improvement is seen transplant remains a salvage option. Similarly, if TIPS cannot be performed due to technical limitations, then liver transplant is an option if there are no other medical or social barriers. Regardless of intervention, anticoagulation should be continued as long as the patient does no suffer complications of bleeding that preclude ongoing therapy.
In conclusion, BCS is a very rare cause of ALF. Early anticoagulation followed by TIPS and/or liver transplant for ALF-BCS are likely the main modalities to be employed in the management of Western patients with this disease. Despite these technical advances, ALF-BCS still carries a very high mortality; we hope that our description of ALF-BCS will inspire larger, prospective studies to benefit these critically ill patients.
Acknowledgments
We gratefully recognize the assistance of Corron Sanders, PhD, and the staff at UT Southwestern and the site investigators and coordinators who contribute so much to this study. We also recognize the patients and families who allow us to gather these data.
Financial Support
ALFSG is supported by a cooperative research agreement from the National Institute of Diabetes, Digestive and Kidney Diseases, grant number U-01-58369. Additional support from the Tips Family Fund at the Northwestern Foundation and the Jeanne Roberts Fund from the Southwestern Foundation.
ABREVIATIONS
- BCS
Budd Chiari Syndrome
- ALF
Acute Liver Failure
- ALFSG
Acute Liver Failure Study Group
- TIPS
Transjugular intrahepatic portosystemic shunt
- IVC
Inferior Vena Cava
- PCV
Polycythemia vera
- US
Ultrasound
- CT
Computed tomography
- MRI
Magnetic resonance imaging
Footnotes
Conflicts of Interest – the authors have no conflicts of interest to disclose
Contributor Information
Justin Parekh, Department of Surgery, UT Southwestern Medical Center.
Vlad M Matei, Department of Ophthalmology, UT Southwestern Medical Center.
Alejandro Canas-Coto, Gastroenterology Division HSJD, University of Costa Rica.
Daniel Friedman, Texas Digestive Disease Consultants.
William M. Lee, Digestive and Liver Diseases Division, UT Southwestern Medical Center
REFERENCES
- 1.Menon KN, Shah V, Kamath PS. The Budd-Chiari syndrome. N Engl J Med. 2004;350:578–585. doi: 10.1056/NEJMra020282. [DOI] [PubMed] [Google Scholar]
- 2.Wang R, Meng Q, Qu L, et al. Treatment of Budd-Chiari syndrome with inferior vena cava thrombosis. Exp Ther Med. 2013;5:1254–1258. doi: 10.3892/etm.2013.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Plessier A, Valla DC. Budd-Chiari syndrome. Semin Liver Dis. 2008;28:259–269. doi: 10.1055/s-0028-1085094. [DOI] [PubMed] [Google Scholar]
- 4.Marudanayagam R, Shanmugam V, Gunson B, et al. Aetiology and outcome of acute liver failure. HPB (Oxford) 2009;11:429–434. doi: 10.1111/j.1477-2574.2009.00086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harmanci O, Kav T, Peynircioglu B, et al. Long-term follow-up study in Budd-chiari syndrome: single-center experience in 22 years. J Clin Gastroenterol. 2013;47:706–712. doi: 10.1097/MCG.0b013e31824ffd63. [DOI] [PubMed] [Google Scholar]
- 6.Segev DL, Nguyen GC, Locke JE, et al. Twenty years of liver transplantation for Budd-Chiari syndrome: a national registry analysis. Liver Transpl. 2007;13:1285–1294. doi: 10.1002/lt.21220. [DOI] [PubMed] [Google Scholar]
- 7.Ulrich F, Pratschke J, Neumann U, et al. Eighteen years of liver transplantation experience in patients with advanced Budd-Chiari syndrome. Liver Transpl. 2008;14:144–150. doi: 10.1002/lt.21282. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Pagan JC, Heydtmann M, Raffa S, et al. TIPS for Budd-Chiari syndrome: long term results and prognostics factors in 124 patients. Gastroenterology. 2008;135(3):808–815. doi: 10.1053/j.gastro.2008.05.051. [DOI] [PubMed] [Google Scholar]
- 9.Lee WM, Larson AM, Stravitz RT. AASLD position paper: the management of acute liver failure. Hepatology. 2012;55:965–967. doi: 10.1002/hep.25551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akyildiz M, Karasu Z, Dheir H, et al. Fulminant Budd-Chiari syndrome associated with polycythemia rubra vera and factor V Leiden mutation. Eur J Intern Med. 2006;17:66–67. doi: 10.1016/j.ejim.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 11.Amitrano L, Guardascione MA, Schiavone EM, et al. Hepatic vein thrombosis leading to fulminant hepatic failure in a case of acute non-promyelocytic myelogenous leukemia. Blood Coagul Fibrinolysis. 2006;17:59–61. doi: 10.1097/01.mbc.0000198049.19763.09. [DOI] [PubMed] [Google Scholar]
- 12.Castro I, Rios JJ, Iniesta N, et al. Acute and fulminant Budd-Chiari syndrome in a well-anticoagulated patient with primary antiphospholipid syndrome. Lupus. 2005;14:979–980. doi: 10.1191/0961203305lu2243xx. [DOI] [PubMed] [Google Scholar]
- 13.De Groot CJ, van Goor GM, Stolk M, et al. Liver failure after delivery. Gut. 2005;54:672–709. doi: 10.1136/gut.2004.045252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eckel F, Huber W, Heidecke C, et al. Fulminate intracardiac thrombosis associated with Budd-Chiari syndrome and inferior vena cava thrombosis. VASA. 2002;31:62–65. doi: 10.1024/0301-1526.31.1.62. [DOI] [PubMed] [Google Scholar]
- 15.Fickert P, Ramschak H, Kenner L, et al. Acute Budd-Chiari syndrome with fulminant hepatic failure in a pregnant woman with factor V Leiden mutation. Gastroenterology. 1996;111:1670–1673. doi: 10.1016/s0016-5085(96)70031-8. [DOI] [PubMed] [Google Scholar]
- 16.Hastings GS, O'Connor DK, Pais SO. Transjugular intrahepatic portosystemic shunt placement as a bridge to liver transplantation in fulminant Budd-Chiari syndrome. J Vasc Interv Radiol. 1996;7:616. doi: 10.1016/s1051-0443(96)70818-7. [DOI] [PubMed] [Google Scholar]
- 17.Karti SS, Yilmaz M, Kosucu P, et al. Early medical treatment is life-saving in acute Budd-Chiari due to polycythemia vera. Hepatogastroenterology. 2003;50:512–514. [PubMed] [Google Scholar]
- 18.Korkmaz C, Kasifoglu T, Kebapci M. Budd-Chiari syndrome in the course of Behcet's disease: clinical and laboratory analysis of four cases. Joint Bone Spine. 2006;74:245–248. doi: 10.1016/j.jbspin.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 19.Kuo PC, Johnson LB, Hastings G, et al. Fulminant hepatic failure from the Budd-Chiari syndrome: a bridge to transplantation with transjugular intrahepatic portosystemic shunt. Transplantation. 1996;62:294–296. doi: 10.1097/00007890-199607270-00024. [DOI] [PubMed] [Google Scholar]
- 20.Moreira V, Aller R, De Luis DA, et al. Fulminant acute Budd-Chiari syndrome stemming from an adrenal tumor. J Clin Gastroenterol. 1997;24:110–112. doi: 10.1097/00004836-199703000-00015. [DOI] [PubMed] [Google Scholar]
- 21.Powell-Jackson PR, Ede RJ, Williams R. Budd-Chiari syndrome presenting as fulminant hepatic failure. Gut. 1986;27:1101–1105. doi: 10.1136/gut.27.9.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sandle GI, Layton M, Record CO, et al. Fulminant hepatic failure due to Budd-Chiari syndrome. Lancet. 1980;1:1199. doi: 10.1016/s0140-6736(80)91666-9. [DOI] [PubMed] [Google Scholar]
- 23.Shih KL, Yen HH, Su WW, et al. Fulminant Budd-Chiari syndrome caused by renal cell carcinoma with hepatic vein invasion: report of a case. Eur J Gastroenterol Hepatol. 2009;21:222–224. doi: 10.1097/MEG.0b013e328305ba06. [DOI] [PubMed] [Google Scholar]
- 24.Shrestha R, Durham JD, Wachs M, et al. Use of transjugular intrahepatic portosystemic shunt as a bridge to transplantation in fulminant hepatic failure due to Budd-Chiari syndrome. Am J Gastroenterol. 1997;92:2304–2306. [PubMed] [Google Scholar]
- 25.Stinson J, Tomkin G, McDonald G, et al. Recurrent disseminated intravascular coagulation and fulminant intra hepatic thrombosis in a patient with anti-phospholipid syndrome. Am J Hematol. 1990;35:281–282. doi: 10.1002/ajh.2830350413. [DOI] [PubMed] [Google Scholar]
- 26.Wong RKM, Wai CT. A bolt out of the blue: a case of unexpected acute liver failure. Ann Acad Med Singapore. 2006;35:504–507. [PubMed] [Google Scholar]
- 27.Larson AM, Polson J, Fontana RJ, et al. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42:1364–1372. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- 28.Rautou PE, Moucari R, Cazals-Hatem, et al. Levels and initial course of serum alanine aminotransferase can predict outcome of patients with Budd-Chiari syndrome. Clin Gastroenterol Hepatol. 2009 Nov;7(11):1230–1235. doi: 10.1016/j.cgh.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 29.Schiodt FV, Davern TJ, Shakil AO, et al. Viral hepatitis-related acute liver failure. Am J Gastroenterol. 2003;98:443–453. doi: 10.1111/j.1572-0241.2003.t01-1-07223.x. [DOI] [PubMed] [Google Scholar]
- 30.Murad SD, Plessier A, Hernandez-Guerra M, et al. Etiology, management, and outcome of the Budd-Chiari syndrome. Ann Intern Med. 2009;151:167–175. doi: 10.7326/0003-4819-151-3-200908040-00004. [DOI] [PubMed] [Google Scholar]
- 31.Ostapowicz G, Fontana RJ, Schiodt FV, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947–954. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- 32.Plessier A, Sibert A, Consigny Y, et al. Aiming at minimal invasiveness as a therapeutic strategy for Budd-Chiari syndrome. Hepatology. 2006;44:1308–1316. doi: 10.1002/hep.21354. [DOI] [PubMed] [Google Scholar]
- 33.Seijo S, Plessier A, Hoekstra J, et al. Good long-term outcome of Budd-Chiari syndrome with a step-wise management. Hepatology. 2013;57:1962–1968. doi: 10.1002/hep.26306. [DOI] [PubMed] [Google Scholar]
- 34.Murad SD, Valla DC, de Groen PC, et al. Determinants of survival and the effect of portosystemic shunting in patients with Budd-Chiari syndrome. Hepatology. 2004;39:500–508. doi: 10.1002/hep.20064. [DOI] [PubMed] [Google Scholar]


